A Rare Case of Metastatic Primary Peritoneal Adenocarcinoma Presenting as Breast Cancer
Watson, G.A.*, Greally, M., Murphy, D.J., Doyle, A., Quinn, C., Walshe, J.M
Affiliation
Departments of Medical Oncology and Pathology, St. Vincent’s University Hospital, Elm Park, Dublin, Ireland
Corresponding Author
Geoffrey Alan Watson, Department of Medical Oncology and Pathology, St. Vincent’s University Hospital, Elm Park, Dublin 4, Ireland, E-mail: Geoff_watson7@hotmail.com
Citation
Watson, G.A., et al. A Rare Case of Metastatic Primary Peritoneal Adenocarcinoma Presenting as Breast Cancer. (2016) Int J Cancer Oncol 3(2): 1-4.
Copy rights
© 2016 Watson, G.A. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Ovarian cancer; Carcinoma; Malignancy; Breast; Metastasis; WT1
Abstract
Metastasis to the breast as a presenting feature of extra mammary malignancy is extremely uncommon. Often in this setting, the distinction between primary and secondary breast malignancy can be challenging. Ovarian or primary peritoneal metastasis to the breast is suggestive of disseminated disease and associated with a guarded prognosis, thus accurate differentiation is crucial for early and appropriate intervention. We report the case of a patient presenting with what initially appeared to be locally advanced breast cancer but subsequent review of pathology and imaging confirmed the presence of metastatic primary peritoneal cancer.
Introduction
Case Report
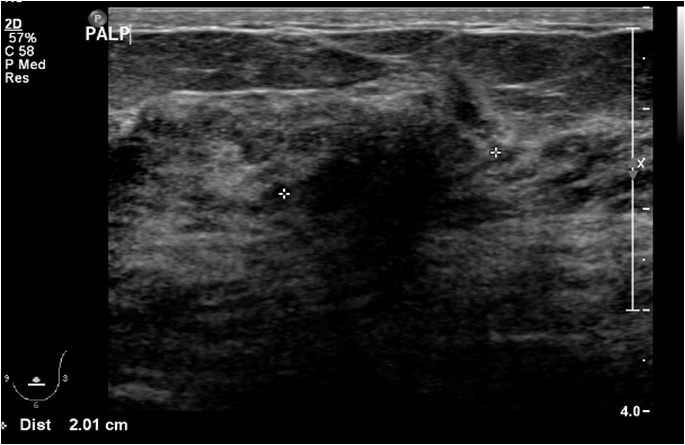
A 61 year old female presented to the triple assessment breast clinic with a three day history of left breast pain following minor trauma. Physical examination revealed an enlarged, pale, and oedematous left breast with nipple retraction and skin dimpling. Mammogram revealed a heterogeneously dense breast parenchyma without a definite mass. Breast ultrasound demonstrated an ill-defined 2cm hypoechoic mass in the left breast with an abnormal ipsilateral axillary lymph node, features concerning for locally advanced breast cancer.(Figure 1).
Figure 1: Targeted left breast ultrasound shows a 2cm hypoechoic mass with irregular margins and posterior acoustic shadowing.
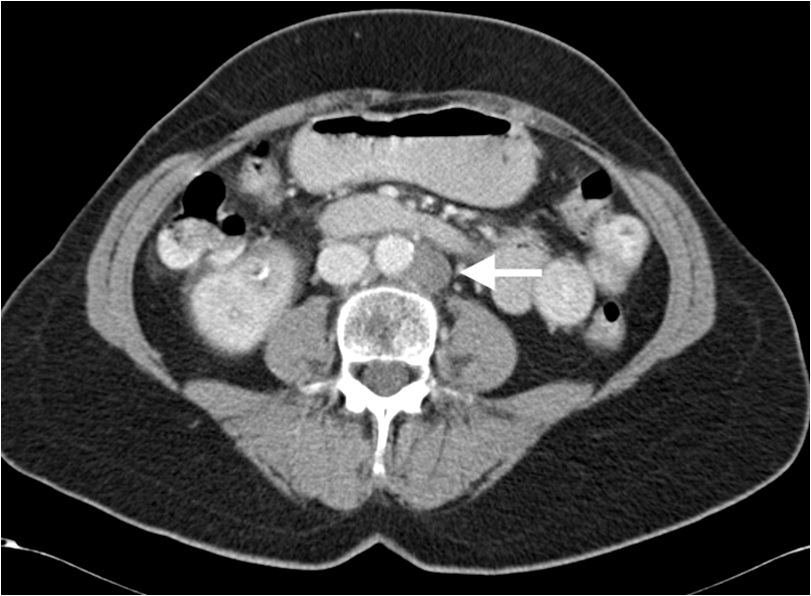
Both the breast mass and axillary lymph nodes were biopsied, and pathological analysis revealed an invasive ductal carcinoma with a nodal metastasis, ER (Oestrogen Receptor) positive, HER2 (Human Epidermal Growth Factor Receptor 2) negative. The breast demonstrated multifocal enhancement in the inferior aspect on Magnetic Resonance Imaging (MRI) and multiple abnormal left axillary nodes were noted. A staging CT of thorax, abdomen and pelvis (TAP) revealed para-aortic lymphadenopathy at the level of the renal arteries, measuring up to 1.8 cm in short axis. A CT guided biopsy of a para-aortic lymph node was positive for metastatic disease. There were no other sites of disease reported. (Figure 2).
Figure 2: Axial abdominal CT with oral and IV contrast shows an enlarged left paraortic retroperitoneal node (arrow).
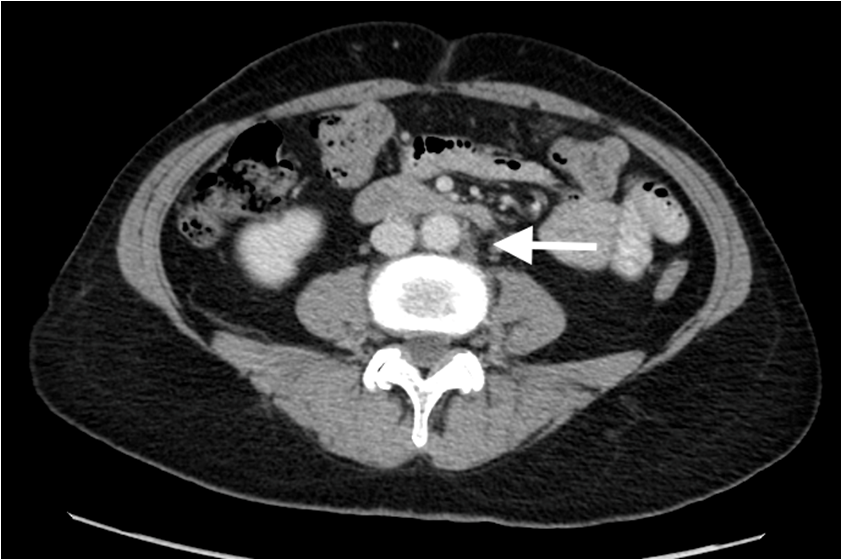
Thus at this time, the clinical picture was Stage IV breast cancer to para-aortic lymph nodes. As local disease control in the breast was a concern, chemotherapy was administered. The patient received dose dense Adriamycin and Cyclophosphamide followed by Taxolchemotherapy, which she tolerated without complication. During chemotherapy, her breast mass reduced but was still palpable. She was then commenced on anti-hormonal therapy, the aromatase inhibitor anastrozoleaiming for ongoing disease control. Follow-up breast imaging after chemotherapy showed stromal thickening in the left breast with abnormal lymph nodes in the left axilla without a focal breast mass, and physical examination revealed resolution of the changes observed at presentation. Restaging CT scan demonstrated reduced retroperitoneal adenopathy; the index para-aortic node reduced in size from 1.8 cm to 0.9 cm (Figure 3).
Figure 3: Partial disease response. Axial CT with oral and IV contrast shows interval reduction in size of left paraortic retroperitoneal node (arrow).
As her disease bulk was low, the role of mastectomy was discussed for local disease control. At that point, her pathology was reviewed by another senior breast pathologist and the possibility of a gynecological primary, rather than primary breast cancer, was raised. WT-1 testing was completed and found to be positive both in the para-aortic lymph node and breast thus suggesting a gynaecological origin. Of note, this woman had a hysterectomy with removal of ovaries 12 years previous for abnormal vaginal bleeding. This surgical specimen was reviewed in light of these findings, however no malignancy was identified. Subsequent review of her imaging from the time of diagnosis revealed very subtle thickening in the omentum, suggestive of a primary peritoneal cancer. When we presented these findings to our patient, she stated that on retrospect she had been experiencing right groin pain and abdominal discomfort which had disappeared on her cytotoxic treatment. A positron emission tomography (PET) scan demonstrated low grade FDG uptake in portocaval and proximal right external iliac regions, consistent with findings on CT imaging. Serum CA-125 at this time was elevated at 53.3 u/ml (normal < 35 u/ml). She was referred to our surgical gynecology colleagues for assessment regarding surgery, however the patient reported a very low symptom burden and thus declined surgical intervention. She remained on anastrozole for two years from the time of her initial diagnosis with a normal CA-125.
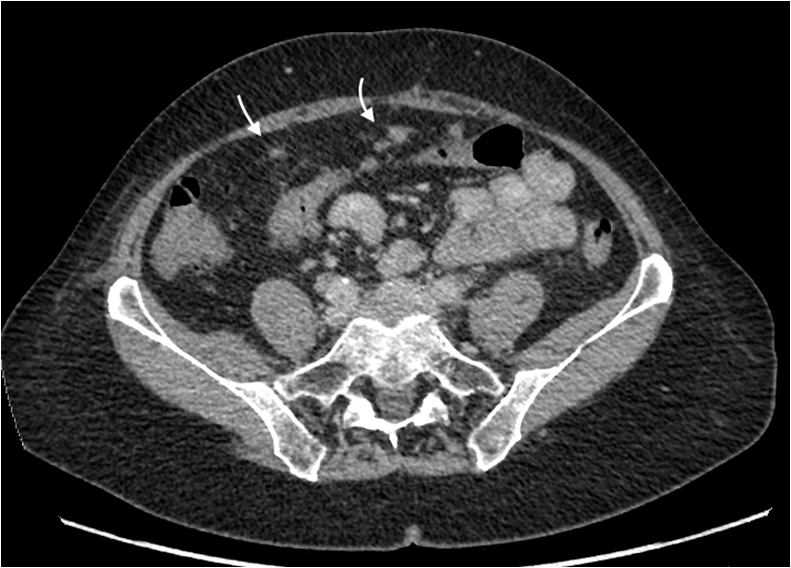
At two years, she was admitted to hospital with acute cholecystitis. A CT Abdomen also revealed the development of multiple peritoneal soft tissue nodules, features consistent with peritoneal carcinomatosis. (Figure 4).
Figure 4: Axial CT abdomen shows multiple peritoneal soft tissue nodules (curved arrows) consistent with peritoneal carcinomatosis.
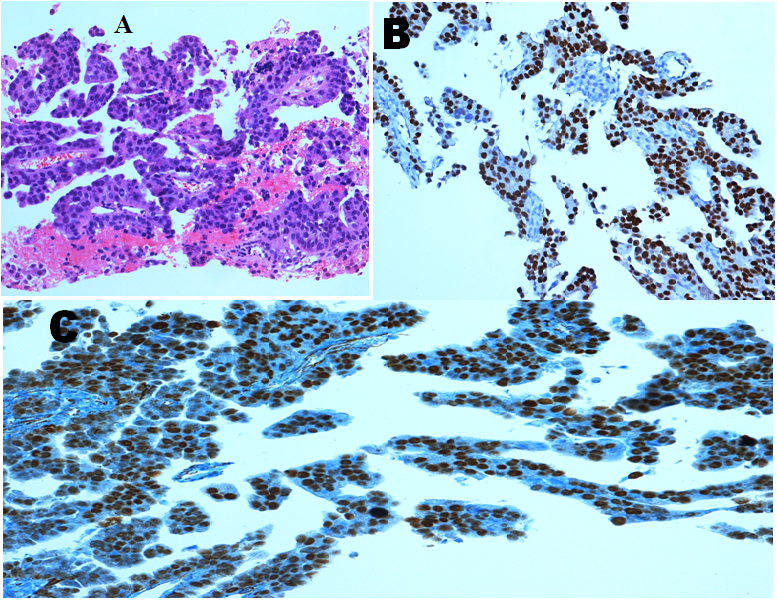
She underwent laparoscopic cholecystectomy, and pathology revealed metastatic adenocarcinoma with features consistent with a peritoneal primary present in multiple submucosal and subserosal lymphatic spaces. Repeat oestrogen receptor (ER) andWT1 testing was performed on this specimen and both were positive. (Figure 5).
Figure 5: Peritoneal biopsy showing (A) metastatic adenocarcinoma (B) Positive Oestrogen Receptor Stain (C) Positive WT1 Stain.
Four years since diagnosis this lady continues to have platinum sensitive peritoneal cancer although the intervals on “break” from therapy have shortened. She was referred for genetic testing although this is very clearly one related process, metastatic peritoneal cancer to breast. She tested BRCA mutation negative. Now four years since diagnosis, our patient remains alive with good quality of life four years since diagnosis of Stage IV primary peritoneal cancer to breast.
Discussion
Metastases to the breast are extremely uncommon, accounting for only 0.5 - 1.3% of breast malignancy[1-5]. The most common sites of origin are lymphoma, melanoma, sarcoma and those from the gastrointestinal and genitourinary tracts in men[2-5]. This presentation is usually associated with advanced disease stage[3,4].
There have been isolated reports of ovarian or peritoneal cancer metastasizing to the breast[1]. The first case was described in 1907 by Sitzenfrey[6]. In a study performed by Hadju and Urban, 4,051 breast cancer patients were identified and the overall incidence of primary gynecologic cancers metastatic to the breast was 0.17%, with only 0.07% of metastatic disease originating from a primary ovarian tumor[3]. The largest literature review by Baykal et al. described 37 cases[7]. Dissemination of ovarian or peritoneal cancer occurs largely via intraperitoneal implantation, but also through lymphatic and haematogenous spread[8]. The most common sites of distant metastasis are the liver and the lung, but certain factors may increase the susceptibility of metastases to the breast, including the absence of hereditary breast–ovarian cancer[5-8].
While diagnosis may be challenging it is important to differentiate between metastatic ovarian/peritoneal cancer and primary breast tumors not only to guide therapy, but also to prevent unnecessary surgical intervention. Treatment options for localized primary breast or ovarian cancer include surgery, radiation and systemic therapy (hormonal therapy and chemotherapy). However in more advanced disease, surgical intervention is less relevant.
Metastatic lesions to the breast tend to be more superficial and less fixed to surrounding tissues than primary breast lesions. Recent studies have shown that 85% of patients present with a solitary tumor, while only 4% have diffuse involvement[1,6]. The most common location is the upper outer quadrant (62% of patients)[1,9].
While various imaging modalities may aid in achieving a correct diagnosis, breast metastasis from primary ovarian/ peritoneal tumors share many morphological features with primary breast cancers, and this was observed in the present case. On mammography, metastatic lesions may appear as discrete, well-circumscribed masses while primary breast cancers typically appear speculated and display architectural distortion that metastases typically do not[1,7,9]. Micro calcifications may rarely be seen in ovarian cancer metastases due to the presence of psammoma bodies[10,11]. Microscopically, both cancers display marked nuclear atypia, brisk mitotic activity and papillary architectural patterns[2,11].
Immunohistochemistry testing with tissue-specific markers may be helpful in determining the primary site of origin when an extra mammary malignancy is suspected. Ovarian carcinoma and breast carcinoma share many immunohistochemical features (e.g., ER, PR, HER2/neu, CA125, CK7, CK20)[11]. However, other candidate markers (e.g., GCDPF-15/BRST2, WT1, PAX8, PAX2, and mammaglobin) may also be useful[11]. WT1 has been identified as a reliable marker in differentiating metastatic ovarian/peritoneal cancer from other primary sites due to its high sensitivity and low potential for aberrant expression[11,12-14]. The most common histological variant of ovarian cancer associated with metastatic disease to the breast is papillary serous Adenocarcinoma[10]. WT1 is expressed in approximately 95% of serous ovarian carcinomas[15].
Secondary breast involvement from an ovarian/peritoneal tumor may be a harbinger of widespread dissemination. Approximately 70% of patients presenting with a metastatic breast lesion have widespread metastatic disease at the time of diagnosis and prognosis is generally poor[4]. In previous studies, survival ranged from 13 days to 3.5 years , with most patients dying within 1 year[1,5,15]. Klein et al. 2010 suggest inflammatory metastatic disease to the breast conferred a graver prognosis, with a median survival of 6 months[1].
Conclusion
Ovarian or peritoneal metastasis to the breast is rare, and diagnosis may be difficult as metastases share many common features with primary breast cancer. Clinical presentation, imaging and immunohistochemistry may aid in reaching a correct diagnosis. In our study WT1 testing was a vital component in reaching the correct diagnosis. Accurate differentiation is necessary because treatment differs significantly for patients with ovarian/peritoneal metastasis to the breast, as compared with patients with primary breast cancer. These metastases to the breast appear to confer a poor prognosis as patients often have widespread advanced disease. Despite this, our patient is alive four years since diagnosis, perhaps due to her low volume of disease at initial presentation. Physician awareness of this clinical entity is paramount in allowing early recognition and the initiation of appropriate therapy and optimizing patient outcomes.
Consent: Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of interest:
The authors declare no conflict of interest.
References
- 1. Klein, R., Brown, A., Gomez-Castro, C.M., et al. Ovarian Cancer Metastatic to the Breast Presenting as Inflammatory Breast Cancer: A Case Report and Literature Review. (2010) J Cancer 1: 27–31.
- 2. Wadhwa, J., Dawar, R., Kumar, L. Ovarian carcinoma metastatic to the breast. (1999) Clinical Oncology 11(6): 419–421.
- 3. Hajdu, S.I., Urban, J.A. Cancers metastatic to the breast. (1972) Cancer 29(6): 1691-1696.
- 4. Vizcaíno, I., Torregrosa, A., Higueras, V., et al. Metastasis to the breast from extramammary malignancies: a report of four cases and a review of literature. (2001) Eur Radiol 11(9):1659-1665.
- 5. Ozguroglu, M., Ersavasti, G., Ilvan, S., et al. Bilateral inflammatory breast metastases of epithelial ovarian cancer. (1999) Am J Clin Oncol 22(4): 408–410.
- 6. Kayikçioglu, F., Boran, N., Ayhan, A., et al. Inflammatory breast metastases of ovarian cancer: a case report. (2001) Gynecologic Oncology 83(3): 613–616.
- 7. Baykal, C., Tulunay, G., Özfuttu, A., et al. Breast and ovarian carcinoma in the same patient, metastasis or dual primaries? (2007) Turkish Journal of Cancer 37(1): 27-30.
- 8. Cormio, G., Rossi, C., Cazzolla, A., et al. Distant metastases in ovarian carcinoma. (2003) Int J Gynecol Cancer 13(2): 125–129.
- 9. Moore, D., Wilson, D., Hurteau, J., et al. Gynecologic cancers metastatic to the breast. (1998) J Am Coll Surg 187(2): 178–181.
- 10. Yamasaki, H., Saw, D., Zdanowitz, J., et al. Ovarian carcinoma metastasis to the breast: a case report and review of the literature. (1993) Am J Surg Pathol 17(2): 193–197.
- 11. Mhawech-Faucegliaa, P., Kaya, B., Lib, C.J., et al. Metastatic ovarian papillary serous carcinoma to the breast: Diagnosis and pitfalls. (2013) Gynecol Oncol Case Reports 4: 35–37.
- 12. Goldstein, N.S., Bassi, D., Uzieblo, A. WT1 is an integral component of an antibody panel to distinguish pancreaticobiliary and some ovarian epithelial neoplasms. (2001) Am J Clin Pathol 116(2): 246–252.
- 13. Lee, B.H., Hecht, J.L., Pinkus, J.L., et al. WT1, estrogen receptor, and progesterone receptor as markers for breast or ovarian primary sites in metastatic adenocarcinoma to body fluids. (2002) Am J Clin Pathol 117(5): 745–750.
- 14. Al-Hussaini, M., Stockman, A., Foster, H., et al. WT-1 assists in distinguishing ovarian from uterine serous carcinoma and in distinguishing between serous and endometrioid ovarian carcinoma. (2004) Histopathology 44(2): 109–115.
- 15. Micha, J., Goldstein, B.H., Epstein, H.D., et al. Ovarian cancer metastatic to the breast. (2006) Gynecol Oncol 102(2): 386–390.