Brain Death Scintigraphy using Tc-99m DTPA: Does Equivocal Cerebral Flow Matter?
Nishanta B. Baidya1, Anja van der Kolk1, Joshua Medow2, Lance T. Hall1,3*
Affiliation
1University of Wisconsin, Department of Radiology, Section of Nuclear Medicine and Molecular Imaging, Madison, WI
2University of Wisconsin, Department of Neurological Surgery, Madison, WI
3Emory University, Department of Radiology and Imaging Sciences, Division of Nuclear Medicine and Molecular Imaging, Atlanta, GA
Corresponding Author
Lance T. Hall, MD, Department of Radiology and Imaging Sciences, Emory University Hospital, 1364 Clifton Rd, Atlanta, GA 30302, Tel: +1-404-712-4843/ Fax: +1-404-712-7435; E-mail: lance.t.hall@emory.edu
Citation
Lance T. Hall., et al. Brain Death Scintigraphy Using Tc-99m DTPA: Does Equivocal Cerebral Flow Matter? (2020) Int J Neurol Brain Dis 7(1): 11-16.
Copy rights
© 2020 Lance T. Hall. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Brain Death; Cerebral Perfusion; DTPA; HMPAO; ECD
Abstract
Objective: To eliminate the “equivocal flow” group from brain death scintigraphy with Tc-99m DTPA by demonstrating that patients with equivocal cerebral flow will eventually fall in the brain death groups who have no cerebral flow.
Methods: We retrospectively reviewed 100 consecutive cerebral perfusion studies requested for confirmation of brain death (100 patients) performed at the University of Wisconsin from October 2009 to December 2017. Two patients died before they could obtain the cerebral perfusion study and 4 patients had duplicate medical record numbers or perfusion studies, leaving a total of 94 evaluable patients.
Patients were categorized into 3 groups: no cerebral flow present, equivocal cerebral flow present and cerebral flow present. Medical records were reviewed to determine if any patients with equivocal flow survived.
Results: Of the 94 evaluable patients, 70 had no cerebral flow present, 16 had equivocal cerebral flow present and 8 had cerebral flow present. Ninety-three of 94 patients in all 3 groups were clinically declared brain dead by the neurointensivist team during the same hospital admission. Fifteen of 16 patients in the equivocal flow group were clinically declared brain dead within 48 hours of the brain death scintigraphic study. One patient with equivocal flow present had clinical care withdrawn and died prior to the completion of clinical brain death determination. There was no difference in the final outcome among the no flow and equivocal flow groups.
Conclusions: All patients with equivocal flow died shortly after the cerebral perfusion study and thus the equivocal flow category and clinical uncertainty associated with it may be eliminated. Furthermore, given the lack of any false negative studies with Tc-99m DTPA, there may be no significant advantage to using the more expensive and logistically challenging cerebral perfusion agents Tc-99m HMPAO and Tc-99m ECD.
Introduction
Diagnosis of brain death is determined by clinical neurological exam and includes cranial nerve function, limb responses, and apnea testing[1-3]. Confirmatory ancillary tests such as electroencephalography (EEG) and cerebral perfusion scintigraphy can be performed in addition to the neurological exam, especially when the clinical exam is inconclusive. For example, cerebral perfusion scintigraphy may be used in addition to the clinical neurological exam and EEG when patients are in barbiturate coma. Additionally, cerebral perfusion scintigraphy can be used to help expedite the clinical diagnosis of brain death in cases where organ donation is contemplated.
Cerebral perfusion scintigraphy can be performed using brain-specific perfusion agents such as Tc-99m - hexamethylpropyleneamine oxime (HMPAO or exametazime or Ceretec) and Tc-99m -ethylene L-cysteinate dimer (ECD or Neurolite) or brain non-specific transient perfusion agents such as Tc-99m diethylenetriaminepentaacetic acid (DTPA), Tc-99m glucoheptonate, and Tc-99m pertechnetate[4-6]. Some Nuclear Medicine physicians prefer brain-specific agents over brain-nonspecific agents as they are less dependent on the quality of the bolus injection, timing, and patient positioning[7-11]. Additionally, brain specific agents allow the option to obtain multiple planar views to include the posterior fossa and/or SPECT imaging which some feel is more definitive to confirm presence or absence of cerebral blood flow[12]. Furthermore, some believe the possibility of faint superior sagittal sinus (SSS) uptake or possible faint blood flow to the posterior fossa may be difficult to visualize with anterior only images typically acquired using Tc-99m DTPA. This can result in an equivocal flow categorization that may complicate or delay brain death determination.
To address the first diagnostic challenge of faint superior sagittal sinus uptake with Tc-99m DTPA, which can be interpreted as “equivocal flow,” we first describe 3 types of cerebral perfusion patterns. They are 1) cerebral flow is present, 2) no cerebral flow is present, and 3) equivocal flow is present. We define equivocal flow as any visual uptake of radiotracer in the superior sagittal sinus without uptake elsewhere or feint uptake elsewhere in an indeterminate location or pattern not typical of normal cerebral flow. We will prove it is feasible to eliminate the equivocal flow group when using anterior only imaging with Tc-99m DTPA by demonstrating patients with equivocal flow will follow in the brain death groups who have no cerebral flow. To address the second diagnostic challenge of posterior fossa attenuation with anterior only imaging, we will demonstrate that all patients without identified cerebral flow on anterior only imaging have the same outcome of brain death. Thus, the two primary aims of this retrospective study of cerebral perfusion scintigraphy are to 1) determine if the “equivocal flow” category and associated clinical uncertainty in diagnosis can be eliminated and 2) determine if any patients without cerebral flow identified survive and thus potentially have clinically relevant posterior fossa blood flow not identified with anterior only imaging with Tc-99m DTPA. A secondary aim is to re-affirm the clinical efficacy of using brain non-specific Tc-99m DTPA with anterior only imaging and thus potentially avoid the use of more expensive and logistically demanding brain-specific agents.
Materials and Methods
Study Patients
We retrospectively reviewed a clinical database of 100 consecutive patients with requested brain death scintigraphy for confirmation of brain death in our institution from October 2009 to December 2017. This retrospective study received an exempt status from the University of Wisconsin Institutional Review Board and no consent from the patients or patients’ family was required.
Of the 100 consecutive cerebral perfusion study requests, 2 patients died before they could obtain the cerebral perfusion study, 2 patients had duplicate medical record numbers, and 2 patients had two serial cerebral flow studies, leaving a total of 94 evaluable patients. Out of 94 patients, 51 patients were female, 43 patients were male and the age of those patients ranged from 12 days old to 74 years old. All 94 patients completed the Tc-99m DTPA cerebral perfusion study. Two patients received a second brain death scintigraphic study with Tc-99m DTPA and 2 different patients received an additional brain death scintigraphic study with Tc-99m HMPAO in order to confirm the result from the prior Tc-99m DTPA brain death scintigraphic study.
Image Acquisition and Processing
All Tc-99m DTPA cerebral perfusion studies were obtained with patients remaining in their critical care beds using a single head gamma camera with low energy high resolution collimator/GE multi purpose square detector (GE Healthcare, Waukesha, Wisconsin, USA). The dose of Tc-99m Tc-DTPA was approximately 16-24 mCi (+/- 20%) / 592-740 Mbq (+/- 20%) for adult patients and adjusted for weight for pediatric patients. The camera face was aligned perpendicular to the patients’ heads. A radioactive marker was used to outline the patients’ heads and neck to verify the entire head and carotid arteries were included in the field of view. For the anterior projection a caudal tilt was included so that the detector face was in line with the forehead and the tip of the nose. Once the position of the patient’s head as well as the detector head placement were satisfactory, the image acquisition was started. Six seconds after initiating image acquisition, the Tc-99m DTPA was injected as a bolus dose, preferably through a central venous line. Data acquisition was performed as: 1-second images for 120 seconds, followed by two 1-minute static images obtained sequentially, with 128 x 128 matrix and zoom x 1. Images were sent to picture archiving communication system (PACS). Adequacy of the bolus injection was verified by documenting carotid artery and extra-cerebral blood flow prior to the patient leaving the imaging suite.
Data Review
The scintigraphic imaging findings and clinical course of all 94 evaluable patients were reviewed. All patient’s clinical outcomes were correlated with the cerebral perfusion patterns, described as flow, no flow, or equivocal flow as detailed below and summarized in Table 1. The amount of time taken by the neurointensivist team to clinically declare brain death following the cerebral flow studies was also compared between the patient groups with no flow and equivocal flow (Table 2). Finally, the amount of time from completion of the cerebral perfusion study to harvesting of organs from organ donors was also measured with attention to the no flow and equivocal flow groups (Table 3).
Categorization of Patients
Patients were categorized based on cerebral perfusion results as “cerebral flow is present,” “no cerebral flow is present,” and “equivocal flow is present.” Cerebral flow present indicates radiotracer uptake in any of the intra-cerebral or cerebellar arterial vasculature, even in cases of abnormal but present flow. No cerebral flow present indicates no radiotracer uptake in any of the intra-cerebral or cerebellar arterial vasculature and no radiotracer uptake in the superior sagittal sinus or atypical locations. The equivocal flow group as previously defined indicates any visual uptake of radiotracer in the superior sagittal sinus without uptake elsewhere in the cerebral vasculature or faint uptake elsewhere in an indeterminate location or pattern not typical of normal cerebral flow.
All patients were admitted into the Neuro-Intensive Care Unit at the University of Wisconsin Hospital and received care under specialized Neuro-Intensivists. The clinical determination of brain death was performed by a team of Neuro-Intensivists based on the patients’ neurological exam over time and use of an apnea test as well as other confirmatory tests as appropriate. Results of the cerebral perfusion studies were communicated to the Neuro-Intensivists at the conclusion of the study and these results were known at the time of brain death declaration.
Results
The brain death scintigraphic imaging findings of all 94 patients were reviewed and categorized into one of three flow patterns: cerebral flow present, no cerebral flow present, and equivocal flow (Table 1). Meticulous review of cerebral blood flow studies with equivocal flow was mandatory in order to avoid inadvertent declaration of definite flow or no flow.
Table 1: Number of patients with different flow patterns and number of patients who were declared brain dead during the same hospital admission.
|
Study Groups |
Number of Patients |
Clinically Brain Dead During Same Hospital Admission |
|
Cerebral Flow Present |
8 |
8 |
|
No cerebral Flow Present |
70 |
70 |
|
Equivocal Cerebral Flow Present |
16 |
*15 |
|
Total Patients |
94 |
93 |
*One patient with equivocal flow had clinical care withdrawn and subsequently died before completion of brain death determination due to irreversible anoxic brain injury.
The current study demonstrated 74.5% (70/94) patients had no cerebral flow present (Figure 1), 17% (16/94) patients had equivocal flow present (Figures 2 & 3), and 8.5% (8/94) patients had cerebral flow present. Ninety-three of 94 patients were clinically declared brain dead by the Neuro-Intensivist team and all 94/94 patients died during the same hospital admission as the cerebral perfusion study.

Figure 1: “No flow” study.Tc-99m DTPA cerebral perfusion study with anterior dynamic angiographic (top 2 rows) and blood pool (bottom row) phases. Good bilateral carotid bolus is present without any intracerebral flow.
Thirteen of 16 patients (81%) with equivocal flow were clinically declared brain dead within 24 hours of the brain death scintigraphic study. Two of 16 patients (13%) with equivocal flow were clinically declared brain dead after 12 hours of the brain death scintigraphic study (TABLE 2). One of 16 patients with an equivocal flow study had clinical care removed by family request and subsequently died prior to completion of brain death determination. This patient’s apnea test had to be terminated prematurely due to hypotensive response despite maximum vasopressor treatment. This patient otherwise had no brainstem reflexes and did not have self-respiratory drive during the attempted apnea test.
Table 2: Time taken to clinically declare brain dead patients with Equivocal Flow
|
Equivocal Flow |
||
|
16 |
||
|
Clinically Declared Brain Dead |
Care Withdrawn |
|
|
15 |
1 |
1 |
|
<12 Hours |
>12 Hours |
|
|
13 |
2 |
|
Two patients with equivocal flow on Tc-99m DTPA cerebral perfusion studies had undergone further Tc-99m HMPAO cerebral perfusion studies in order to confirm lack of cerebral or posterior fossa blood flow (Table 3) (Figures 2 & 3). One patient obtained the Tc-99m HMPAO cerebral perfusion study after 12 hours following the acquisition of the Tc-99m DTPA cerebral perfusion study and a second patient obtained it after 24 hours. Both of the Tc-99m HMPAO cerebral perfusion studies were negative for any cerebral or posterior fossa blood flow. Both patients were clinically declared brain dead within approximately 4 hours following the acquisition of the second cerebral perfusion study with Tc-99m HMPAO.

Figure 2(A): “Equivocal flow” study. Tc-99m DTPA cerebral perfusion study with anterior dynamic angiographic (top 2 rows) and blood pool (bottom row) phases. Minimal tracer uptake in the left lateral cerebral region (red arrow) likely represents scalp collateral perfusion.

Figure 2(B): Tc-99m HMPAO cerebral perfusion study obtained after the Tc-99m DTPA “equivocal flow” study in figure 2 (A). There is no evidence of intracerebral radiotracer uptake on early or delayed imaging. This patient was reported as having no cerebral perfusion and was clinically declared brain dead.

Figure 3(A): “Equivocal flow” study. Tc-99m DTPA cerebral perfusion study with anterior dynamic angiographic (top 2 rows) and blood pool (bottom row) phases. Minimal tracer uptake in the right lateral cerebral region (red asterisk) with trace activity in the superior sagittal sinus (red arrow).
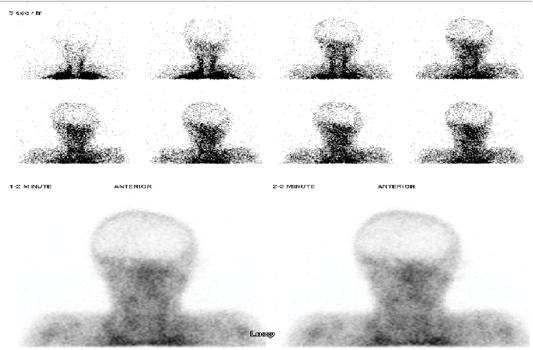
Figure 3(B): 99mTc-HMPAO cerebral perfusion study obtained after the 99mTc-DTPA “equivocal flow” study in figure 3 (A). There is no evidence of intracerebral radiotracer uptake on early or delayed imaging. This patient was reported as having no cerebral perfusion and was clinically declared brain dead.
Table 3: Time lag to harvest organs from patients with Equivocal Flow
|
Patients with Equivocal Flow with Tc-99m DTPA Cerebral Perfusion Study |
Time Lag to Obtain Tc-99m HMPAO Cerebral Perfusion Study |
Time Lag to Harvest Organs |
|
Patient 1 |
> 12 hours |
58 |
|
Patient 2 |
> 24 hours |
46 |
Discussion
Brain Death and Brain Death Scintigraphic Study
The ability to both accurately and expeditiously provide a clinical diagnosis of brain death is crucial in many patients, particularly in patients whom may be organ donors. Complex and fluctuating donor hemodynamics increase chances of compromising organ viability for transplantation as well as the inevitable hormonal and inflammatory cascade that can result in graft dysfunction and increased chances of rejection. These risks increase over time[13-15]. A reliable confirmatory test such as cerebral perfusion scintigraphy can help diminish these risks, but only if it can be performed and interpreted rapidly and accurately.
Brain specific cerebral perfusion agents such as Tc-99m HMPAO and Tc-99m ECD are increasing in popularity but there is no clear evidence they are more accurate than the brain non-specific agent Tc-99m DTPA[16]. According to the Society of Nuclear Medicine and Molecular Imaging (SNMMI) Brain Imaging Council, although some institutions still use brain non-specific agents such as Tc-99m DTPA, Tc-99m glucoheptonate, and Tc-99m pertechnetate for assessment of cerebral perfusion, they are less common than Tc-99m HMPAO and Tc-99m ECD[12]. Some authors argue Tc-99m DTPA is not as good as brain specific agents as the former is bolus dependent and it does not have cerebral retention unlike brain specific agents, therefore it lacks specificity[17]. Authors have also argued about lack of adequate image acquisition using Tc-99m DTPA cerebral flow studies unlike brain specific tracers which will allow delayed imaging as well as brain SPECT imaging[12]. However a retrospective study carried out with direct comparison of Tc-99m DTPA and Tc-99m HMPAO for brain death gave identical results for these two agents[18].
Normally, when cerebral and/or cerebellar blood flow is present, it will be readily identified on angiographic phase images and later there will be significant venous drainage into the SSS on blood pool images. In some cases, only feint SSS uptake on blood pool images can be seen along with lack of cerebral or cerebellar flow on angiographic phase images. Under normal intracranial pressures (ICP), it is possible that an alternate venous drainage pathway can lead to SSS uptake from extracranial venous drainage via emissary veins into the superior sagittal sinus. Likewise in normal anatomy the posterior fossa veins terminate as bridging veins, which collect into three groups: a galenic group that drain into the vein of Galen; a petrosal group that drains into the petrosal sinuses; and a tentorial group that drains into the transverse, straight, or superior petrosal sinus[19]. However, under conditions of high ICP, anastomosing vessels from the scalp will follow the path of least resistance and drain elsewhere given the very low pressure in the venous system outside of the cranial vault. Of concern is the possibility this feint SSS uptake can be from feint cerebral or cerebellar blood flow that is not readily identified on angiographic phase images. Of particular concern is potential feint blood flow to the posterior fossa that may be difficult to visualize with anterior only images. Thus, any uptake in the SSS can result in an equivocal flow categorization, which is another potential pitfall of using Tc-99m DTPA for brain death scintigraphy. An additional diagnostic conundrum with Tc-99m DTPA can occur when anterior only planar imaging is utilized. With anterior only imaging, the posterior fossa can be significantly attenuated and obscured from view by the bony skull base. It is possible that with absence of cerebral blood flow, there may be blood flow in the posterior fossa that may not be identified without additional lateral images obtained during the angiographic phase. This blood flow could potentially sustain brain stem function for a brief period of time and thus complicate the clinical diagnosis of brain death. It could also be argued that feint blood flow to the posterior fossa is not sufficient to sustain brain stem functioning for any appreciable length of time and thus brain death is inevitable. For example, a study report has shown all patients with partial persistent flow in the posterior fossa finally met the clinical criteria for brain death including the cessation of the brain stem function[20].
At our institution, brain death scintigraphic studies with Tc-99m DTPA are performed directly on the patient’s bed with a single head camera, unlike studies with Tc-99m HMPAO or Tc-99m ECD SPECT whereby patients may need to be moved from their beds onto the scanner bed if multiple image projections or SPECT is anticipated. Our technique reduces risk of injury to patients (especially head and/or spine injury patients), as well as risk of dislodging vital tubes such as the endotracheal tube, external ventricular drain, central lines, etc. Another benefit is that duration of imaging with this approach is very short, which is important in these patients who are often very unstable and risk organ viability for potential organ donors. Studies with Tc-99m HMPAO and Tc-99m ECD may require delayed imaging after injection, and if SPECT is used the imaging time is significantly longer. Needless to say this additional time required and patient movement imposes higher risks on patient safety and organ procurement in potential organ donors. A downside to our approach is the inability to obtain lateral images during the angiographic phase or high quality lateral images on a delayed phase given the distance of the patient’s head from the camera. However, this shortcoming did not result in any change in diagnostic outcomes.
In addition to differences in imaging times and logistics, there is a significant difference in costs of the various agents used in cerebral perfusion imaging. At our institution the costs of Tc-99m HMPAO and Tc-99m ECD are at least 6 times and 8 times, respectively, the cost of Tc-99m DTPA, which is about USD 560. Furthermore, maintaining the shelf life of Tc-99m HMPAO is more challenging. If not stabilized with methylene blue it needs to be used within half hour of reconstitution, while addition of methylene blue will slightly increase the shelf life to 4 hours[13].
Significance of the Current Study
Our study shows that there is no difference in the final outcome (clinically declared brain death) in equivocal flow and no flow study groups in cerebral perfusion studies using Tc-99m DTPA. This is a novel embodiment of the retrospective study to eliminate the equivocal flow category from cerebral perfusion studies that has not been reported previously. Elimination of the equivocal flow category will help streamline interpretation of brain death scintigraphic study results with only 2 groups, viz cerebral flow present and no cerebral flow present. This can reduce clinical uncertainty and help expedite brain death determination. Additionally, despite theoretical concerns for potential miss of subtle posterior fossa blood flow with anterior only image acquisition, no patients without identified cerebral flow survived and thus this is likely a clinically insignificant risk. We can leverage these findings to reduce delays in diagnosis and avoid use of more expensive and logistically demanding agents like Tc-99m HMPAO and Tc-99m ECD, which might not be available in all institutions on demand, especially during non-business hours.
In this study there were 2 patients with equivocal flow with Tc-99m DTPA who subsequently obtained additional Tc-99m HMPAO cerebral perfusion studies, which confirmed no cerebral or posterior fossa blood flow. We argue this subsequent use of more expensive and logistically demanding Tc-99m HMPAO imaging added unnecessary delays and costs and could have been avoided had we eliminated the equivocal flow category. Our proposal to eliminate the equivocal flow category in Tc-99m DTPA cerebral perfusion imaging also emphasizes its potential utility in expediting harvesting of organs from potential donors and minimizing hospital costs by providing an earlier brain death determination and reducing costly days of intensive care.
Limitations
The retrospective nature of this relatively small study limits evaluation of outcomes. Additionally, the patient population included in this study all subsequently died and this limits comparison of sub-groups. Finally, although we sought to make indirect comparisons and inferences to the use of Tc-99m HMPAO and Tc-99m ECD, with exception of only 2 patients, we did not have data to directly compare these agents.
Conclusion
This study has shown that there is no difference in the final outcome (clinical brain death) in equivocal flow and no flow categories in cerebral perfusion studies using Tc-99m DTPA with anterior only imaging in the patient’s own bed. We suggest using only two diagnostic categories when interpreting brain death studies: 1) cerebral flow present and 2) no cerebral flow present. We also argue against the routine use of more expensive and logistically demanding agents such as Tc-99m HMPAO and Tc-99m ECD.
Disclosure
The current study is a retrospective study therefore it did not require any financial support as well as consent from the patients or patients’family.
The current study is adhered to the ethical guidelines and ethical approvals.
All authors have approved the final manuscript and hence there are no conflicts of interests.
References
- 1. Flowers, W.M. Jr., Patel, B.R. Accuracy of clinical evaluation in the determination of brain death. (2000) South Med J 93(2): 203–206.
- 2. Heran, M.K., Heran, N.S., Shemie, S.D. A review of ancillary tests in evaluating brain death. (2008) Can J Neurol Sci 35(4): 409–419.
- 3. Wijdicks, E.F., Varelas, P.N., Gronseth, G.S., et al. Evidence- based guideline update: Determining brain death in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology. (2010) Neurology 74(23): 1911–1918.
- 4. Bertagna, F., Barozzi, O., Puta, E., et al. Residual brain viability, evaluated by 99mTc-ECD SPECT in patients with suspected brain death and with confounding clinical factors. (2009) Nucl Med Commun 30(10): 815–821.
- 5. Conrad, G.R., Sinha, P. Scintigraphy as a confirmatory test of brain death. (2003) Semin Nucl Med 33(4): 312–323.
- 6. Larar, G.N., Nagel, J.S. Tc-99m HMPAO cerebral perfusion scintigraphy: considerations for timely brain death declaration. (1992) J Nucl Med 33(12): 2209–2211.
- 7. Abdel-Dayem, H.M., Bahar, R.H., Sigurdsson, G.H., et al. The hollow skull: A sign of brain death in Tc-99m HM-PAO brain scintigraphy. (1989) Clin Nucl Med 14(12): 912-916.
- 8. Costa, D.C., Motteux, I.M., McCready, A.C. Diagnosis of brain death with technetium 99m hexamethylpropylene amine oxime. (1991) Eur J Nucl Med 18(7): 503-506.
- 9. Kurtek, R.W., Lai, K.K., Tauxe , W.N., et al. Tc-99m hexam- ethylpropylene amine oxime scintigraphy in the diagnosis of brain death and its implications for the harvesting of organs used for transplantation. (2000) Clin Nucl Med 25(1): 7-10.
- 10. Laurin, N.R., Driedger, A.A., Hurwitz, G.A., et al. Cerebral perfusion imaging with technetium-99m HM-PAO in brain death and severe central nervous system injury. (1989) J Nucl Med 30(10):1627-1635.
- 11. Sinha, P., Conrad, G.R. Scintigraphic confirmation of brain death. (2012) Semin Nucl Med 42(1): 27–32.
- 12. Bonetti, M.G., Ciritella, P., Valle, G., et al. 99m-Tc HM-PAO brain perfusion SPECT in brain death. (1995) Neuroradiology 37(5): 365–369.
- 13. Bos, E.M., Leuvenink, H.G., van Goor, H., et al. Kidney grafts from brain dead donors: Inferior quality or opportunity for improvement? (2007) Kidney Int 72(7): 797–805.
- 14. Nygaard, C.E., Townsend, R.N., Diamond, D.L. Organ donor management and organ outcome: A 6-year review from a Level I trauma center. (1990) J Trauma 30(6): 728–732.
PubMed│CrossRef│Others
- 15. Oto, T., Excell, L., Griffiths, A.P., et al. Association between primary graft dysfunction among lung, kidney and heart recipients from the same multiorgan donor. (2008) Am J Transplant 8(10): 2132–2139.
- 16. Donohoe, K.J., Agrawal, G., Frey, K.A., et al. SNM Practice Guideline for Brain Death Scintigraphy 2.0. (2012) J Nucl Med Technol 40(3): 198-203.
- 17. Munari, M., Zucchetta, P., Carollo, C., et al. Confirmatory tests in the diagnosis of brain death: comparison between SPECT and contrast angiography. (2005) Crit Care Med 33(9): 2068–2073.
- 18. Spieth, M.E., Ansari, A.N., Kawada, T.K., et al. Direct Comparison of Tc-99m DTPA and Tc-99m HMPAO for Evaluating Brain Death. (1994) Clin Nucl Med 19(10): 867-872.
- 19. Rhoton, A.L. The Posterior Fossa Veins. (2000) Neurosurgery 47(Suppl 3): S69–S92.
- 20. Braun, M., Ducrocq, X., Huot, J.-C., et al. Intravenous Angiography in brain death: report of 140 patients. (1997) Neuroradiology 39(6): 400–405.












