Carcinogenesis and Endocrine Pancreas Deficiency: A Working Frame for Cancer Prevention or Treatment
Affiliation
Department of Neurochemistry, CNRS Gif sur Yvette, France
Corresponding Author
Maurice Israel, MD, PhD,retired CNRS Director, Av. Aristide Briand 2, Bures sur Yvette, 91440, France; E-mail: mauisrael@wanadoo.fr
Citation
Israel, M. Carcinogenesis and endocrine pancreas deficiency, a working frame for cancer prevention or treatment. (2015) Int J Cancer Oncol 2(1): 1-8.
Copy rights
© 2014 Israel M. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Cancer metabolism; Pancreas; Beta cells; Insulin; GABA; Alpha cells; Glucagon; Nutrition-cancer; Incretin
Abstract
In Cancer, a metabolic rewiring of biochemical pathways supports tumor cell anabolism, while body reserves are depleted by catabolic hormone. Wepreviously analyzed in detail the downstream phosphorylation of enzymes supporting the metabolic rewiring observed in cancer and found thatthe hybrid-anabolic or catabolic status of tumor cellsenzymeswould be explained by alteration of the endocrine pancreas. It is like if beta cells secreting anabolic insulin failed to silence with gamma aminobutyric acid (GABA), neighboring alpha and delta cells, releasing catabolic glucagon and somatostatin respectively. Moreover, the GABA deficiency, would alsofail to turn off insulin release leading to a progressive desensitization of insulin receptors on differentiated cells, selectively sensitive to glucagon. This explains the depletion of tissue reserves for the benefit of mitotic cells with new,non-desensitized insulin receptors,such cellswill then display the hybrid rewired metabolic response. At this initialstage the metabolic advantagegained by these stem cells is reversible,giving new therapeutic possibilities for preventing their transformation into aggressive tumors. We propose that stem cells that repair tissues after an injurymight start the carcinogenic process, if there is an associated pancreaticfailure that rewires their metabolic pathways. This is discussed in relation to Nutrition, Diabetes type 2 and to factors increasing cancer incidence
Introduction
There is a general consensus considering that pesticides, like many other compounds that can be accidentally absorbed (aniline, asbestos etc…) are triggers for various cancers. We are also aware that nutritional life styles (excess of sugar), metabolic syndrome, diabetes type 2, obesity, or specific vitamin B deficiencies, may in the long run favor cancer. Moreover, addictions to tobacco or betel nut are known to increase the incidence of various cancers. This is also the case for physical causes (UV light or radioactivity). All these carcinogens add up their effects to a genetic susceptibility for cancer that is observed in families with a greater incidence of tumors of the breast or colon etc… With such a variety of triggers one may consider cancer as a multi factorial disease. However, the description of a metabolic plan, that is typical of cancer, which gives to tumor cells a metabolic advantage over differentiated cells, allows us to focus our attention on some common mechanisms that would induce a metabolic process associated to the formation of tumors in affected organs. If we want to describe such a general process that would lead to cancer, we must take into account external triggers, genetic aspects, metabolic signaling, metabolism, cellular populations and the properties of tumor cells. Giving a hypothetical scenario for carcinogenesis may at least provide a frame for a possible prevention and to the suggestion of possible metabolic changes to add up to current chemotherapy.
Oncogenes that target anabolic signaling pathways,
We recall that a retrovirus can capture a gene, from a host cell and transmit it to a new host, an up regulation of the gene product, now under viral control, will eventually cause tumors. The captured gene has become a viral oncogene (v-oncogene), which derives from a normal host gene the proto-oncogene. Hence, a virus can perturb the expression of a cellular gene the proto-oncogene, by modifying its expression, or its regulation, or by transmitting a mutated form of the proto-oncogene captured from the previous host. Independently of any viral infection, a similar tumorigenic mechanism takes place, if the proto-oncogene is translocated in another chromosome; and transcribed under the control of stronger promoters. In this case, the proto-oncogene becomes an oncogene of cellular origin (c-oncogene). Another mode for converting a proto-oncogene into an oncogene occurs when a retrovirus simply inserts its strong promoters in front of the proto-oncogene enhancing its expression.
The main point to consider is that retroviral oncogenes and cellular oncogenes modify a major signaling pathway: the MAP kinases mitogenic pathways, at different steps. At the ligand level we find tumors such Wilm's kidney cancer, resulting from an increased expression of insulin like growth factor (IGF); we have also the erbB or V-int-2 oncogenes expressing respectively NGF and FGF growth factor receptors. The receptor for these ligands activates the tyrosine kinase signals, as would do insulin acting on the insulin receptor.
At the Tyrosine kinase level, The Rous sarcoma virus transmits a tyrosine kinase Src that increases tyrosine phosphorylation activity, and leads to a chicken leukemia. Similarly, in murine leukemia, a virus captures and retransmits the tyrosine kinase abl. The tyrosine kinase step can also be enhanced if abl is translocated and expressed with the bcr gene on chromosome 22, as a fusion protein (Philadelphia chromosome); in this particular case abl is a cellular oncogene.
Further ahead, Ras exchanging protein for GTP/GDP, and then the Raf serine-threonine kinases are proto-oncogenes giving corresponding oncogenes. Finally, at the level of transcription factors activated by MAP kinases, one finds cjun, cfos or cmyc; the latter, is for example, activated by the insertion of an avian leucosis virus. All this indicates that retroviral attacks boost the MAP kinases as would do inflammatory cytokine, or increased insulin signals coupled to glucose transport and glycolysis.
A branch of the MAP kinase mitogen pathway opens also the PI3 kinase pathway, PTEN phosphatase counteracts this effect, thus acting as a tumor suppressor. We recall that a DNA virus, the Epstein-Barr virus of infectious mono nucleosis, gives also the Burkitt lymphoma; the effect of the virus is to enhance PI3 kinase. Downstream, we also observe tumors in relation to mTOR (the target of an immuno suppressor rapamycine) mTOR controls protein translation; it additionally inhibits PP2A phosphatase. This phosphatase regulated by methylation of its catalytic unit counteracts the kinases; PP2A is itself a target of the simian SV40 and Polyoma viruses. Schematically, one may consider that the different steps of MAP kinase pathways are targets for retroviruses, while the different steps of PI3 kinase pathway are targets for DNA viruses. The viral-driven enhanced function of these pathways mimics the effects of their prolonged activation by their usual triggers, insulin or IGF for example, which brings us back to the endocrine controls of metabolism.
Cell lysis and anabolic-repair: lytic products sensed as nutrients by the endocrine system
We know that each tissue and organ, including brain, contains a population of stem cells, their mitosis will take place, to compensate a cell loss, following a variety of toxic aggressions. The stem cell hypothesis appears some 50 years ago in few histology books. André Gernez[1] applies the concept for the first time to cancer and neurological diseases. He compares tissues "to colonies of bees in which, only the queens renew the colony, while most bees are sterile workers". This concept radically changed ideas on tissue repair and regeneration. The mitosis of a stem cell gives two different daughters; one forms back a stem cell, maintaining the stem cell population, while the other daughter that differentiates is sterile, which maintains the function of the organ. The process explained the constant mass of organs. The situation is different in cancer, since the two daughter cells inherit a mitotic capability explaining the geometric increase of the tumor. Probably, a variety of tissue aggressions trigger apoptosis (pesticides, reactive oxygen radicals, toxins, mitochondria uncouplers, stress etc...). The disruption of cells releases many substances, amino acids, carbohydrates mitogens, that are in a way considered as "nutrients" by sensors of endocrine glands, which respond by releasing anabolic hormones that start the repair process. The pancreas releases insulin, the hypophysis Growth hormone (GH), which elicits the release of IGF from the liver… These hormones and other Growth factors act on tyrosine kinase receptors inducing mitosis and metabolic processes generating energy through oxidative glycolysis; anabolism forms the substances for building up new cells.
Cells that renew tissues after an aggression, may adopt metabolic features giving them a selective advantage over normal cells. This occurs if signaling regulations are for some reason out of control. The resulting metabolic changes will first induce epigenetic modifications stabilizing the metabolic advantage of these dividing cells; a pre-cancer situation develops, it is in principle reversible. Then, inevitable mutations select the most successful cells, leading to cancer. In a way, stem cells of each organ are in a given environment, if the environment is not correctly ruled by nutritional hormones, stem cells adopt a metabolic plan that is advantageous for them, which enhances their mitotic developments, mutations will select the most successful but aggressive populations associated to cancer[2-6].
Hormonal control of catabolism or anabolism
The action of insulin and other anabolic hormones is transmitted to cells via membrane tyrosine kinase receptors; we have seen that several oncogenes enhance this signaling route. The tyrosine kinase receptor receptors stimulate MAP kinase and PI3 kinase signaling pathways, controlling mitosis and cell survival. This receptor activates an intermediate protein kinase: PKB, which will phosphorylate specific serine sites and inhibit protein kinases. This will, in synergy with protein phosphatases, dephosphorylate the enzymes that were phosphorylated during catabolism by PKA and Src; a detailed analysis was studied in ref[7]. Insulin will stimulate glycolysis, by promoting the incorporation of the glucose transporter in membrane; dephosphorylated glycogen synthase is now activated, forming glycogen, conversely the dephosphorylation of glycogen phosphorylase, blocks glycogenolysis. The model exemplified here for enzymes involved in glycogen metabolism was validated on many more enzymes controlling protein and lipid synthesis[7].
In anabolism, glycolysis is stimulated by the increaseof fructose 2-6 bis phosphate. Stem cells can divide; there is an increase of protein synthesis, of nucleotide synthesis, for DNA and RNA, of lipids to form new cell membranes.
Similarly to stem cells, subjected to anabolic actions, tumor cells, will incorporate glucose, display a high glycolysis and form new molecular building blocks for new mitotic daughter cells. On the other hand, it can be assumed that the mobilization of reserves observed in cancer for feeding the tumor, depends of catabolic hormones, as it is the case for starvation. In such situations, catabolic hormones: glucagon, epinephrine and cortisol, mobilize reserves of the organism to form glucose, and ketone bodies that are both nutrients supplied here to tumor cells. Glucagon is released by the alpha cells of the pancreas, epinephrine and cortisol by the adrenal gland. Glucagon and epinephrine bind to cell membrane receptors coupled to G proteins (GPCR)of the Gs type, which stimulate adenylate cyclase and cyclic AMP (cAMP) synthesis. The increase in cAMP stimulates a protein kinase A (PKA) a serine-threonine kinase, which activates Src tyrosine kinase. This elicits the phosphorylation of protein kinases that ultimately phosphorylate enzymes, which may then mobilize glycogen, protein, and lipid body reserves. Thus, glucose is produced by glycogenolysis from liver and muscle glycogen. Glycogenolysis depends on the enzyme glycogen phosphorylase, which is activated by phosphorylation; conversely, the enzyme glycogen synthase will be blocked by phosphorylation. Catabolic hormones also trigger a synthesis of glucose, (gluconeogenesis) in the liver; using amino acids, mainly alanine coming from muscle proteolysis, which is activated by cortisol. Alanine will then be transaminated to pyruvate, which will then be carboxylated to oxalo acetate. The latter, gives phosphoenol pyruvate and follows the reverse path of glycolysis until glucose. To ensure gluconeogenesis, one must also avoid that oxaloacetate at the origin of the pathway gets consumed inthe Krebs cycle, whose entry will be locked by a phosphorylation of pyruvate dehydrogenase (PDH)[8]. On the other hand, one must also avoid a backward reaction from phosphoenolpyruvate to pyruvate; this requires inhibiting pyruvate kinase (PK) by phosphorylation[9]. The blockade of PDH and PK mediated by catabolic hormones is maintained in tumor cells that are on the other hand submitted to the anabolic action mediated by anabolic hormones via the tyrosine kinase receptors.Tumor cells must then overcome the blockade of PDH and a PK that remain phosphorylated. We are somehow in a hybrid situation for the tumor cells, the response is catabolic for PK and PDH; they are blocked by phosphorylation, while others enzymes get normally dephosphorylated (glycogen synthase, glycogen phosphorylase or Acetyl CoA carboxylase for example) as in anabolism[7]. This hybrid situation reconnects metabolic pathways in tumor cells and will give them a metabolic advantage, allowing them to plunder the tissue reserves mobilized by catabolic hormones[7]. How do we explain this very peculiar response?
A pancreatic GABA failure explains cancer metabolic rewiring
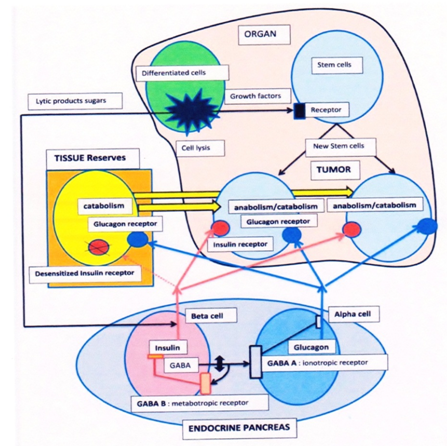
What would be the consequences of a failure of this GABA dependent regulation of the endocrine pancreas? It may occur if glutamate decarboxylase (GAD) the GABA synthetizing enzyme is inhibited by pesticides for example. We also suspect that vitamin B6 deficiencies affect GABA synthesis, since B6 is the cofactor of GAD. Such deficiencies might be provoked by compounds forming adducts with vitamin B6 aldehyde (hydrazines, amines, pyrroles) example: isoniazide or gyromitrine from gyromitra mushrooms, in several cases there was a carcinogenic effect. Finally, autoimmune diseases affecting GAD have been described. All these possible causes that decrease the effect of GABA or its release from beta cells would alter the switch off mechanism for glucagon that will then be released, together with insulin. A hybrid catabolic / anabolic message would be sent to cells; and indeed, tumor cells display a dual hybrid response. This is shown by the blockade of PK and PDH as for catabolism, paradoxically associated to a very active glycolysis and citrate condensation, feeding synthetic processes as for anabolism. To overcome the PK and PDH blockade, tumor cells rewire the system, they use for their citrate condensation, acetyl CoA coming from ketone bodies provided by tissue reserves, while oxaloacetate (OAA) is formed via phosphoenol pyruvate carboxy kinase or malate dehydrogenase. Below the citrate condensation, a new stop in the Krebs cycle favors the citrate efflux from mitochondria, which starts via ATP citrate lyase and acetyl CoA carboxylase the synthesis of fatty acids and lipid membranes for mitotic cells; malonyl CoA inhibits the entry of fatty acids in mitochondria, rendering tumor cell dependent of ketone bodies and lysolipids sources[7]. Why then enzymes of differentiated cells, hepatocytes or adipocytes, respond preferentially to catabolic hormones; as if, in cancer, differentiated cells, were relatively insensitive to insulin signals. In a way, this resembles to a situation found in diabetes type 2, in which there is a desensitization of insulin receptors, interiorized by endocytosis before proteolysis. Insulin resistance is often associated to chronic inflammation; in diabetes type 2, there is indeed an elevated cancer risk[10]. Normally, beta cells turn off insulin release terminating its action, which avoids an insulin desensitization process, provoked by a persistent insulin release. To terminate insulin release, the released GABA acts on metabotropic GABA Bauto receptors of beta cells that are coupled to Gi proteins. This closes after several steps the insulin release mechanism[11]. Hence, GABA release from beta cells functions as an autocrine inhibitor, turning off insulin release. If GABA release gets deficient there will then be a persistent release of insulin, which desensitizes insulin receptors on differentiated cells that will respond preferentially to catabolic hormones. In mitotic cells, the situation is very different, since they respond to both insulin and glucagon, adopting a hybrid anabolic/catabolic rewiring. The simplest explanation is that in contrast to differentiated cells, submitted to insulin receptor desensitization; new stem cells express new insulin receptors that have not been desensitized. And since these cells have also Gs coupled receptors, they will display the dual anabolic /catabolic response and start the rewiring process (Figure 1). Moreover, in adrenal medulla, a deficient GABA release stimulates epinephrine release, which inhibits somatostatin. Glucagon also stimulates cortisol release.
The figure represents:- an organ in which a tumor develops; -the endocrine pancreas; -tissuereserves. In the organ, cells are disrupted by external or internal toxic agents; they release lytic products, sugars, growth factors. These products are sensed as a "food intake" by endocrine glands, the pancreas releases anabolic insulin, the growth factors and mitogens induce the mitosis of stem cells in the injured organ, starting arepair process, one daughter cell differentiates to replace adisruptedcell the other gives back a stem cell, maintaining the stem population, this rule is not respected if a tumor develops.
A GABA deficiency in pancreatic beta cells has two consequences:
emsp; First beta cells release insulin but are unable to inhibit with GABA the alpha and delta cells via theirinotropic GABA A receptors, there will be a concomitant glucagon release from alpha cells. Second, GABA also fails to inhibit GABA B metabotropic autoreceptors of beta cells; these are coupled to Gi proteins and normally turnoff insulin release, the persistent insulin release leads to a desensitization of insulin receptors of differentiated cells. In tissue reserves(the desensitized insulin receptor is interiorized in the cell and represented inactive); cells in body stores will thus respond preferentially to catabolic glucagon, which depletes reserves. In contrast,new stem cells with non-desensitized insulin receptors receive a dual anabolic–catabolic signal and rewire their metabolic pathways in the "cancer mode" they gain a metabolic advantage enabling them to plunder body reserves, this enhances the mitotic activity of daughter stem cells, the population increases, mutations follow, selecting the successful and aggressive population. These modified stem cells form a tumor in an organ they were meant to repair.
We have mentioned the increased incidence of cancer associated to diabetes type 2, we should also consider here an important nutritional regulation affected in this disease, which may have a role in nutrition and cancer: that is the secretion of incretins GIP and GLP1 peptides from the intestine they anticipate for the pancreas that food is taken. Incretins will then elicit the release of insulin from beta cells even before glycaemia gets elevated. Following the works of Bayliss and Starling on secretin[12] and the observation of Moore[13] on the glucose lowering effect of gut extracts, incretins were purified[14].They have a crucial role in relation to food intake and hormonal control of metabolism; see review[15]. The oral intake of glucose releases more efficiently insulin than an equivalent glucose increase in the blood; incretins stimulate beforehand the pancreas. GLP1 acts on beta cell G protein coupled receptors of the Gs type increasing the release of insulin. In this case cAMP activates PKA and also EPAC, (exchange factor activated by cAMP). The action of PKA on the KATP channel is inhibitory and closes it, which depolarizes the beta cells opening the voltage dependent calcium channels, which triggers insulin release; in addition a calcium induced release of calcium from internal stores is activated by EPAC, which boosts insulin release.
This effect of incretin mediated via Gs coupled receptors is attenuated in diabetes type 2; the peak release of insulin following a meal is blunted. In parallel we have seen that the GABA deficit fails to activate Gi coupled metabotrobic autoreceptors (GABA B) on beta cells that terminate the insulin release process, leading to a persistent release of insulin and to desensitization, of insulin receptors on differentiated cells[16-18]. Moreover, we know that leptin receptors of the hypothalamus control lipid storage or energy expenditure via JAK- STAT signals. Downstream the leptin receptor, one finds several proteins IRS or Grb2 that are common to the insulin signaling cascade, it is then probable that a persistent insulin release resulting from the GABA deficiency, will interfere or desensitize leptin receptor signals, leading to obesity, as when leptin receptors are invalidated[19]. In sum, the GABA deficiency described would lead in the different circumstances, to diabetes type 2, to obesity, or to cancer.
It was interestingly pointed out that GABA is also secreted by ductal pancreatic cells and that it was particularly involved in the case of pancreatic tumors. It was indeed found that GABA and an anti-inflammatory compound (celocoxib) conjugated their effects and prevented the progression of pancreatic cancer[20-23].
Conclusion
The Figure 1 represents a possible carcinogenic scenario and the metabolic features associated to an endocrine pancreas failure, which will give a selective advantage to a population of stem cells that will be transformed. An aggression of organs breast liver or other, by external agents or internal toxins could be a starting point, often associated to inflammation. The lysis of cells releases in the blood stream sugars and many cellular compounds that are then sensed by the endocrine system and the pancreas as an intake of food. This will elicit the release of anabolic insulin and IGF that stimulate the mitosis of stem cells, starting a regeneration of the lysed tissue. If the pancreatic GABA dependent selection switch between anabolism and catabolism is altered, there will be a concomitant release of catabolic glucagon and anabolic insulin; moreover, the decrease of GABA will fail to terminate the release of insulin. Differentiated cells will then become relatively insensitive to insulin as occurs in diabetes type 2 and will essentially respond to catabolic signals depleting body reserves. Stem cells that express new, not desensitized, insulin receptors receive a dual anabolic–catabolic signal and rewire their metabolic pathways in the hybrid mode that was described. They gain in this way a selective advantage over the other cells in the organ since they can plunder body stores and continue to divide. In the long run mutations stabilize their metabolic advantage selecting the most aggressive population. The pancreatic alteration has given to these stem cells committed to repair an organ, a selective advantage that leads to a tumor in any organ that they should have repaired.
Abbreviations
GABA : Gamma amino butyric acid; KATP : K+ channels inhibited by ATP; MAPK : Mitogen-activated protein kinase pathway ; PI3 kinase : Phosphatidylinositide 3-kinasepathway; RAS : Small GTP-binding proteins; GPCR : G protein-coupled receptor, Gs type stimulates, Gi inhibits adenylate cyclase ; Protein kinase A (PKA); EPAC: Exchange protein activated by cAMP; PKB : Protein kinase B; IGF : Insulin like growth factor; PK : Pyruvate kinase; PDH: Pyruvate dehydrogenase; OAA : Oxaloacetate ; GLP-1:Glucagon like peptide ; GIP glucose dependent insulinotropic peptide.
References
- 1. Gernez, A. Néo-postulats biologiques et pathologiques. (1975) Editions « La vie Claire ».
- 2. Israël, M. On the origin of cancers by means of metabolic selection. Tumor cell targets. (2012) Biomed Res 23(2): 5-8.
- 3. Israël, M., Schwartz, L. On the metabolic origin of cancer: Substances that target tumor metabolism. (2011) Biomed Res 22 (2): 132-166.
- 4. Israël, M., Schwartz, L. The metabolic advantage of tumor cells. (2011) Mol Cancer 10 (70): 1-2.
- 5. Israël, M. A possible primary cause of cancer: deficient cellular interactions in endocrine pancreas. (2012) Mol Cancer 11: 63-68.
- 6. Israël, M. A primary cause of cancer: GABA deficiency in endocrine pancreas. (2012) Cancer Therapy 8: 171-183.
- 7. Israël, M. Signaling and metabolism in cancer: endocrine pancreas deficiency and hybrid anabolism-catabolism, drugs that undo the process. Cancer Therapy 10: 1-12.
- 8. Madhok, B.M., Yeluri, S., Hughes, T.A., et al. Dichloro acetate induces apoptosis and cell-cycle arrest in colorectal cancer cells. (2010) Br J Cancer 102(12): 1746-1752.
- 9. Mazurek, S., Eigenbrodt, E. The tumor metabolome. (2003) Anticancer Res 23(2A): 1149-1154.
- 10. Hemminki, K., Li, X., Sundquist, J., et al. Risk of cancer following hospitalization for type 2 diabetes. (2010) Oncologist 15(6): 548-555.
- 11. Braun, M., Wendt, A., Buschard, K., et al. GABAB receptor activation inhibits exocytosis in rat pancreatic-cells by G-protein-dependent activation of calcineurin. (2004) J Physiol 559(Pt2): 397-409.
- 12. Bayliss, W.M., Starling, E.H. The mechanism of pancreatic secretion. (1902) J Physiol 28(5): 325-353.
- 13. Moore, B. On the treatment of diabetes mellitus by acid extract of duodenal mucous membrane. (1906) Biochem J. 1(1): 28-38.
- 14. Zunz, E., La Barre, J. Contribution à l'étude des variations physiologiques de la sécrétion interne du pancréas. (1929) Arch Int Physiol Biochim 31(2): 20-24.
- 15. Yutaka Seino, Mitsuo Fukushima, Daisuke Yabe. GIP and GLP-1, the two incretin hormones: Similarities and differences. (2010) J Diabetes Invest 1(1-2): 8-22.
- 16. Israël, M. Comment on cancer Metabolism and on the role of the endocrine pancreas. (2014) J Clin Med Res 6(6): 490-491.
- 17. Israël, M. Cancer metabolism: An alteration of the anabolic-catabolic switch. (2014)OA Cancer 2(1): 1-4.
- 18. Israël, M. An alteration of the endocrine pancreas involved in cancer. (2014) Cancer Causes Control 25(12): 1733-1736.
- 19. Garofalo, C., Surmacz, E. Leptin and cancer. (2006) J Cell Physiol 207(1): 12-22.
- 20. Al-Wadei, M.H., Al-Wadei, H.A., Schuller, H.M. Effects of chronic nicotine on the autocrine regulation of pancreatic cancer cells and pancreatic duct epithelial cells by stimulatory and inhibitory neurotransmitters. (2012) Carcinogenesis 33(9): 1745-1753.
- 21. Al-Wadei, M.H., Al-Wadei, H.A., Schuller, H.M. Gamma-amino butyric acid (GABA) prevents the induction of nicotinic receptor-regulated signaling by chronic ethanol in pancreatic cancer cells and normal duct epithelia. (2013) Cancer Prev Res (Phila) 6(2): 139-148.
- 22. Schuller, H.M., Al-Wadei, H.A., Majidi, M. GABA B receptor is a novel drug target for pancreatic cancer. (2008) Cancer 112(4): 767-778.
- 23. Al-Wadei, H.A., Al-Wadei, M.H., Schuller, H.M., et al. Celecoxib and GABA cooperatively prevent the progression of pancreatic cancer in vitro and in xenograft models of stress-free and stress-exposed mice. (2012) PLoS One 7(8): e43376.