Avascular Necrosis of Humeral Head after Thalidomide Use: A Report of Two Cases
Ahmad Rezaeian
Affiliation
Department of Otorhinolaryngology, Head and Neck Surgery, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Corresponding Author
Ahmad Rezaeian, Department of Otorhinolaryngology, Head and Neck Surgery, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran, Tel: +989133156278/ Fax: +983136688597; E-mail: dr.ahmadrezaeian@gmail.com
Citation
Rezaeian, A. Avascular Necrosis of Humeral Head after Thalidomide Use: A Report of Two Cases. (2018) Int J Hematol Ther 4(1): 31- 33.
Copy rights
© 2018 Rezaeian, A. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Thalidomide; Multiple myeloma; Necrosis Humeral bone
Abstract
Background: Thalidomide is an immunomodulatory drug which is used for treatment of certain cancers such as multiple myeloma. Multiple Myeloma (MM) is a cancer that creates a specific type of white blood cell called plasma cell and accumulates these cancerous cells in bone marrow. To our knowledge, there is no report of avascular necrosis of humeral head after thalidomide use; hence, the aim of present study was to report two cases of avascular necrosis of humeral head after thalidomide use in MM patients.
Case reports: Twenty five patients with history of MM were evaluated for the adverse effects of thalidomide from 2008 to 2014 at Department of Orthopedics, Isfahan University of Medical Sciences-Isfahan- Iran. All cases were assessed by X-ray and bone scan.
Conclusion: Out of 25, two cases were diagnosed with avascular necrosis of humeral head with pain in shoulders. It seems that thalidomide can cause necrosis in humeral head.
Introduction
Multiple Myeloma (MM) is a disabling cancer that is part of a spectrum of diseases ranging from monoclonal gammopathy of unknown significance to plasma cell leukemia. MM is characterized by several features, including low blood counts, bone and calcium problems, infections, kidney problems, monoclonal gammopathy, light chain amyloidosis and solitary plasmacytoma[1].Thalidomide was first introduced for patients with refractory multiple myeloma. Due to the promising results achieved in these patients, the drug was subsequently used in the earlier stages of the disease[2]. In this paper, we review the available clinical data regarding the adverse effects of thalidomide in the patients with MM.
Case Report
Case One
A 63-year-old man was referred to the Orthopedics Department by an oncologist owing to right upper limb pain. He had been diagnosed with MM 18 months ago and had been under treatment by thalidomide (200 mg/day) for 16 months. The patient used acetaminophen (pro re nate) for the shoulder and joint pain. He had a remarkable medical history, including peptic ulcer disease and gastrointestinal bleeding and was under treatment by protein pump inhibitor (Omiperazol 20 mg/day) and histamine receptor blocker (Famotidine 10 mg/day). There was no report and history of sickle cell anemia, gaucher disease, alcohol use, hypercoagulopathy and use of bisphosphonate. The patient did not use any corticosteroids, too. He suffered from increasing shoulder pain and reported having a long period of suffering. Pain was more in the right shoulder, particularly in the range of motion. During the increasing pain, a limited range of motion in daily activities was observed. Limitations extended over months.
Clinical evaluation
Clinical examinations showed right shoulder muscular atrophy. In all range of motions, including flexion, abduction, rotation limitation and pain on was remarkable.
Radiographic evaluation
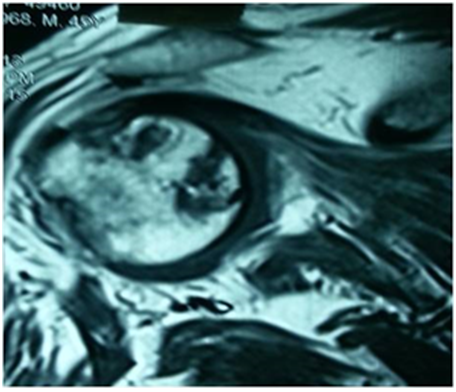
Standard radiological diagnoses include conventional x-ray images. True anterior-posterior (AP) view (Figure 1) showed subchondral sclerosis and a radiolucent area in humeral head. Also, there were some degenerative changes in the articular surface of humeral head. MRI determined the avascular necrosis of humeral head (Figure 2).
Figure 1: True anterior-posterior (AP) view in case 1: subchondral sclerosis and radiolucent area in humeral head.
Figure 2: MRI view in case 1: the avascular necrosis of humeral head.
Case Two
A 59-year-old female was referred to the Orthopedics Department by an oncologist owing to left upper limb and lumbar pain. He had been diagnosed with MM 13 months ago and had been under treatment by thalidomide (200 mg/day) for 13 months. The patient used acetaminophen (PRN) for the shoulder and joint pain. She had a remarkable medical history, including diabetic mellitus, for which she used metformin 500 mg/day. There was no report and history of sickle cell anemia, gaucher disease, alcohol use, hypercoagulopathy and use of bisphosphonate. The patient did not use any corticosteroids, too. She suffered from increasing lumbar and shoulder pain and reported having had this condition since three months ago. Pain was more in the left shoulder, particularly in the range of motion. Owing to left shoulder pain, the patient had limited mobility and loss of function in daily activities.
Clinical evaluation
Clinical examinations showed left shoulder muscular atrophy. In all range of motions, including flexion, abduction, rotation limitation and pain on was remarkable. Also, tenderness was noted in lumbar spine examination.
Radiographic evaluation
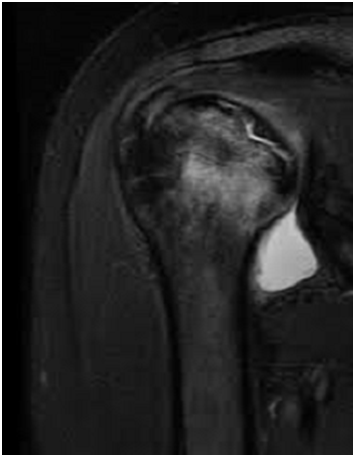
Standard radiological diagnoses include conventional x-ray images. True anterior-posterior (AP) view (Figure 3) revealed subchondral sclerosis and a radiolucent area in humeral head. Also, there were some degenerative changes in the articular surface of humeral head. MRI determined the avascular necrosis of humeral head (Figure 4).
Figure 3: True anterior-posterior (AP) view in case 2: the subchondral sclerosis and radiolucent area in humeral head.
Figure 4: MRI view in case 2: the avascular necrosis of humeral head.
Discussion
In this study, we found avascular necrosis in two Iranian patients with multiple myeloma disease who were treated with thalidomide (200 mg/day) for more than 10 months. Therefore, thalidomide may cause necrosis in the articular surface, although this case report is the first study reporting avascular necrosis in treatment with thalidomide in multiple myeloma patients. Thalidomide is an immunomodulatory and anti-inflammatory drug which stops neoangiogenesis in malignant tumors, although its mechanism is still unknown[3,4]. We have this hypothesis that thalidomide prevents neoangiogenesis in cartilage; therefore, it may be caused by avascular necrosis. Other studies have shown that thalidomide is an endogenous metabolite of estradiol and 2-methoxyestradiol and suppresses disease in collagen-induced arthritis by exerting antiangiogenic effects[5,6]. In the study by Song et al[7], thalidomide exacerbated zoledronate-induced first-stage BRONJ (Bisphosphonate-Related Osteonecrosis of the Jaws) and caused osteonecrosis of the jaw and inhibition of angiogenesis. Also, thalidomide has teratogenic effects and causes orthopedic malformations. In the past, thalidomide was used for sedation in pregnant woman; therefore, the children were born with many musculoskeletal malformations. Musculoskeletal presentations of this children included spinal defects such as block vertebrae, spondylolysis, scoliosis, malformations of intervertebral disc and dysgenesia of sacrum[8-11]. In the study by Panousis evaluating thalidomide effect on musculoskeletal injury in an experimental model, thalidomide prolonged the survival of experimental models with musculoskeletal injury by preventing mononuclear apoptosis. Therefore, thalidomide can be used for management of severe traumas[12]. Thalidomide has several adverse effect on the patients with multiple myeloma, but the most common complication is toxic neuropathy. Other side effect are less common and are controlled easily by drugs[13]. In the present study, the patients were treated with a low dose of thalidomide. More adverse effects of thalidomide are also dose-dependent. Offidani et al[14] showed an association between thalidomide dose and side effects in multiple myeloma; therefore, the most common side effects such as constipation, somnolence and fatigue were not dose-dependent. However, peripheral neuropathy was dose-dependent. Also, a daily dose of thalidomide (150 mg) had minimal side effects. The study by Talamo, Giampaolo, et al[15] had an opposite result with our study, they concluded that avascular necrosis is a rare complication in multiple myeloma so male sex, dexamethasone dose and younger age increased risk of avascular necrosis and didn’t have relation between thalidomide and avascular necrosis.
Conclusion
In conclusion, Avascular necrosis is a rare and non-common complication in multiple myeloma patients and this complication occur in the long term of disease. other studies concluded that using of corticosteroids can induce this complication in the multiple myeloma but these studies didn’t evaluate effect of thalidomide in the multiple myeloma or concluded non effect of thalidomide in avascular necrosis, also in our study patients didn’t use corticosteroids in during of therapy and were new case of multiple myeloma (diagnosed 16 or 18 months before complication). It’s seen, this avascular necrosis in our patients was new complications that may be induce with thalidomide usage.
The other adverse effects of thalidomide are rare but may occur. Moreover, musculoskeletal side effects may occur as a result of using thalidomide in multiple myeloma, but in a dose-dependent manner. Therefore, there was no report of avascular necrosis in Iranian multiple myeloma patients as a result of using thalidomide (200 mg/day), but we found two cases with avascular necrosis in using thalidomide possibly due to antiangiogenic effects on articular cartilage. Hence, future studies are required to confirm our findings.
Conflict of interest: The authors declare no conflict of interest whatsoever arising out of the publication of this manuscript
Funding: No funding
References
1. Stamo, A., Grigor’eva, V. Clinical characteristics of pain syndrome in patients with multiple myeloma. (2016) Zh Nevrol Psikhiatr Im S S Korsakova 116(10): 11-15.
PubMed||CrossRef||Others
2. Musto, P., Anderson, K., Attal, M., et al. Second primary malignancies in multiple myeloma: an overview and IMWG consensus. (2016) Ann Oncol 28(2): 228-245.
3. Bertolini, F., Mingrone, W., Alietti, A., et al. Thalidomide in multiple myeloma, myelodysplastic syndromes and histiocytosis. Analysis of clinical results and of surrogate angiogenesis markers. (2001) Ann Oncol 12(7): 987-990.
PubMed||CrossRef||Others
4. Zangari, M., Elice, F., Tricot, G. Immunomodulatory drugs in multiple myeloma. (2005) Exp Opinion Invest Drugs 14(11): 1411-1418.
5. Josefsson, E., Tarkowski, A. Suppression of type II collagen-induced arthritis by the endogenous estrogen metabolite 2-methoxyestradiol. (1997) Arthritis Rheum 40(1): 154-163.
6. Oliver, S., Cheng, T.P., Banquerigo, M.L., et al. The effect of thalidomide and 2 analogs on collagen induced arthritis. (1998) J Rheumatol 25(5): 964-969.
7. Song, Z., Dong, W., Yin, L., et al. Effect of thalidomide on development of bisphosphonate-related osteonecrosis of the jaws in rat. (2015) Nan Fang Yi Ke Da Xue Xue Bao 35(8): 1084-1089.
8. Lenz, W., Knapp, K. Thalidomide embryopathy. (1962b) Arch Environm Health 5: 100-105.
PubMed||CrossRef||Others
9. Brent, R.L., Holmes, L.B. Clinical and basic science lessons from the thalidomide tragedy: what have we learned about the causes of limb defects? (1988) Teratology 38(3): 241-251.
10. Ruffing, L. The spine in the thalidomide-embryopathy. (1980) Fortschr Med 98(11): 405-409.
PubMed||CrossRef||Others
11. Edwards, D., Nichols, P. The Spinal Abnormalities in Thaltdomide Embryopathy. (1977) Acta Orthop Scand 48(3): 273-276.
12. Panousis, K., Nikolaou, V.S., Tsaganos, T., et al. Thalidomide prolongs survival after experimental musculoskeletal injury, through an effect on mononuclear apoptosis. (2014) J Surg Res 188(1): 198-205.
13. Novosad, O.I., Kriachok, I.A., Kadnikova, T.V., et al. Experience of using thalidomide in the treatment of patients with multiple myeloma. (2009) Lik Sprava (3-4): 79-86.
PubMed||CrossRef||Others
14. Offidani, M., Corvatta, L., Marconi, M., et al. Common and rare side-effects of low-dose thalidomide in multiple myeloma: focus on the dose-minimizing peripheral neuropathy. (2004) Eur J Haematol 72(6): 403-409.
15. Talamo, G.P., Angtuaco, E.J.C., Walker, R.C., et al. Avascular necrosis of femoral and/or humeral heads in multiple myeloma: results of a prospective study of patients treated with dexamethasone-based regimens and high-dose chemotherapy. (2005) J Clin Oncol 23(22): 5217-5223.