Cervical Neoplasia in Women Living With HIV at Cervical Cancer Screening Clinics in Mutare, Eastern Zimbabwe
Munyaradzi Mukuzunga1, Elizabeth Chadambuka1, Elliot Chikaka1, Patron Mafaune2, Auxilia Chideme-Munodawafa1*
Affiliation
- 1Faculty of Health Sciences, Africa University, Mutare, Zimbabwe
- 2Provincial Medical Director at Ministry of Health, and Child Care
Corresponding Author
Auxilia Chideme-Munodawafa, Ph. D., Faculty of Health Sciences, Africa University, PO Box 1320, Mutare, Zimbabwe, Tel: +263 772136880; E-mail: amunodawafa1@gmail.com / chidemea@africau.edu
Citation
Mukuzunga, M.,et al. Cervical Neoplasia in Women Living With HIV at Cervical Cancer Screening Clinics in Mutare, Eastern Zimbabwe. (2016) J Gynecol Neonatal Biol 2(2): 38-45.
Copy rights
© 2016 Mukuzunga, M. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Cervical neoplasia; Cervical cancer; HIV
Abstract
Title: Cervical neoplasia in women living with HIV at cervical cancer screening clinics in Mutare, Zimbabwe, 2015.
Introduction: Cervical cancer in H IV infected women occurs at a younger age and progresses faster compared to those without the HIV infection. In resource limited setting with HIV prevalence like Zimbabwe targeted screening of women living with HIV is an option. The aim of the study was to determine the prevalence of cervical neoplasia and the risk factors amongst HIV infected women attending cervical screening clinics.
Methods: A cross sectional study was conducted at visual inspection with acetic acid and cervicography (VIAC) clinics, in Mutare city in Eastern Zimbabwe from May to July 2015. Two hundred and forty four women living with HIV who were screened for cervical cancer were enrolled in the study. Data were collected by an interviewer administered structured and pretested questionnaire. Data was entered and analysed using Epi info version 7.0.
Results: Out of the 242 participants, 77(31.8%) had cervical abnormality. For the abnormalities 28 underwent cryotherapy, 29 for LEEP and 20 with invasive cancer were referred and attended to in gynaecological clinic. History of genital warts [AOR 5.80(2.00; 16.90)], having more than one lifetime sexual partner [AOR 3.20 (1.16; 8.86)], first pregnancy after the age of 18 [AOR 0.32(0.10; 0.97)], CD4 count ≥ 250 copies/mm3 at antiretroviral commencement [AOR 0.27(0.09; 0.85)] and a latest CD4 count ≥ 500 cell/mm3 [AOR 0.25(0.09; 0, 68)] were independent factors associated with cervical neoplasia.
Conclusion: The prevalence of cervical neoplasia among women living with HIV is high and cervical screening should be part and parcel of management of these women. The need of early initiation of highly active antiretrovirals cannot be over-emphasised.
Introduction
Globally cervical cancer is the second most common cancer amongst women[1]. The developing world bears the brunt of the disease, with 85% of cases occurring outside the developed world. Screening using the Pap smear has reduced the burden of cervical cancer in the developed world to rates as low as 8.5 per 100 000 women in the USA. In 2013, the top 8 countries for cervical prevalence per 100 000 standardised rate were Malawi 75.9 per 100 000 standardised rate, Mozambique at 65.0 , Comoros 61.3, Zambia 58.0, Zimbabwe 56.4, Tanzania 54.0, Swaziland 53.1 and Burundi 49.3, clearly showing the high burden in Sub Saharan Africa[2].
According to World Health Organisation[3], about 270000 women die of cervical cancer every year in the low and middle income countries, this accounts for more than 80% of the total deaths. Cervical cancer is the commonest cause of cancer mortality in Africa (10.4% of all cancer deaths) and contributes 20% of all cancer deaths in African women[4]. The most common cancers amongst black Zimbabwean women in 2008, according to cancer registry[5] were cervical cancer 33.8%, breast cancer at 11.6%, and Kaposi Sarcoma 10.7%. Vaccination for HPV in Zimbabwe is at piloting stage with 2 districts involved and results of the pilot study only available in 2017, when decision will be made on rolling out of program.
Mutare Provincial Hospital is the referral centre for the 7 district hospitals in Manicaland Province, which is on the Eastern border of Zimbabwe with Mozambique. The hospital is located in Mutare City, the provincial capital city. The Anti-Retroviral Treatment (ART) program was rolled out in 2004 and the VIA program in July 2013. Manicaland had HIV prevalence for those between 15 - 49 years of 14.1% and 17.9% amongst women according to demographic health survey, 2010 - 11[6]. Mutare Provincial Hospital recorded 92 new cases of cervical cancer, 84 follow up cases and 79 deaths in 2013(T5, 2013). There is no routine screening for cervical cancer for women living with HIV and attending HIV treatment clinic.
Cervical cancer screening in resource limited areas in the context of high HIV prevalence in Sub Saharan Africa and care has adopted the “see and treat” approach. This is when a woman at a single visit has cervical cancer screening using VIAC and any precancerous lesions observed are treated instantly with cryotherapy. Any abnormal cervical lesions that cannot be treated by cryotherapy are referred according to prescribed protocol. In Accra, Ghana[7], an urban setting, that offered immediate treatment after VIAC had no major complications, was acceptable and feasible. Ramogola-Masire, de Klerk, Monare, Ratshaa, Friedman & Zetola in Botswana agreed that “see and treat” was feasible, efficient and able to sustain high outputs[8].
Integration of this approach within HIV treatment and care clinic, feasibility was demonstrated in Zambia by Pfaendlar et al[9] and Perham et al[10] in Zambia, though the latter used a low cost HPV based screening method rather than VIA. VIA is attractive because it does not require laboratory infrastructure and staff, cervical cancer screening results are instant allowing immediate treatment, easy to learn and use. It is a more cost effective population based screening method compared to conventional cervical cytology as demonstrated in Western Kenya among women living with HIV[11].
Problem statement
The prevalence of cervical neoplasia amongst women living with HIV is not known either. Knowledge of risk factors for cervical cancer amongst women living HIV allow for determination of predictors for cervical neoplasia. Predictors of cervical neoplasia knowledge would allow for risk profiling and targeted screening among HIV infected women where routine screening of all these women is unsustainable due to cost.
Absence of screening for pre-cancerous cervical changes leads to late presentation of cervical cancer in advance state which is not curable leading to an increase in both morbidity and mortality. The high death proportion amongst cervical cancer cases recorded at Mutare Provincial Hospital in 2013, 79/176 (44.9%) is a proxy of late presentation when screening was absent. The death of women from cervical cancer is magnified in those living with HIV because of both occurrence at an earlier age and rapid progressing of disease in this population therefore there is need to explore the factors associated with cervical cancer amongst women living with HIV.
Justification
An appreciation of risk factors and predictors of cervical neoplasia in a vulnerable population (HIV infected women) in Mutare will help facilitate target counselling and screening for precancerous lesion using the available resources. This knowledge can help formulation of linkage of ART and VIAC clinics to ensure high risk women are afforded screening as part of management of HIV infection.
Low prioritisation of cancer is due to lack of information which highlights the extent of cancer risk. Hence highlighting the prevalence and risk factors for cervical cancer among women living with HIV has the potential to move efforts to have routine screening of all HIV infected women or targeted screening. Screening for cervical cancer assumes a major role in fighting cervical cancer in this community where HIV prevalence in high at 15% yet there is no HPV vaccination.
The determination of the prevalence of cervical neoplasia among women living with HIV will give ammunition to lobbying for cervical cancer to be treated as an important opportunistic infection of HIV/AIDS and enable cervical cancer screening programs to tap into funding from HIV/AIDS pool.
Broad objective
To determine the factors associated with pre-invasive and invasive cervical cancer in HIV infected women at Mutare VIAC clinics, 2015.
Specific objectives
1. To establish prevalence of cervical neoplasia amongst women living with HIV at Mutare VIAC clinics, 2015.
2. To identify the socio-demographic factors associated with cervical neoplasia amongst HIV infected women at Mutare VIAC clinics, 2015.
3. To determine the clinical factors associated with cervical neoplasia amongst HIV infected women at Mutare VIAC clinics, 2015.
4. To determine the sexual and reproductive factors associated with cervical neoplasia amongst HIV infected women at Mutare VIAC clinics, 2015.
5. To assess knowledge of cervical cancer among HIV infected women attending Mutare VIAC clinics, 2015.
Chapter III Methodology
Study design
A cross sectional study was done.
Inclusion criteria
All women18 years or above living with HIV attending VIAC clinic were eligible to participate in the study.
Exclusion criteria
The following were excluded from the study
• age below 18
• HIV negative women
• women after a total hysterectomy
• pregnancy
Study setting
The study was conducted at Mutare Provincial Hospital VIAC clinic and Mutare New Start Centre VIAC clinic. New Start Centre is a centre run by Population Service International in partnership with City of Mutare. New Start Centre offers voluntary testing and counselling for HIV, family planning services, antiretroviral therapy services, TB diagnosis, CD4 count testing and cervical cancer screening using VIAC. VIAC program is sponsored by DFID.
Study population
The study population was adult females aged 18 and above who were HIV infected and undergoing cervical cancer screening by visual inspection with 5% acetic acid at the two VIAC clinics.
Questionnaire
The questionnaire was developed in English and was translated to the local language Shona. The Shona version was back translated to English to confirm whether the meaning was maintained through translation. The two English versions were similar. Questionnaire was pretested at Sakubva Clinic Opportunistic Infection Clinic, and necessary adjustment made both to content and language. Questions asking for duration, for example “how long have you been on antiretroviral treatment” was changed to “when did you start antiretroviral treatment, that is year and month” as recommended at pretesting.
VIAC procedure
The client for cervical screening was taken into a private room, the procedure room. Here the client was asked predetermined questions on demographic, reproductive history, sexual behaviour and any symptoms of sexually transmitted infections with data captured electronically. VIAC procedure was done by a trained midwife. Acetic acid 5% was sprayed on the cervix and assessed as well as taking pictures (cervicograph).
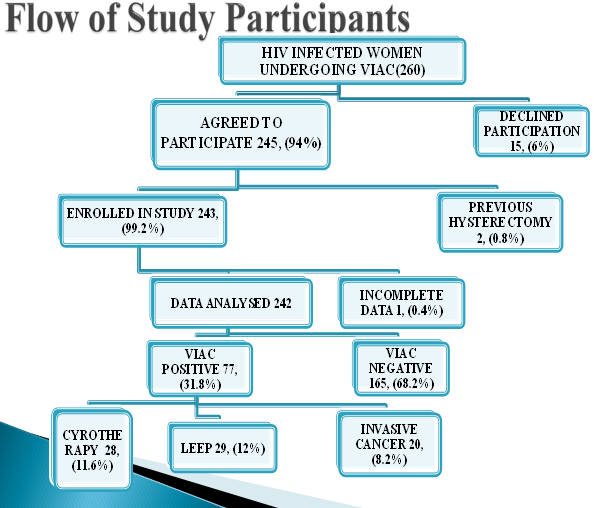
The women were told of the findings with the aid of the cervicograph magnified on computer screen and what needed to be done, either treatment or review date. Those with lesions that required cryotherapy, it was administered by the midwife. Those who required LEEP, cervical biopsy and any other gynaecological were referred to the gynaecology outpatient. Please see patient flow below
Figure 1:
Quality control of VIAC was assured by periodic sampling and reviewing of the cervicographs by a team of midwives, doctors trained in VIAC and the provincial gynaecologist. When midwives were unsure of VIAC findings during procedure they consulted the doctor responsible for the VIAC clinic or the gynaecologist.
Sample size
The sample size was calculated using Stat Cal in Epi Info version 7. Using 95% confidence, 80% power, percentage outcome in unexposed i.e. being woman who are HIV negative with cervical squamous intraepithelial neoplasia, 3.3%, and percent5.4( 2.9 - 8.8)] according to a study by Ezechi, Petterson, Okolo, Ujah & Ostergen in Nigeria the sample size came to 233. Assuming a non-response rate of 10%, then maximum sample size = 233*100/90 = 260
Sampling
Women attending the two VIAC clinics in Mutare were given a talk on cervical cancer disease, visual inspection with acetic acid procedure and then offered VIAC. All those who were living with HIV were told about the study during the processes of VIAC procedure and offered participation in the study. Those who agreed to participate in the study signed a consent form for participating in the study and responded to an interviewer administered questionnaire.
Data collection
The nurses/midwives who were working in the two VIAC clinics were trained on the questionnaire. They helped collect data using a pre-tested interviewer administered questionnaire, in language of participant`s choice (English or Shona). Information on the socio-demographic, sexual and reproductive characteristics, HIV treatment history was collected. HIV clinic data like initial CD4 count, latest CD4 count, types of anti-retroviral drugs the women were on and previous antiretroviral drugs if there was any change, were obtained from medical records or cards wherever it was appropriate and they were available. The VIAC findings were entered into the questionnaire. The study participants were assessed for knowledge on cervical cancer. Any knowledge gaps were filled in and misinformation and/or misconceptions corrected. Participants were thanked for participation in study and reminded of their review date for next VIAC screening.
Data analysis
Information from the questionnaires was checked for completeness before it was entered into Epi info version 7. Data cleaning was performed by running simple frequency distributions, summary statistics and cross-tabulation to identify and correct out-of-range, missing and inconsistent values. Quantitative variables were summarized using median, Q1 and Q3, or mean and standard deviation as was appropriate. Categorical variables were tabulated using frequencies and percentages. A bivariate analysis was carried out; including the calculation of crude Odds Ratios (ORs) and the precision of these population estimates were provided by constructing 95% Confidence Intervals (C.I.).
The prevalence of cervical abnormality among the women living with HIV was calculated. The women`s knowledge on cervical cancer and screening was described using associated percentages. Analysis was made to assess the association of socio-demographic factors, sexual and reproductive characteristics, and HIV clinical factors with cervical abnormality. Stratification was done to determine existence of confounding and/or effect modification. The Chi-square for differing Odds Ratios by stratum was used to test if they were statistically different. Multivariate regression was done to find predictors or independent factors of cervical abnormality in HIV infected women by creating a model. The results were presented as Odd Ratios (OR) or Adjusted Odd Ratios (AOR) with their 95% confidence intervals.
Permission
Permission was sought and granted from Faculty of Health Science (FHS) at Africa University, Africa University Review Ethics Committee (AUREC), Provincial Medical Director (PMD) Manicaland Province and Medical Superintendent Mutare Provincial Hospital and Mutare New Start Centre Management.
Ethical considerations
Detailed information about the study was given to women who met the study criteria. Those who agreed to participate in the study signed a written informed consent before study questionnaire was administered. Confidentiality of data obtained was ensured and maintained with records kept under lock and key.
Results
There were 260 women living with HIV who were offered to enter into the study. Of these 15 declined because of time constraints, 2 were excluded because they had undergone hysterectomy and 1 questionnaire was excluded from analysis because it was incomplete. The response rate was about 94 % (245/260).
Table 1: The socio-demographic characteristics by VIAC status of women living with HIV at Mutare VIAC clinics, 2015.
| Exposure | Category | VIAC positive N = 77 n (%) | VIAC negative N = 165 n (%) |
|---|---|---|---|
| Age | Median years (Q1; Q3) | 39(33;43) | 42(35;48) |
| < 30 | 10(13) | 14(8.5) | |
| 30 – 40 | 30(39) | 52(31.5) | |
| 40 – 50 | 28(36) | 60(36) | |
| > 50 | 9(12) | 39(24) | |
| Educational level | None/ Primary | 21(28) | 54(32.7) |
| Secondary | 50(65) | 97(58.8) | |
| Tertiary | 6(8) | 14(8.5) | |
| Residence | Rural | 31(41) | 67(41) |
| Farm | 3(4) | 9(5) | |
| Urban | 43(56) | 89(54) | |
| Marital status | Married/cohabiting | 37(48.1) | 79(47.8) |
| Divorced | 9(11.7) | 18(10.9) | |
| Widow | 23(29.9) | 59(35.8) | |
| Single | 8(10.4) | 9(5.5) |
Socio-demographic characteristics
Slightly above a third of the participants 88(36%), were between 40 and 50 years followed by those aged 30 - 40 years who were 82(34%). The median age for the respondents was 41 years [Q1 = 34; Q3 = 48]. Those with primary or no education were 75(31%) and majority had attained secondary education 145(61%) but only 20(8%) had obtained tertiary education. Most participants were urban dwellers 132(54.5%), with 98(40.5%) living in rural areas and a mere 12(5%) lived on farms. Only 4 women were into tobacco use. Most women were married or cohabiting 116(48%).(Table 1)
Sexual and reproductive characteristics
Mean age of menarche was 14.7 years [standard deviation (sd) = 2.7]. Mean age of first sexual contact was 19.4[sd = 3.3], while 99(40.9%) women had their first sexual contact by 18 and 47(19.4%) women before 16. The mean age at first pregnancy was 19.97 [sd = 4.17] and 157 (64.9%) had more than one life sexual partner. The mean number of lifetime sexual partners was 3.7 (sd = 6.7), while for those with cervical abnormality was 4.5(sd = 5.4) and those without 3.4(sd = 7.3). The overall mean number of lifetime sexual partners was 3.8(sd = 6.7), 4.5(sd = 5.4) for those with cervical abnormality and 3.4(sd = 7.3) for those without. Mean parity was 3.24[sd = 2.02] with 60 (24.8%) women who gave birth to at least 5 children yet 16 (6.6%) had never given birth. Most women 192(79.3%) had some exposure to hormonal contraception. (Table 2)
Table 2: The sexual and reproductive characteristics of women living with HIV at Mutare VIAC clinics, 2015.
| Exposure | Category | VIAC positive N = 77 n (%) | VIAC negative N = 165 n (%) |
|---|---|---|---|
| Age at first pregnancy | < 18 years | 23(31.5)* | 29(18.4)** |
| >18 years | 50(68.5)* | 129(81.6)** | |
| Age at first sexual contact | < 16 years | 12(16) | 12(7) |
| >16 years | 65(84) | 153(93) | |
| Menarche | Mean (SD) years | 14.6(1.7) | 14.7(1.6) |
| Contraception | Hormonal use | 64(83.1) | 128(77.6) |
| No hormonal use | 13(16.9) | 37(22.4) | |
| Lifetime partners | 1 | 20(26) | 65(39.4) |
| 2 | 22(28.6) | 42(25.5) | |
| 3 | 6(7.8) | 22(13.3) | |
| 4 | 6(7.8) | 11(6.7) | |
| > 4 | 23(29.8) | 25(15.5) | |
| Mean (sd) | 4.5(5.4) | 3.4(7.3) | |
| Parity | Nulliparity | 5(7) | 11(7) |
| Primiparous | 11(14) | 23(14) | |
| Multiparous | 60(79) | 131(79) | |
| History of STI | Yes | 55(71.4) | 63(38.2) |
| No | 22(28.6) | 102(61.8) | |
| History of genital warts | Yes | 44(57.1) | 22(13.6) |
| No | 33(42.9) | 140(86.4) | |
| Use of condom in last encounter | Yes | 51(66.2) | 93(56.4) |
| No | 26(33.8) | 72(43.6) |
Note: N for * is 73 and for ** is 158, because some of the women never got pregnant.
A history of STIs was reported by almost half the women 118 (48.8%) and more than a quarter that is 66 (27.6%) reported a history of genital warts. Those who had not used any condoms in their last sexual encounter were 98 (40.5%). A total of 24 (9.9%) women had their first sexual contact before the age of 16, which legally is rape.
Clinical factors
The majority of the respondent 214 (89.5%) were on ART. The mean CD4 count at ART commencement was 206 cells/mm3 [sd = 126] and mean latest CD4 was 559 cells/mm3 [sd = 311]. The length of period on of ART had a median of 42 months [Q1 = 17; Q3 = 67]. Those on first line were 212 (97.7%) and 211(99.5%) were on tenofovir (TDF) & lamuvidine (3TC) and nevirapine (NVP) or efarvirenz (EFV). (Table 3)
Table 3: The clinical characteristics of women living with HIV at Mutare VIAC clinics, 2015.
| Exposure | Category | VIAC positive N = 77 n(%) | VIAC negative N = 165 n(%) |
|---|---|---|---|
| ART exposure | Never on ART | 9(12) | 16(10) |
| < 12 months | 13(19) | 30(20) | |
| ≥ 12 - 36 months | 12(18) | 35(24) | |
| ≥ 36 - 48 months | 10(15) | 15(10) | |
| ≥ 48 months | 32(48) | 68(46) | |
| Baseline CD4 | Median (Q1;Q3) | 117(79;204) | 233(146;315) |
| Most recent CD4 | Median (Q1;Q3) | 391(264;563) | 594(400;719) |
Prevalence of cervical neoplasia
From the 242 women, 77 (28 underwent cryotherapy, 29 for LEEP and 20 with invasive cancer were referred and attended to in gynaecological clinic.) had cervical neoplasia on VIAC, making a prevalence of 31.8% [95% CI = (26.0; 38.1)]. The cervical neoplasia consisted of 28 (36.4%) treated by cryotherapy, 29 (37.7%) needed LEEP and 20 (26.0%) were classified as invasive cancer (visible tumour or ulcer).
Knowledge of cervical cancer
The respondents were largely aware that cervical cancer is preventable with a figure of 175 (73.5%), though 25 (10.5%) said the cancer was not preventable, and 38 (16%) did not know. Methods cited as prevention for cervical cancer were: early screening 68 (28.1%); condom use 55 (22.7%); HPV vaccination 3 (1.2%); male partner circumcision one and other methods apart from these were 53 (21.9%) responds. Other methods mentioned included not inserting herbs and fingers into the vagina, being faithful to one partner, avoiding vaginal douching, stopping smoking and delaying sexual debut.
More than three quarters 187 (77.3%) were aware that VIAC was screening method for cervical cancer, 49 (21.9%) reported Pap smear, none mentioned HPV DNA screening and 49 (20.3%) said they did not know any screening method despite undergoing VIAC. Knowledge of risk factor was good with the different factors reported as follows: multiple partners 144 (59.5%), early sexual debut 92 (38.0%), HIV infection 84 (34.7%), sexually transmitted infections 84 (34.7%), smoking 5 7(23.6%), HPV infection 5 (2.1%) and those who were not aware of any factor 40 (16.5%).
The socio-demographic characteristic of age, level of highest education, place of residence, age at menarche, tobacco use and marital status were all not significantly associated with abnormal cervical finding after bivariate analysis as shown in the table 4.
Table 4: Socio-demographic factors association with abnormal cervical finding on VIAC in women living with HIV at VIAC clinics in Mutare.
| Exposure | Disease | OR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| Age ( < 40) | Yes | 40 | 66 | 1.62 (0.94 ; 2.80) | 0.08 |
| No | 37 | 99 | |||
| Education( ≤ primary) | Yes | 21 | 54 | 0.78 (0.43; 1.43) | 0.41 |
| No | 55 | 110 | |||
| Menarche (< 14) | Yes | 21 | 28 | 1.81 (0.96; 3.45) | 0.06 |
| No | 56 | 137 | |||
| Residence (urban) | Yes | 43 | 89 | 1.08 (0.63; 1.86) | 0.78 |
| No | 34 | 76 | |||
| Marital status | Yes | 37 | 79 | 1.03 (0.60; 1.78) | 0.91 |
| No | 39 | 86 | |||
There was strong association between lifetime history of Sexually Transmitted Infection (STI) [OR; 4.05 (2.25; 7.27), p value ≤ 0.001] which means those with a history of STI were about 4 times more likely to have cervical abnormality compared to those who did not experience an STI. Having a life history of genital warts [OR; 8.48 (4.49; 16.04), p value ≤ 0.001] increased the chance of having cervical neoplasia by about eight and half times. Any woman who had their first pregnancy before 18 [OR; 2.05 (1.08; 3.87), p value = 0.03] doubled their risk of having abnormal cervical finding.
Table 5: Sexual & reproductive factors associated with abnormal cervical finding on VIAC in women living with HIV, VIAC clinics in Mutare
| Exposure | Disease | OR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| Use of hormonal contraception | Yes | 64 | 128 | 1.42 (0.71; 2.86) | 0.32 |
| No 37 | 13 | 37 | |||
| Regular condom use | Yes | 51 | 93 | 1.51 (0.86; 2.67) | 0.15 |
| No | 26 | 72 | |||
| Age at first sexual contact(< 16) | Yes | 12 | 12 | 2.35 (1.005; 5.51) | 0.04 |
| No | 65 | 153 | |||
| Age at first pregnancy( ≥ 18) | Yes | 23 | 29 | 2.05 (1.08; 3.87) | 0.03 |
| No | 50 | 129 | |||
| Parity ( ≥ 5) | Yes | 60 | 122 | 1.24(0.66; 2.36) | 0.50 |
| No | 17 | 43 | |||
| Lifetime history of STIs | Yes | 55 | 63 | 4.05 (2.25; 7.27) | 0.00 |
| No | 22 | 102 | |||
| Lifetime history of genital warts | Yes | 44 | 22 | 8.48 (4.49; 16.04) | 0.00 |
| No | 33 | 140 | |||
| Lifetime sexual partners (< 4) | Yes | 48 | 129 | 0.46 (0.26; 0.83) | 0.01 |
| No | 29 | 36 | |||
| Lifetime sexual partners( ≥ 2) | Yes | 57 | 100 | 1.85 (1.02;3.37) | 0.04 |
| No | 20 | 65 | |||
sexual and reproductive factors or exposures were related to cervical neoplasia.
If a woman had more than one lifetime sexual partner [1.85 (1.02; 3.37) p value = 0.04] they had a 1.85 times more chance of cervical neoplasia compared to those with only one lifetime sexual partner. However, if the number of their lifetime sexual partners was less than 4 [OR; 0.46 (0.26; 0.83)] they were about 54% less likely to have cervical abnormality compared to those with 4 and more lifetime sexual partners. (Table 5)
Women who had their first sexual encounter whilst below age of consent, sixteen were 2.35 times more likely to have neoplasia compared to those who had their sexual debut after the age of 16 [OR 2.35, 95% CI = (1.01 - 5.51), p value = 0.04]. Women with a baseline CD4 ≥ 250 cells/mm3 were about 79% less likely to present with cervical abnormality compared to those who had an initial CD4 count < 250 cells/mm3 [OR = 0.21 (0.09; 0.46), p value < 0.001]. A current or latest CD4 count ≥ 500 cells/mm3 meant women were 71% less likely to have cervical neoplasia compared to those with a latest CD4 less than 500 cells/mm3 [OR = 0.29 (0.15, 0.58); p-value < 0.001). Therefore, a good immune function was protective against cervical abnormality. Other HIV clinical factors like whether one was on ART or not and duration of ART had no significant association with cervical neoplasia as shown in Table 6.
Table 6: Clinical factors association with abnormal cervical finding on VIAC in women living with HIV at VIAC clinics in Mutare.
| Exposure | Disease | OR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| Use of ART | Yes | 67 | 147 | 0.81 (0.34; 1.93) | 0.63 |
| No6 | 9 | 16 | |||
| Duration of ART(36 months) | Yes | 25 | 65 | 0.76 (0.42; 1.37) | 0.36 |
| No | 42 | 83 | |||
| CD4 at ART commencement( ≥ 250) | Yes | 9 | 56 | 0.21(0.09; 0.46) | 0.00 |
| No | 49 | 64 | |||
| Latest CD4 count( ≥ 500) | Yes | 18 | 74 | 0.29(0.15; 0.58) | 0.00 |
| No | 34 | 41 | |||
Confounding and effect modification Age is a recognised confounder or effect modifier of relationship between a variable or exposure and an outcome. Stratification was done to elicit for presence of confounding and/or effect modification. (Table 7)
Table 7: History of genital warts was stratified by age to check for Effect Modification or confounding for women living with HIV at VIAC clinics in Mutare, 2015.
| History of genital warts | |
|---|---|
| OR < 40 years | 18.26(6.32;52.81) |
| OR ≥ 40 years | 4.80(2.07;11.09) |
| Crude OR | 8.48(4.49;16.04) |
| Breslow Day test X2(homogeneity) | 3.75 |
| & p value | 0.054 |
| Conclusion | the crude is between the stratum ORs so it’s EM |
Multiple regression/independent factors
A stepwise descending procedure was applied in the multivariate analysis, to obtain a model that best predicted the presence of abnormal cervical finding or cervical neoplasia. All the exposures with irrespective of their OR on Bivariate analysis were included in the initial multivariate model. Exposures that were not significantly associated with occurrence of cervical neoplasia or abnormal VIAC and did not add any significant prediction were sequentially removed.
Participants who had a history of genital warts were about 5.8 times more likely to have cervical neoplasia compared to those who did not have a history of genital warts and it was statistically significant [AOR = 5.80 (2.00; 16.90), p value < 0.001]. Women who had a baseline CD4 count equal to or greater than 250 ( ≥ 250) copies/mm3 were 73% less likely to develop cervical abnormality compared to those with baseline CD4 < 250 [AOR = 0.27 (0.09; 0.85), p value = 0.02]. Moreover, those women living with HIV with latest CD4 ≥ 500 copies/mm3 were 75% less likely to have VIAC positive finding compared to those women living with HIV with latest CD4 < 500 copies/mm3 and it was statistically significant [AOR = 0.25 (0.09; 0,68), p value = 0.01]. The women who had their first pregnancy when they were at least 18 were 68% less likely to develop cervical neoplasia compared to those who got pregnant before they turned 18 and it was statistically significant, [AOR = 0.32 (0.10; 0.97), p value = 0.04]. Participants who had 2 or more ( ≥ 2) lifetime sexual partners were 3.2 times more likely to present with cervical neoplasia compared to those who had just one lifetime sexual partner and it was statistically significant [AOR = 3.2 (1.16; 8.86), p value = 0.03]. (Table 8)
Table 8: Independent Factors Associated with cervical neoplasia on VIAC in women living with HIV at Mutare VIAC clinics, 2015.
| VARIABLE | Crude OR (95% CI) | P- value | Adjusted OR(95%CI) | P- value |
|---|---|---|---|---|
| Lifetime history of genital warts | 8.48(4.49; 16.04) | ≤ 0.001 | 5.80(2.00; 16.90) | ≤ 0.001 |
| CD4 at ART commencement ≥ 250 | 0.21(0.09; 0.46) | ≤ 0.001 | 0.27(0.09; 0.85) | 0.02 |
| Latest CD4 count ( ≥ 500) | 0.29(0.15; 0.58) | ≤ 0.001 | 0.25(0.09; 0,68) | 0.01 |
| Age at first pregnancy( ≥ 18) | 0.09(0.26; 0.92) | 0.02 | 0.32(0.10; 0.97) | 0.04 |
| Lifetime sexual partners ( ≥ 2) | 1.85(1.02;3.37) | 0.04 | 3.20(1.16;8.86) | 0.03 |
Discussion
The prevalence of cervical neoplasia in women living with HIV was 31.8%, which is very high and warrants targeted screening of HIV infected women for cervical cancer. This targeted cervical cancer screening would be part and parcel of HIV treatment programmes as a standard of care as concluded by Denslow et al[12]. The “see and treat” approach, employing VIAC with cryotherapy would be ideal for resource limited areas like Sub Saharan Africa. It is cost effective and can be run by nurses in HIV care clinics, with supervision of physicians. The approach has worked in a rural setting in India, Sankaranaravan et al[13], in urban setting in Accra, Ghana, Blumnthal et al[7] and was safe, effective and acceptable according to Ramogola-Masire et al[8] in Botswana. Pfaendlar et al[9] in Zambia and Odafe, Torpev, Khamofu, Oladele, Adedokun Okove[14] in Abuja Nigeria both have shown feasibility of “see and treat” approach integration into the care of HIV infected women.
The high cervical neoplasia prevalence of 31.8% in the study is comparable to the ones found by Omar et al,[15] of 38% in Soweto, South Africa and by Ali-Risasi et al[1] in Kinshasa, Democratic Republic of Congo of 31.1% [95% CI (24.0 % – 39.7 %)]. However lower prevalence rates of different magnitudes have been reported by Gedefaw et al[16] in Southern Ethiopia 22.1% [(95% CI: 18.3-25.9)] by Ezechi et al[17] in South Western Nigeria 19.2% and by Jaquet et al[18] in Cot`dvoire 9%.
These differences in prevalence could be due to different oncogenic HPV profiles in different regions, noting that HPV infection and persistence is a necessary factor for development of cervical neoplasia though not a sufficient factor, Kumar & Bhasker[19]. Stricker et al[20] have noted indirect evidence point at that HPV16 is less likely to cause the development of cervical neoplasia in HIV-infected as it does in uninfected women making presence of particular HPV serotypes in a population key.
HPV infections are not the only factor responsible for development of cervical neoplasia but other behavioural and demographic factors do contribute, according to Aggarwal[21]. Comparing this study and the one by Ononogbu et al[22] in Nigeria they have similar factors: mean age of sexual debut 19.4(sd = 3.3) and 19(4), mean parity 3.2(sd = 2.0) and 4(2), married 48% and 52%, attained at least secondary education 69% and 77% but differences occur on mean number of lifetime sexual partners 3.7(sd = 6.7) and 1(sd = 1), cervical neoplasia prevalence 31.8% and 6% respectively.
Women with 2 or more lifetime sexual partners [adjusted OR 3.2(1.15; 8.86), p- 0.03] were at greater risk of cervical neoplasia compared to women with one life sexual partner Studies have established the increased risk of cervical neoplasia for women who are HIV infected with multiple lifetime sexual partners including Anastos et al[23] in Rwanda, Adam et al[24] in Soweto South Africa and Kafuruki et al[25] in Mwanza, Tanzania. They all concurred that multiple partners was an independent risk factor for cervical neoplasia.
The level of immunological status had a bearing on the presence of cervical neoplasia with both the baseline CD4 or at commencement of ART and latest CD4 count independently associated with abnormal VIAC finding [OR = 0.27 95% CI = (0.09; 0.85),p value = 0.02] and [OR = 0.25, 95% CI = (0.09; 0,68), p value = 0.01] respectively. This is supported by Kafuruki et al[25], Teixeira et al[26] and Vasconcelos de Andrade et al[27] in Brazil who all showed that a low baseline CD4 was predictive of cervical neoplasia. Latest CD4 count ( ≥ 500 cells/cm3) in the study was protective against cervical neoplasia, [OR = 0.25 95% CI = (0.09; 0,68), p value = 0.01]. Women who had sexual debut before 18 were associated with a positive VIAC finding [OR 2.01, 95% CI (1.12; 3.63), p = 0.02] but this was not a predictor of cervical neoplasia after multiple
Level of education, occupation or income were reported to affect risk of VIAC positivity by Kahesa et al[28], but we found no association and our findings were supported by Atashili et al,[29] in Cameroon. This could be due to the high literacy rate in Zimbabwe and economic situation placing majority in same income bracket irrespective of level of education or occupation. Moreover these women getting free VIAC screening may mean same income level with those with a different or higher income accessing cervical cancer screening at a charge elsewhere.
Conclusion
Cervical cancer screening can be part and parcel of care for women living with HIV through the “see and treat” utilising VIAC and cryotherapy. There is need for further decentralisation of the cervical cancer screening services to ensure access for all women living with HIV. The Manicaland Provincial Medical Director and the Medical Superintendent need to strength and increase HIV testing and counselling including Provider Initiated Testing And Counselling (PITC) to ensure that women who are infected with HIV can be diagnosed and started on ART early, while their CD4 count is still high and thereby prevent development of cervical neoplasia, and also strength pre-VIAC counselling so that women undergoing the procedure are well aware about the disease (about a quarter could not mention cervical screening method despite undergoing the screening.
References
- 1. Ali-Risasi, C., Verdonck, K., Padalko, E., et al. Prevalence and risk factors for cancer of the uterine cervix among women living in Kinshasa, the Democratic Republic of the Congo: a cross-sectional study. (2015) Infect Agent Cancer 10: 20.
- 2. Ferlay, J., Soerjomataram, I., Dikshit, R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. (2015) Int J Cancer 136(5): E359-E386.
- 3. Stewart, B., Wild, C.P. World cancer report 2014.
- 4. Ferlay, J., Shin, H.R., Bray, F., et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. (2010) Int J Cancer 127(12): 2893-2917.
- 5. Chokunonga, E., Borok, M.Z., Chirenje, Z.M., et al. Zimbabwe National Cancer Registry. Annual Report 2012 (2014) Harare pg: 1-56.
- 6. Zimbabwe Demographic and Health Survey 2010-11. (2012) Zimbabwe National Statistics Agency (ZIMSTAT) and ICF International.
- 7. Blumenthal, P.D., Gaffikin, L., Deganus, S., et al. Cervical cancer prevention: safety, acceptability, and feasibility of a single-visit approach in Accra, Ghana. (2007) Am J Obstet Gynecol 196(4): 407.e1-8.
- 8. Ramogola-Masire, D., de Klerk, R., Monare, B., et al. Cervical cancer prevention in HIV-infected women using the “see and treat” approach in Botswana. (2012) J Acquir Immune Defic Syndr 59(3): 308-313.
- 9. Pfaendler, K.S., Mwanahamuntu, M.H., Sahasrabuddhe, V.V., et al. Management of cryotherapy-ineligible women in a “screen-and-treat” cervical cancer prevention program targeting HIV-infected women in Zambia: lessons from the field. (2008) Gynecol Oncol 110(3): 402-407.
- 10. Parham, G.P., Mwanahamuntu, M.H., Sahasrabuddhe, V.V., et al. Implementation of cervical cancer prevention services for HIV-infected women in Zambia: measuring program effectiveness. (2010) HIV Ther 4(6): 713-722.
- 11. Mabeya, H., Khozaim, K., Liu, T., et al. Comparison of conventional cervical cytology versus visual inspection with acetic acid (VIA) among HIV-infected women in western Kenya. (2012) J Low Genit Tract Dis 16(2): 92-97.
- 12. Denslow, S.A., Rositch, A.F., Firnhaber, C., et al. Incidence and progression of cervical lesions in women with HIV: a systematic global review. (2014) Int J STD AIDS 25(3): 163-177.
- 13. Sankaranarayanan, R., Rajkumar, R., Esmy, P.O., et al. Effectiveness, safety and acceptability of ‘see and treat’ with cryotherapy by nurses in a cervical screening study in India. (2007) Br J Cancer 96(5): 738-743.
- 14. Odafe, S., Torpey, K., Khamofu, H., et al. Integrating cervical cancer screening with HIV care in a district hospital in Abuja, Nigeria. (2013) Niger Med J 54(3): 176-184.
- 15. Omar, T., Schwartz, S., Hanrahan, C., et al. Progression and Regression of Pre-malignant Cervical Lesions in HIV-infected Women from Soweto: A Prospective Cohort. (2011) AIDS 25(1): 87-94.
- 16. Gedefaw, A., Astatkie, A., Tessema, G.A. The prevalence of precancerous cervical cancer lesion among HIV-infected women in Southern Ethiopia: a cross-sectional study. (2013) PloS one 8(12): e84519.
- 17. Ezechi, O.C., Ostergren, P.O., Nwaokorie, F.O., et al. The burden, distribution and risk factors for cervical oncogenic human papilloma virus infection in HIV positive Nigerian women. (2014) Virology Journal 11: 5.
- 18. Jaquet, A., Horo, A., Charbonneau, V., et al. Cervical human papillomavirus and HIV infection in women of child-bearing age in Abidjan, Cote d’Ivoire, 2010. (2012) Br J Cancer 107(3): 556-563.
- 19. Kumar, R.V., Bhasker, S. Potential opportunities to reduce cervical cancer by addressing risk factors other than HPV. (2013) J Gynecol Oncol 24(4): 295-297.
- 20. Strickler, H.D., Burk, R.D., Fazzari, M., et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus–positive women. (2005) J Natl Cancer Inst 97(8): 577-586.
- 21. Aggarwal, P. Cervical cancer: Can it be prevented? (2014) World J Clin Oncol 5(4): 775-780.
- 22. Ononogbu, U., Almujtaba, M., Modibbo, F., et al. Cervical cancer risk factors among HIV-infected Nigerian women. (2013) BMC Public Health 13: 582.
- 23. Anastos, K., Hoover, D.R., Burk, R.D., et al. Risk factors for cervical precancer and cancer in HIV-infected, HPV-positive Rwandan women. (2010) PLoS One 5(10): e13525.
- 24. Adam, Y., McIntyre, J.A., de Bruyn, G. Incidence of cytological abnormalities within 24 months of a normal cervical smear in Soweto, South Africa. (2012) S Afr Med J 103(1): 34-39.
- 25. Kafuruki, L., Rambau, P.F., Massinde, A., et al. Prevalence and predictors of cervical intraepithelial neoplasia among HIV infected women at Bugando Medical Centre, Mwanza-Tanzania. (2013) Infect Agent Cancer 8: 45.
- 26. Teixeira, N.C., Araújo, A.C., Correa, C.M., et al. Prevalence and risk factors for cervical intraepithelial neoplasia among HIV-infected women. (2012) Braz J Infect Dis Apr 16(2): 164-169.
- 27. de Andrade, A.C., Luz, P.M., Velasque, L., et al. Factors associated with colposcopy-histopathology confirmed cervical intraepithelial neoplasia among HIV-infected women from Rio De Janeiro, Brazil. (2011) PloS one 6(3): e18297.
- 28. Kahesa, C., Kjaer, S.K., Ngoma, T., et al. Risk factors for VIA positivity and determinants of screening attendances in Dar es Salaam, Tanzania. (2012) BMC public health 12: 1055.
- 29. Atashili, J., Adimora, A.A., Ndumbe, P.M., et al. High prevalence of cervical squamous intraepithelial lesions in women on antiretroviral therapy in Cameroon: Is targeted screening feasible? (2012) Cancer epidemiol 36(3): 263-269.