Beneficial Effects of Chlorogenic Acids on Essential Hypertension
Jacob Yumha Laiz
Affiliation
Molecular and Clinical Pharmacology Program, Institute of Biomedical Sciences, Faculty of Medicine, University of Chile, Chile Santiago, Chile
Corresponding Author
Dr. Ramón Rodrigo, Molecular and Clinical Pharmacology Program,Institute of Biomedical Sciences, Faculty of Medicine, University of Chile, Independencia 1027,Santiago, Chile, Tel: 56-2-29786126; E-mail: rrodrigo@med.uchile.cl
Citation
Rodrigo, R., et al. Beneficial Effects of Chlorogenic Acids on Essential Hypertension. (2016) Int J Food Nutr Sci 3(1): 213- 217.
Copy rights
© 2016 Rodrigo, R. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Chlorogenic acids, Essential hypertension, Reactive oxygen species, Nitric oxide, Endothelium, Oxidative stress, AntioxidantsJacob Yumha Laiz, Ramon Rodrigo
Abstract
Hypertension is a worldwide condition considered as the most important risk factor in the development of the cardiovascular disease. The contribution of the oxidative stress in its pathophysiology is a well known fact. Polyphenols are potent antioxidant compounds able to mitigate the damage produced by reactive oxygen species. Chlorogenic acids are polyphenols occurring in many dietary sources but primarily in green coffee extracts. Several studies have shown their antioxidant and anti-inflammatory properties. In the last decades there have been performed several clinical trials showing that the use of these compounds decrease the systolic and diastolic blood pressures probably by increasing the bioavailability of nitric oxide and improving the endothelium function. These properties could be the bases for supporting an important adjuvant therapy for the treatment of essential hypertension
Introduction
Increasing body of evidence has involved the contribution of oxidative stress in the mechanism of essential hypertension[1]. Accordingly, it has been demonstrated that patients with hypertension have increased production of reactive oxygen species (ROS) [2,3] and lower levels of antioxidant species[4]. In addition, it has been found that ROS production is enhanced, redox-dependent signaling is amplified, and antioxidant bioactivity is reduced in the arteries of hypertensive humans[5], all accounting for a role of vascular oxidative stress in the pathogenesis of essential hypertension[6-8].
The ROS play a physiological role in the homeostasis of the vascular wall by regulating the vascular tone and endothelial function[6,9]. This regulation is related to the activation/down-regulation of metabolic pathways modulated by low-to-moderate concentration of ROS[10]. However, when there is an imbalance between ROS production and antioxidant species the endothelium shifts its actions generating a reduction in the vasodilation of the wall and an increasing in the proinflammatory state and prothrombotic setting[11].
In the vascular wall different sources of ROS coexist, which can be classified as enzymatic and nonenzymatic compounds. NADPH oxidase is the primary biochemical source of superoxide in the vascular endothelium. Nevertheless, there are other enzymes that contribute to the oxidative stress including xanthine oxidase, mitochondrial enzymes and uncoupled endothelial nitric oxide synthase (eNOS)[11].
The main generator of ROS in the vasculature is NADPH oxidase (NOX) that produces superoxide anion by reducing molecular oxygen. This enzyme is up-regulated in hypertension primarily by molecular signs[11]. The superoxide anion can interact with some molecules to form other free radicals or can react with biomolecules generating a disruption in the biological processes of the cell. For example, L-arginine and tetrahydrobiopterin (BH4) are two essential cofactors of eNOS. Their deficiency or oxidation (BH4) are associated with uncoupling of the L-arginine-nitric oxide (NO) pathway resulting in decreased formation of NO and increased eNOS-mediated generation of superoxide instead. Furthermore, superoxide can combine with NO to form peroxynitrite that can also oxidize and destabilize eNOS to produce more superoxide[12,13] leading to a positive feed-back reaction. In turn, the xanthine oxidase enzyme catalyzes the last two steps of purine metabolism. During this reaction, oxygen is reduced to superoxide. There is evidence that in spontaneously hypertensive rats (SHR) the xanthine oxidase is up-regulated, thus leading to increased ROS production and increased vascular tone[14].
The mitochondria are a major source of ROS. It has been studied that a part of the superoxide that is produced in the intermembrane space may be carried to the cytoplasm[15], being the complex I the main source of superoxide generated in the mammalian mitochondria. Normally, the other complexes do not produce significant levels of superoxide. Aside from this increased ROS source, it has been reported a reduction in antioxidant enzymatic activity in patients with hypertension[16].
There are some factors able to protect the tissues against oxidative stress, being NO one of the most important endogenous factors. The latter is known to play an important role as a key paracrine regulator of vascular tone.
Physiologically, NO inhibits leukocyte–endothelial cell adhesion, vascular smooth muscle cell (VSMC) proliferation and migration, and platelet aggregation, all contributing to maintain the health of the vascular wall. Therefore, taking into account the diverse beneficial effects of NO, it is conceivable that decreased NO bioavailability in the vasculature reduces vasodilatory capacity, thereby contributing to the development of hypertension.
Except the vasorelaxing and antiproliferative properties per se, NO plays an important role in antagonizing the effects of angiotensin II (AT-II), endothelins and ROS[11].
NO not solely antagonizes the effects of AT-II on vascular tone, cell growth, and renal sodium excretion, but also down-regulates the synthesis of angiotensin I converting enzyme (ACE) and AT1 receptors expression [1,11].
It is noteworthy that exogenous antioxidants are also able to abrogate the effect of increased ROS in the vascular wall, thereby leading to beneficial effects. On this line, polyphenols can be used to decrease the oxidative stress. In the last decades there were studies demonstrating that these compounds have antioxidant and anti-inflammatory properties. They can participate in the modulation of different cellular pathways to improve the endothelial function[17]. For example they can enhance the bioavailability of NO by different mechanisms including activation of eNOS by the PI3-kinase/Akt pathway[18]. In addition, they can inhibit ROS generators enzymes such as NADPH and xanthine oxidase and increase the levels of glutathione[17]. On the other hand, there is also evidence that they can prevent the COX-dependent formation of endothelium-derived contracting factors[19].
Chlorogenic acids (CGAs) are one of the polyphenols that have gained interest during the last years. There are several studies that related CGAs properties with an improvement of glucose and insulin metabolism[20]. Knowing that hypertension is the most important risk factor in the development of cardiovascular diseases (CV)[21], it could represent a relevant target of reducing the cardiovascular risk; in agreement with several studies showing that the use of green coffee extracts (GCEs) causes decreased values of blood pressure in humans[22]. Those effects have been attributed to the polyphenols present in the GCEs. As CGAs are the major polyphenols present in coffee, this effect was attributed primarily to them. All of these properties could be probably explained by their capacity to improve the bioavailability of nitric oxide and endothelial function. However it is important to considerer that even though there are not many studies about the possible negative effects of CGAs in humans, some experiments in rats have shown more incidence in carcinomic effects when treated with caffeic acid[23]. That fact probably indicates that it should be considerer a balance between the positive and negative effects of these compounds.
The aim of this chapter was to present an update of the studies supporting a role of CGAs to counteract the oxidative stress and herein the development of essential hypertension.
The Role of Chlorogenic Acid in the Essential Hypertension.
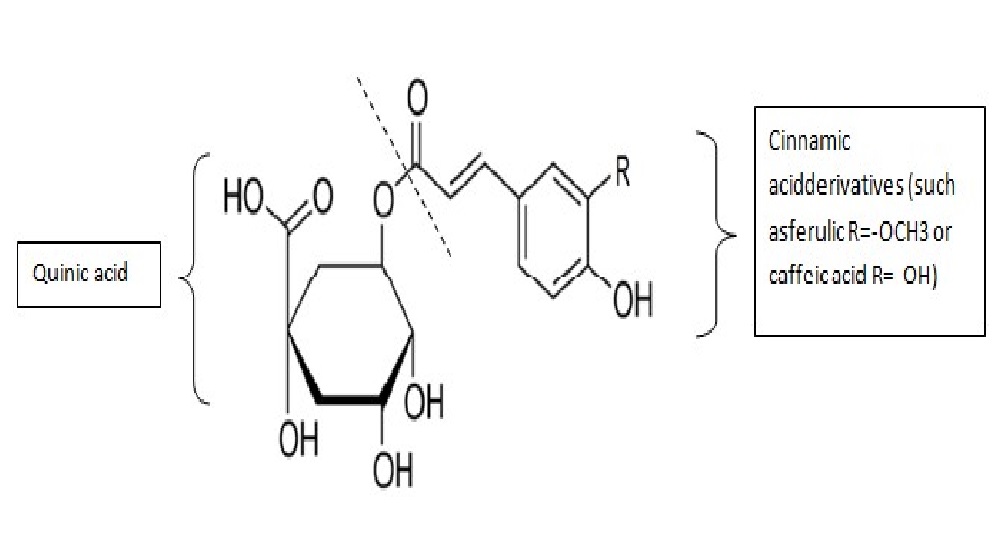
The CGAs are a family of polyphenols formed through a reaction of esterification between a trans-cinnamic acid and a quinic acid (Figure 1). They are found in many dietary sources such as fruits, vegetables and seed. The most commonly CGA found in coffee, is 5-O-caffeoylquinic acid, often called “chlorogenic acid”, being one of the most important source of CGAs[24]. Moreover, due to the high consumption of coffee worldwide, CGAs are one of the major founts of polyphenols in the human diet.
Figure 1: Molecular structure of chlorogenic acids. These compounds are formed through a reaction of esterification between a trans-cinnamic acid derivativeand a quinic acid. The capacity of resonance of the phenol group allows them to be a free radical scavenger.
Recently, there have been several studies that analyze the importance of these compounds in some diseases. One of the most interest areas is their possible protection in cardiovascular diseases.
There is evidence that the use of CGAs may improve the glucose metabolism and insulin sensitivity[20], thereby reducing the relative risk of type 2 diabetes[25,26]. In addition, it probably improves the reduction of weight in obesity[27,28] and is related with the reduction of the relative risk of stroke in women patients[29,30].
Mechanism of CGAs against Essential Hypertension
Like polyphenols CGAs have antioxidant and anti-inflammatory properties but their mechanism is not completely understood[22]. The total effect in the blood pressure is produced by a combination of various CGAs metabolites with different power of action[31]. With a common dietary intake of GCEs, CGAs are hydrolyzed in the small intestine to form the two principal components of the CGAs which are trans-cinnamic acid (such as caffeic and ferulic acids) and quinic acids. Some studies have analyzed that the major percentage of CGAs present in an infusion of coffee are formed by a caffeoylquinic acids (86 %)[32-44]. The hydroxylation takes place in the small intestine but only one third of the CGAs is absorbed there. The majority of the CGAs metabolites are absorbed in the large intestine after interacting with the microflora where caffeic acid can be converted to ferulic acid by a transferase or be reduced to dihydrocaffeic acid[22].
Studies in SHR suggested that the most powerful hypotensive effect is produced by ferulic acid with an approximately 9 times better decrease than caffeic acid and 17 times better decrease than quinic acid[31]. For that reason we will emphasize our analysis in the ferulic acids (FAs).
The hypotensive effect of FAs could be probably explained from their antioxidant properties. They can act not solely as scavengers[35] but also as non-selective NOX antagonists[36,37], contributing to a minor production of superoxide and less formation of ROS. This condition will protect the eNOS from disruption and increase the bioavailability of NO[38], leading to an improvement in the endothelial function. In addition, there is evidence that they can enhance acetylcholine induced endothelial dependent vasodilation[38] and inhibit the vascular proliferation of SMVC induced by angiotensin II[39].
Caffeic acid shows similar properties of FAs like their regulation of some function of angiotensin II. In SHR it was analyzed that these effects were due to a blocking of metabolic pathways involved in the process like JAK/STAT cascade or Ras/Raf signaling[40]. Additionally, it has been observed that caffeic acid could decrease the levels of Rac 1 GTPase, which can participate in the oxidative stress by contributing to generation of ROS[36,41].
Furthermore, CGAs might interact with the renin-angiotensin aldosterone system by inhibiting ACE activity as shown both in vitro and in vivo[41-43].
As polyphenols, the anti-inflammatory properties of CGAs could not be explained by their antioxidant properties. They probably will regulate some signaling pathways such as iκb/NF-κb or even interfere with the COX-2 activation. In a study of hepatic ischemia reperfusion injury in rats, CGAs shows protection properties. In addition they inhibited the translocation of the factors NF-κB and IRF-1 and induce the Nrf2[44].
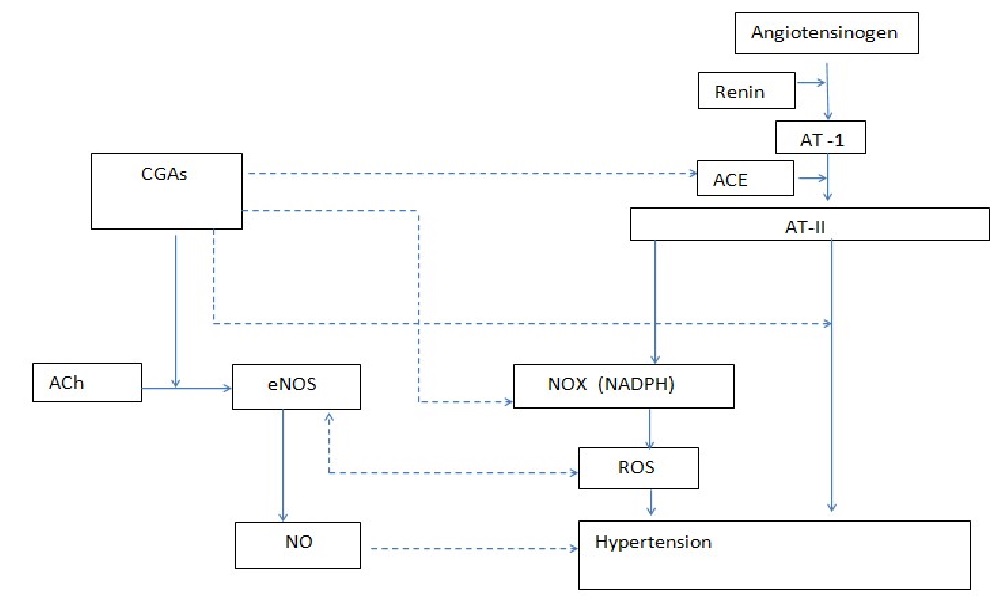
However this area has been less explored and not well elucidated yet but it may have importance in the long-term regulation and could be an important therapeutic strategy for atherosclerotic disease[31,44]. Figure 2
Figure 2: A flux diagram showing the molecular mechanisms of CGAs against essential hypertension. Abbreviations: CGAs (Chlorogenic acids), AT-1 (Angiotensin I), ACE (A-1 converter enzyme), AT-II (Angiotensin II), Ach (Acetylcholine), eNOS (endothelial nitric oxide synthase), NO (Nitric oxide), ROS (Reactive oxygen species).
⤏:inhibition
⟶:stimulation
Studies in Human Patients
Numerous studies investigating the effect of CGAs in blood pressure have been performed however, in a recent review of meta-analysis of randomized clinical trials (RCTs), only 5 of them where found eligible and with duration of more than 4 weeks. They demonstrated a significant reduction in the systolic (MD: -4.31 mmHg) and diastolic (MD: -3.68 mmHg) blood pressures compared with the placebo/control group[46]. Patients selected in two RCTs were normotensive whereas in three of them they had mild essential hypertension. There were no adverse effects reported in those studies.
However these studies have some limitations that are important to expose. One of them is that all of them took place in Asia (four in Japan and one in India). This shows that it is unclear if the properties of CGAs can be present in occidental people. In addition, the studies have different methodology, design, duration (ranges 4 to 26 weeks) and have small sample size which can probably biased the results[47-50].
Perspectives
It seems that CGAs could have some beneficial effects on essential hypertension, based on their biological properties. There is a biomolecular basis that supports this theory and studies that have positive results. However, it is necessary to diminish the limitations of those studies in order to improve their eligibility. For example, it is imperative to analyze these results in people with descent other than Asian, have a bigger sample size, increase the duration of the studies in order to investigate if it is really no adverse effects, determine the minimum effective dose of CGA which can reduce the blood pressure and unified the methodology and design of the studies in order to reduce the big heterogeneity that they have between each other.
Nonetheless, the use of CGA could be very useful in patients with incipient or low hypertension due to the molecular basis of their action that antagonizes with the remodeling mechanisms in blood vessels. This effect is more debatable in patients with high or long term hypertension.
Although there is a reduction in the blood pressure values, the impact of that reduction in the clinical practice is modest at best. It is likely that the use of CGA could be focused on the prevention rather than the treatment of established hypertension.
Conclusion
Cumulated evidence shows that oxidative stress participates in the pathophysiology of the essential hypertension. In this context, is reasonable to propose that polyphenols such as CGAs could have protective effects in this disease. The use of CGAs has been related to a vasodilatory response probably mediated by an increase of NO bioavailability in the vascular wall. This leads to a modest decrease of the systolic and diastolic blood pressure values. Although the results of RCTs are controversial due to the big heterogeneity in the designs between each other, it seems consensual that CGAs can reduce the blood pressure values.
The clinical impact of this reduction is still uncertain but it probably could support a prevention strategy or as a complement to the conventional established therapies for treatment of essential hypertension (adjunct therapy).
Finally, it is important to note that the molecular mechanisms involved in the vasodilatory response are not elucidated yet and there are probably more molecular pathways able to participate in the antioxidant function. For example, the regulation of the metabolic Keap1/Nrf2 pathway which is activated in low-to-moderated ROS concentration, thus giving rise to a very important target of study because it promotes the antioxidant response cell. As CGAs acts as free radical scavengers, they could decrease the concentration of ROS and indirectly activate this pathway.
References
- 1. Rodrigo, R., González, J., Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. (2011)Hypertens Res 34(4): 431–440.
- 2. Lacy, F., Kailasam, M.T., O’Connor, D.T., et al. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. (2000)Hypertension 36(5): 878-884.
- 3. Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? (2004)Hypertension 44(3): 248-252.
- 4. Briones, A.M., Touyz, R.M. Oxidative stress and hypertension: current concepts. (2010)Hypertension Rep 12(2): 135-142.
- 5. Touyz, R.M., Schiffrin, E.L. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. (2001) J Hypertens 19(7): 1245-1254.
- 6. Paravicini, T.M., Touyz, R.M. Redox signaling in hypertension. (2006)Cardiovasc Res 71(2): 247-258
- 7. Rodrigo, R., Passalacqua, W., Araya, J., et al. Implications of oxidative stress and homocysteine in the pathophysiology of essential hypertension. (2003) J Cardiovasc Pharmacol 42(4): 453-461.
- 8. Bengtsson, S.H., Gulluyan, L.M., Dusting, G.J., et al. Novel isoforms of NADPH oxidase in vascular physiology and pathophysiology. (2003)Clin Exp Pharmacol Physiol 30(11):849-854.
- 9. Lassègue, B., Griendling, K.K. Reactive oxygen species in hypertension; An update. (2004)Am J Hypertens 17(9): 852-860.
- 10. Rodrigo, R., Prieto, J.C., Castillo, R. Cardioprotection against ischaemia/reperfusion by vitamins C and E plus n − 3 fatty acids: molecular mechanisms and potential clinical applications. (2013)Clinical Science 124(1): 1–15.
- 11. González, J., Valls, N., Brito, R., et al. Essential hypertension and oxidative stress: New insights. (2014)World J Cardiol. 6(6):353-366.
- 12. Zou, M.H., Cohen, R., Ullrich, V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. (2004)Endothelium 11(2): 89-97.
- 13. Laursen, J.B., Somers, M., Kurz, S., et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. (2001)Circulation 103(9): 1282-1288.
- 14. Feairheller, D.L., Brown, M.D., Park, J.Y., et al. Exercise training, NADPH oxidase p22phox gene polymorphisms, and hypertension. (2009)Med Sci Sports Exerc 41(7): 1421-1428.
- 15. Han, D., Antunes, F., Canali, R., et al. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. (2003)J BiolChem 278(8): 5557 -5563.
- 16. Zhou, L., Xiang, W., Potts, J., et al. Reduction in extracellular superoxide dismutase activity in African-American patients with hypertension. (2006)Free Radic Biol Med 41(9): 1384-1391.
- 17. Rodrigo, R., Gil, D., Miranda-Merchak, A., et al. Antihypertensive role of polyphenols. (2012)Adv Clin Chem 58: 225-254.
- 18. Nijveldt, R.J., Boelens, P.G., Norren, V.K., et al. Flavonoids: a review of probable mechanisms of action and potential applications. (2001)Am. J. Clin. Nutr 74(4): 418–425.
- 19. Subbaramaiah, K., Chung, W.J., Michaluart, P., et al. Resveratrol inhibits cyclooxygenase- 2 transcription and activity in phorbol ester-treated human mammary epithelial cells. (1998)J Biol Chem 273()34: 21875–21882.
- 20. Van, D.R.M. Coffee and type 2 diabetes: from beans to beta-cells. (2006)Nutr Metab Cardiovasc Dis 16(1):69–77.
- 21. Fernández-Arroyo, S., Camps, J., Joven, J., et al. Managing Hypertension by Polyphenols. (2015)Planta Med 81(8): 624-629.
- 22. Zhao, Y., Wang, J., Ballevre, O., et al. Antihypertensive effects and mechanisms of chlorogenic acids. (2012)Hypertens Res 35(4):370-374.
- 23. Hagiwara, A., Hirose, M., Takahashi, S., et al. Forestomach and kidney carcinogenicity of caffeic acid in F34 rats and C57B1I6N x C3H1eN Fi mice. (1991)Cancer Res 51(20): 5655-5660.
- 24. Higdon, J.v., Frei, B. Coffee and health: a review of recent human research. (2006)Crit Rev Food Sci Nutr 46(2): 101-123.
- 25. Salazar-Martinez, E., Willett, W.C., Ascherio, A., et al. Coffee consumption and risk for type 2 diabetes mellitus. (2004)Ann Intern Med 140(1): 1–8.
- 26. Van, D.R.M., Hu, F.B. Coffee consumption and risk of type 2 diabetes: a systematic review. (2005)JAMA 294(1): 97–104.
- 27. Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. (2007)J Int Med Res 35(6): 900–908.
- 28. Nardini, M., D'Aquino, M., Tomassi, G., et al. Inhibition of human low-density lipoprotein oxidation by caffeic acid and other hydroxycinnamic acid derivatives. (1995)Free Radic Biol Med 19(5):541-552.
- 29. Larsson, S.C., Virtamo, J., Wolk, A. Coffee consumption and risk of stroke in women. (2011)Stroke 42(4): 908–912.
- 30. Lopez-Garcia, E., Rodriguez-Artalejo, F., Rexrode, K.M., et al`. Coffee consumption and risk of stroke in women. (2009) Circulation 119(8): 1116–1123.
- 31. Suzuki, A., Kagawa, D., Ochiai, R., et al. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. (2002)Hypertens Res 25(1): 99–107.
- 32. Sassa, S., Kikuchi, T., Shinoda, H., et al. Preventive effect of ferulic acid on bone loss in ovariectomized rats. (2003)In Vivo 17(3): 277-280.
- 33. Tang, Q.Y., Kukita, T., Ushijima, Y., et al. Regulation of osteoclastogenesis by Simon extracts composed of caffeic acid and related compounds: successful suppression of bone destruction accompanied with adjuvant-induced arthritis in rats. (2006)Histochem Cell Biol 125(3): 215-225.
- 34. Lai, Y.L., Yamaguchi, M. Phytocomponent p-hydroxycinnamic acid stimulates bone formation and inhibits bone resorption in rat femoral tissues in vitro. (2006)Mol Cell Biochem 292(1-2): 45-52.
- 35. Sato, Y., Itagaki, S., Kurokawa, T., et al. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. (2011)Int J Pharm 403(1-2): 136–138.
- 36. 36. Johnson, D.K., Schillinger, K.J., Kwait, D.M., et al. Inhibition of NADPH oxidase activation in endothelial cells by ortho-methoxy-substituted catechols. (2002)Endothelium 9(3): 191–203.
- 37. Kanegae, M.P., Fonseca, L.M., Brunetti, I.L., et al. The reactivity of orthomethoxy-substituted catechol radicals with sulfhydryl groups: contribution for the comprehension of the mechanism of inhibition of NADPH oxidase by apocynin. (2007)Biochem Pharm 74(3): 457–464.
- 38. Suzuki, A., Yamamoto, M., Jokura, H., et al. Ferulic acid restores endothelium-dependent vasodilation in aortas of spontaneously hypertensive rats. (2007)Am J Hypertens. 20(5):508–513.
- 39. Hou, Y.Z., Yang, J., Zhao, G.R., et al. Ferulic acid inhibits vascular smooth muscle cell proliferation induced by angiotensin II. (2004)Eur J Pharmacol 499(1-2): 85–90.
- 40. Li, P.G., Xu, J.W., Ikeda, K., et al. Caffeic acid inhibits vascular smooth muscle cell proliferation induced by angiotensin II in stroke-prone spontaneously hypertensive rats. (2005)Hypertens Res 28(4): 369–377.
- 41. Actis-Goretta, L., Ottaviani, J.I., Fraga, C.G. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. (2006) J Agric Food Chem 54(1): 229–234.
- 42. Liao, C.C., Ou, T.T., Huang, H.P., et al. The inhibition of oleic acid induced hepatic lipogenesis and the promotion of lipolysis by caffeic acid via up-regulation of AMP-activated kinase. (2014)J Sci Food Agric 94(6):1154-1162.
- 43. Ardiansyah., Ohsaki, Y., Shirakawa, H., et al. Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. (2008)J Agric Food Chem 56(8): 2825–2830.
- 44. Yun, N., Kang, J.W., Lee, S.M., Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: molecular evidence of its antioxidant and anti-inflammatory properties. (2012) J Nutr Biochem 23(10): 1249–1255.
- 45. Wu, C., Luan, H., Zhang, X., et al. Chlorogenic Acid Protects against Atherosclerosis in ApoE2/2 Mice and Promotes Cholesterol Efflux from RAW264.7 Macrophages. (2014)PLoS One 9(9):e95452.
- 46. Onakpoya, I,J., Spencer, E.A., Thompson, M.J., et al. The effect of chlorogenic acid on blood pressure: a systematic review and meta-analysis of randomized clinical trials. (2015)J Hum Hypertens 29(2):77-81
- 47. Joneson, T., Bar-Sagi, D. A rac1 effector site controlling mitogenesis through superoxide production. (1998)J Biol Chem 273(29):17991-17994.
- 48. Watanabe, T., Arai, Y., Mitsui, Y., et al. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. (2006)Clin Exp Hypertens 28(5): 439–449.
- 49. Kuzkaya, N., Weissmann, N., Harrison, D.G., et al. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. (2003)J Biol Chem 278(25): 22546-22554.
- 50. Zou, M.H., Cohen, R., Ullrich, V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. (2004)Endothelium 11(2):89-97.