Comparative Study of the Effect of Taurine, Caffeine, and /or Paracetamol on Male Fertility and Teratogenicity in Rats
Asmaa M.Kandil, Wedad A. Hassan, Ahmady Y. Ahmed
Affiliation
Pharmacology Department, National Organization for Drug Control and Research, NODCAR, Giza, Egypt, P.O. BOX 29
Corresponding Author
Ebtehal Mohammad F, Pharmacology Department, national organization for drug control and research, NODCAR, Giza, Egypt, P.O. BOX 29, Tel:+201005222558; E-mail: ebtehal21@yahoo.com
Citation
Ebtehal, M. F. Comparative Study of the Effect of Taurine, Caffeine, And /or Paracetamol on Male Fertility and Teratogenicity in Rats (2018) J Pharm Pharmaceutics 5(1): 1- 7.
Copy rights
© 2018 Ebtehal, M. F. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Taurine; Caffeine; Paracetamol; Fertility; Teratogenicity
Abstract
Caffeine (CF) is one of the popular nutrients that have been implicated as a risk factor for infertility. Taurine (TAU) is the most abundant free amino-acid present in mammalian tissues and cells. Paracetamol (PCM) is an effective oral analgesic. The present study represented to evaluate the effects of CF, TAU and /or PCM on male fertility in rat and the development of their fetuses. The 1st group was kept as a normal control received distilled water. The 2nd, 3rd and 4th groups received orally 250 mg / kg, 40 mg / kg and 270 mg/ kg of TAU, CF and PCM, respectively. In addition, the 5th, 6th, 7th, and 8th groups received TAU+CF, TAU+PCM, CF+PCM and TAU+CF+PCM, respectively, for 45th consecutive days. Then blood samples were taken to determine the testosterone and FSH level, and total pro-oxidant. Moreover, in testes, malondialdehyde and nitrite/nitrate were estimated. Besides, the abnormality of sperms, sperm counts and percentage of sperm motilities were evaluated. These males were allowing to mated with untreated female rats to determined the rate of pregnancy and study any malformation in their fetuses. The results revealed that each of CF and/ or PCM decreased the weights of fetuses, as well as sperm counts and motilities significantly. Besides, percentages of head and tail abnormalities were increased in CF and/or PCM. The histopathological examination revealed presence of marked degenerative changes in testes in CF and/or PCM-treated groups. In conclusion, we observed that TAU almost improved undesirable effects induced by PCM or/and CF.
Introduction
Caffeine (CF), 1, 3, 7-trimethylxanthine, is one of the most popular habitually consumed nutritionalcomponents in the world for its central nervous system and psychoactive effects[1,3]. In fact, it presents in several food and beverage products, such as coffee and tea. In clinical medicine, CF used as the smooth muscle relaxant, diuretic, analgesic, and it is used as adjuvant treatment in brain diseases such as headaches and Parkinson’s disease[2,3]. Effects of CF mediated by its action on cAMP phosphodiesterase, phosphatidylinositol- 3kinase, and its antagonistic actions at the A1 and A2A subtypes of adenosine receptors[4,5].
Taurine (TAU), 2 - aminoethane sulfonic acid, is the most rich free amino-acid present in mammalian tissues and cells, as well as it plays amain role in several essential biological processes in the body. It acts as neurotransmitter, osmo-regulator and antioxidant in most tissues. TAU is essential for development of the central nervous system and the retina, calcium modulation, and reproduction. In addition it is necessary in cell membrane stabilization in the heart, muscle, retina, and throughout the CNS as well immunity[6]. Furthermore, TAU acts as an endogenous antioxidant and this is related to TAU’s scavenging mechanism and reducing cell apoptosis[7-9]. Actually, in the radical scavenging mechanisms the reactive radical specie is inactivated by accepting a hydrogen atom from a hydroxyl group of the phenolacid[10].
Paracetamol (PCM), acetaminophen, is a valuable, safe, good – tolerated, a non-prescription cheap drug, broadly used as antipyretic and analgesic.Its overdose is a common reason of self-poisoning worldwide resulting from its wide availability and accessibility[11,12]. It is fairly absorbed through gastrointestinal tract, metabolized by glucourindation and hydroxylation in the liver and is excreted in urine[13]. Saturated and excess PCM is oxidatively metabolized by hepatic cytochrome p450 (CYP450) system to a toxic metabolite, namely, N-acetyl-p-benzoquinone imine[14]. Metabolites of PCM caused oxidative stress in the cell leading to its terminationwhich leads to fulminant liver failure and also death[15]. These observations designate a possible antigonadotrophic effect of PCM. Further, it is now known that PCM can hinder nitric oxide generation, which is essential for normal reproductive activity in the male rat.
Here we want to investigate the effect of CF and/or TAU on male fertility in rats and the development of their fetuses, as both ingredients are present together in Red Bull Energy Drink. In fact, throughout the last decade, the use of energy drinks has been increasingly looked upon with caution as potentially dangerous due to their perceived strong concentration of CF. In addition, a large number of energy drink intoxications have been reported all over the world including cases of seizures and arrhythmias[16]. It was previously reported that toxic doses of CF may affect conductance and refractoriness on the heart, which results in the development of various arrhythmias[17].
Besides, we study the effect of PCM, over counter analgesic treatment, either lonely or in combination with CF and TAU. Sinceincreased access and use of the over counter drugs in developing countries, coupled with lack of knowledge and awareness about proper use of these medications can lead to potential harmful effects.
Methods
Animals
Adult Sprague-Dawley sexually mature rats of both sexes weighing from 150 - 200 g obtained from the animal house of the National Organization for Drug Control and Research (NODCAR, Giza, Egypt) were used in the present study.Animals were housed adlibitum for at least one week in the laboratory room prior to testing under controlled environmental conditions; constant temperature (25 ± 2°C), humidity(60 ± 10%), and alternating 12 h light/dark cycles. The investigation complies with the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85 - 23, revised 1996).
Sixty four male rats were randomly allocated into eight groups (n = 8). The 1st group was kept as a normal control received 1 ml/kg distilled water. The 2nd, 3rd and 4th groups received 250 mg / kg, 40 mg /kg, and 270 mg/kg; orally of TAU, CF and PCM, respectively. Additionally, the 5th, 6th, 7th, and 8th groups received TAU+PCM, TAU+CF, CF+PCM and TAU+CF+PCM, respectively, for 45th consecutive days.
Estimated Parameters
Effect of the tested treatments on male fertility
The effect of the tested chemicals on male fertility was studied by epididymal spermatozoa examination as well as histopathological examination of the testes.
Epididymal spermatozoa examination
The epididymal content was obtained immediately after sacrificing each rat. The tail of epididymis was cut with a sharp blade, squeezed gently in a clean watch-glass and examined using the following parameters.
(a) Progressive motility of sperms:
The progressive motility of sperms was estimated according to the method reportedby Bearden and Fluquary, (1980)[18]. A small droplet of epididymal content was added to one drop of 2.9% sodium citrate solution on clear glass slide then the slide was gently warmed. Several fields were quickly examined under microscope (X100) and the progressively motility of sperms were estimated as a percentage.
(b) Sperm cell concentration (sperm count):
This was performed according to previous technique[19]. The pipette of counting erythrocytes by haemocytometer was used. The epididymal content was with drawn up to the mark 0.1 and the pipette was then filled up to the mark 101 by 0.9% sodium citrate solutions. The content of the pipette was mixed and shacked well by holding the ends of the pipette between the thumb and the index fingers. A cover slide was placed over the counting chamber and a drop of diluted epididymal content was spread between the haemocytometer chamber and the cover slide. The sperms in 5 large squares were counted using the high power objective lens (X400). The total number in five small squares was multiplied by millions.
(c) Epididymal sperm abnormalities:
A drop from the epididymal content of each rat was immediately taken and mixed with an equal drop of Eosin-Nigrosin stain for detection of dead and malformed sperm. The semen was carefully mixed with the stain, then films were examined at random per slide under x 200 and the sperm abnormalities were recorded.
Determinationof testosterone and FSH levels
Blood samples were taken from each rat on the 45th day to determine the testosterone and FSH levels. Serum levels of both were assayed using Rat ELISA kit (Cusabio®, China) according to the manufacturer procedure.
Biochemical measurements for oxidative stress biomarkers as compared to control rats
In fact, serum total pro-oxidant status was done according to Erel, 2004[19]. Testes contents of malondialdehyde (MDA) and total tissue nitrite/nitrate (NOx) content were estimated according to manufacturer’s prescripts using reagent kits (Biodiagnostic, Giza, Egypt).
Effect of the tested chemicals on rate of pregnancy, weights of fetuses and malformation in fetus
In the last week of treatment, male rats of all groups are allowed to mate with females to determine the pregnancy rate and the effect of the chemicals used on their fetuses.
Females of 11 - 13 weeks old were selected for the present study and vaginal smears were prepared every morning and examined under the light microscope (according to the method of Snell, (1956)[20]) for 5 days to select the female with regular estrus. Two females were selected and caged together with one male overnight under controlled environmental condition of temperature (25 ± 2°C), humidity (60 ± 20%) and light (12 lights-12 dark cycles). The zero day of gestation was determined by the presence of sperms in the vaginal smear[21]. Pregnant rats of all groups were kept under observation till the 20th day of gestation then pregnant rats were sacrificed under light ether anesthesia. All fetuses were examined for the occurrence of any malformation and weighed. Total implantation sites, fetal mortality rate (resorbed or still birth) and living fetuses were recorded.
Histopathological examination:
After fixation of testes in 10% formalin/saline for 24h, testes tissues were embedded in paraffin wax and cut at 5 μm thickness. Sections were processed, stained by Haematoxylin and Eosin stain for histopathological examination using the light microscope.
Statistical analysis
Data were expressed as means ± Standard Error of the Means (SEM). Comparisons between means were carried out using one-way ANOVA followed by Tuke–Kramer multiple comparisons test. Statistical analysis was performed using GraphPad Prism software (version 5); a probability level of less than 0.05 was accepted as statistically significant.
Results
The current study examined toxicological effects of the combination of CF and TAU which are already present in certain product as in Red Bull, as well as the effect of daily receiving of PCM as an over the counter treatment, on testis and fetuses in rats.
Effects of tested treatments on sperm count and motility as well as serum levels of testosterone and FSH
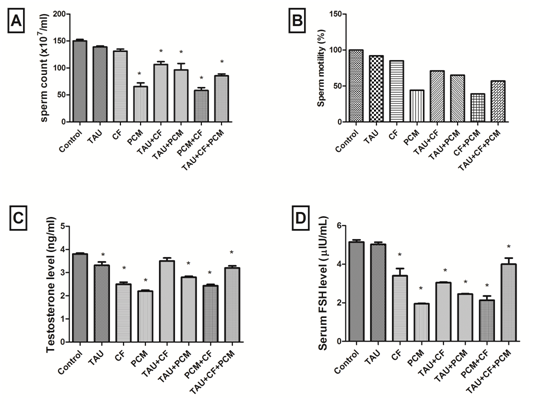
Noteworthy, administration of PCM caused a significant decrease in the sperm count alone (56%) or in combination with CF (61%) as compared to control group. Whereas, addition of TAU improved sperm counts where TAU+PMC declined sperm count by 36% and TAU+CF+PMC declined it by 43% as compared to control group [Figure 1A].
Figure (1): Effects of tested treatments on sperm count as compared with control rats (A). Effects of tested treatments on sperm motility percentage as compared with control rats (B). Effects of tested treatments on testosterone level as compared with control rats (C). Effects of tested treatments on FSH level as compared with control rats (D). The 1stgroup was kept as a normal control received 1ml / kg distilled water. The 2nd, 3rd and 4th groups received 250 mg / kg, 40 mg / kg, and 270 mg/kg; orally of TAU, CF and PCM, respectively. Additionally, the 5th, 6th, 7th and 8th groups received TAU + PCM, TAU + CF, CF + PCM and TAU + CF + PCM, respectively, for 45th consecutive days.
Each bar with vertical line represents the mean ± SEM of 6 - 8 rats per group. *vs. Control group, (one-way ANOVA followed by Tukey – Kramer multiple comparisons test; p < 0.05). Where, TAU; Taurine, CF; Caffeine, PCM; Paracetamol.
Moreover, administration of PCM caused a significant decrease in the sperm motility alone or in combination with CF as compared to control group. While, TAU enhanced sperm motility where TAU+PMC as well TAU+CF+PMC reduced sperm motility as compared to control group [Figure 1B].
Markedly, administration of PCM and CF caused a significant decrease in testosterone level alone by 42% and 34%, respectively, or in combination by 36% as compared to control group. Whereas, addition of TAU improved testosterone level where TAU+PMC declined testosterone level by 26% and TAU+CF+PMC declined it by 16% as compared to control group [Figure 1C].
Likewise, administration of PCM and CF caused a significant decrease in FSH level alone by 62% and 34%, respectively, or in combination by 59% as compared to control group. While, addition of TAU enhanced FSH level where TAU+PMC declined FSH level by 52% and TAU+CF+PMC declined it by 22% as compared to control group [Figure 1D].
Abnormalities of head and tail of sperm formation
In fact, using of Eosin and Nigrosin stain revealed photomicrograph of smear from seminal fluid of none treated rat in control group showing normal sperm formation with head, neck and tail. But photomicrograph from CF-treated group showed coiled tail sperm. Sperms in PCM-treated group demonstrated double head sperms. Further male rats received TAU + PCM showed kinked or coiled tail and round head. Moreover, coiled tail sperm; coiled neck and detached head in rats were given CF+PCM. Meanwhile photomicrograph of CF+TAU-treated group revealed double head and pairing phenomenon. In the last photomicrograph, we found that misshapen head with flattened head sperms in TAU+CF+PCM-treated group [Figure 2].
Figure (2): Effects of tested treatments on sperm formation in male rats showing that abnormalities of head, neck and tail of sperms. The 1st group was kept as a normal control received 1ml / kg distilled water. The 2nd, 3rd and 4th groups received 250 mg / kg, 40 mg / kg, and 270 mg / kg; orally of TAU, CF and PCM, respectively. Additionally, the 5th, 6th, 7th and 8th groups received TAU + PCM, TAU + CF, CF + PCM and TAU + CF + PCM, respectively, for 45th consecutive days.
In fact, using of Eosin and Nigrosin stain revealed photomicrograph of smear from seminal fluid of none treated rat in control group showing normal sperm formation with head, neck and tail. But photomicrograph from CF-treated group showed coiled tail sperm. While rat sperms in PCM-treated group demonstrated double head sperms. Further male rats received TAU + PCM showed kinked or coiled tail and round head. Moreover, coiled tail sperm; coiled neck and detached head in rats were given CF+PCM. Meanwhile photomicrograph of CF+TAU-treated group revealed double head and pairing phenomenon. In the last photomicrograph, we found that misshapen head with flattened head sperms in TAU + CF + PCM - treated group. Where, TAU; Taurine, CF; Caffeine, PCM; Paracetamol.
Effects of tested treatments on oxidative stress biomarkers
Obviously in table 1, administration of PCM and CF caused a significant increase in total pro-oxidant contents alone to 4 folds and 2 folds, respectively, or in combination to 3 folds as compared to control group. Whereas, addition of TAU improved total pro-oxidant contents where TAU+PMC declined it by 3 folds and TAU+CF+PMC declined it by 2 folds as compared to control group.
Similarly, administration of PCM caused a significant rising in MDA content 29%, or in combination with CF by 30% as compared to control group. In addition nitrite/nitrate (NOx) content increased significantly after administration of PCM by 20% as compared to control group.
Table 1: Effects of taurine, caffaiene, & /or paracetamol on oxidative stress biomarkers as compared to control group.
| Groups | Serum Total pro-oxidant (nmol/ml) | Testes Malondialdehyde (nmol/g tissue) | Testes Nitrite/Nitrate (μmol/g tissue) |
|---|---|---|---|
| Control | 6.22 ± 0.703 | 3.74 ± 0.128 | 228 ± 4.71 |
| TAU | 9.94 ± 0.200* | 3.67± 0.25 | 214 ± 3.61 |
| CF | 11.2 ± 0.500* | 4.26 ± 0.04 | 250 ± 12.9 |
| PCM | 26.6 ± 1.23* | 4.84 ± 0.255 * | 274 ± 17.4 * |
| TAU + CF | 12.1 ± 0.724* | 4.19 ± 0.048 | 261 ± 1.37 |
| TAU + PCM | 17.5 ± 0.281* | 4.11 ± 0.11 | 215 ± 1.34 |
| CF + PCM | 18.3 ± 0.185* | 4.85 ± 0.2* | 240 ± 7.88 |
| TAU + CF+PCM | 14.5 ± 0.400* | 4.43 ± 0.157 | 228 ± 10.9 |
Values are expressed as mean ± SEM of 6-8 rats per group. *vs. Control group, (one-way ANOVA followed by Tukey– Kramer multiple comparisons test; p < 0.05). Where, TAU; Taurine, CF; Caffeine, PCM; Paracetamol.
Effect on rate of pregnancy and weights of fetuses
In the present study, figure (3A) revealed that, PCM alone and in combination with CF diminished the rate of pregnancy markedly by 50% as compared to control group. TAU improved this percentage when combined with PCM as well as with CF and PCM to become 67% as compared to control group.
Moreover, figure (3B) illustrated that weight of fetuses decreased significantly in PCM-, CF- and CF+PCM- treated groups as compared to control group by 27%, 22%, and 30%, correspondingly. Additionally, we found that TAU improved the weight of fetuses in TAU+CF-, and TAU+PCM-treated groups to decline by 19%, and 18%, respectively, as compared to control group. At the same time, TAU didn’t enhance reduction in weight (29%) in TAU+CF+PCM-treated group as compared to control group.
Figure (3): Effects of tested treatments which were given to male rats on percentage of pregnancy in female rats as compared with control rats (A). Effects of tested treatments which were given to male rats on weight of fetuses as compared with control rats (B), the fetuses obtained from pregnant rats at 20th day of gestation. The 1st group was kept as a normal control received 1ml / kg distilled water. The 2nd, 3rd and 4th groups received 250 mg / kg, 40 mg / kg, and 270 mg/kg; orally of TAU, CF and PCM, respectively. Additionally, the 5th, 6th, 7th and 8th groups received TAU + PCM, TAU + CF, CF+PCM and TAU + CF + PCM, respectively, for 45th consecutive days.
Each bar with vertical line represents the mean ± SEM of 6 - 8 rats per group. *vs. Control group, (one-way ANOVA followed by Tukey– Kramer multiple comparisons test; p < 0.05). Where, TAU; Taurine, CF; Caffeine, PCM; Paracetamol.
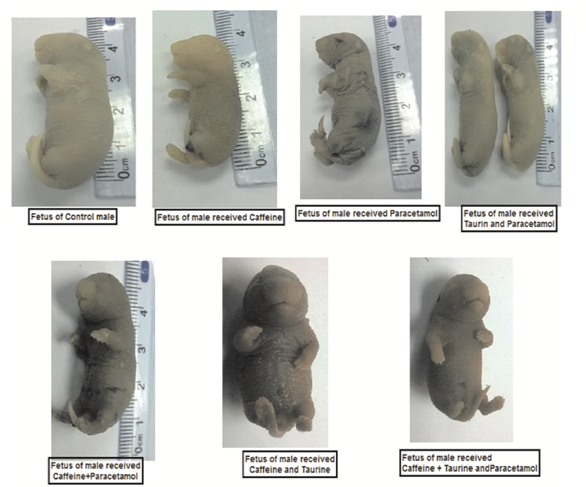
Malformation in fetus
Figure (4) showed that fetuses obtained from control pregnant rats at 20th day of gestation normal fetus showing normal growth. On the other hand, fetuses belonged to PCM demonstrated hematoma and wrinkled skin or contraction, CF revealed loss of foot due to severe hematoma, TAU+CF showed paralysis in forelimb as well as TAU+CF+PCM demonstrated paralysis in forelimb and hematoma on hind limb with contraction in all over the body.
Figure (4): Effects of tested treatments which were given to male rats on malformation of fetuses as compared with control rats, the fetuses obtained from pregnant rats at 20th day of gestation. The 1st group was kept as a normal control received 1ml / kg distilled water. The 2nd, 3rd and 4th groups received 250 mg / kg, 40 mg / kg, and 270 mg/kg; orally of TAU, CF and PCM, respectively. Additionally, the 5th, 6th, 7th and 8th groups received TAU + PCM, TAU + CF, CF + PCM and TAU+CF+PCM, respectively, for 45th consecutive days.
Photomicrographs showed that, fetuses belonged to PCM has hematoma and wrinkled skin or contraction, CF revealed loss of foot, TAU+CF showed paralysis in forelimb as well as TAU + CF + PCM demonstrated paralysis in forelimb and hematoma on hind limb with contraction in all over the body. Where,TAU; Taurine, CF; Caffeine, PCM; Paracetamol.
Histopathological examination of testes of male rats:
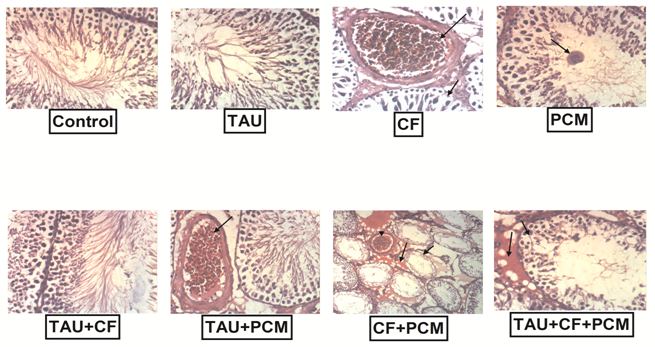
Histopathological findings of tissues were illustrated in [Figure 5]. In fact, the histopathological examination of sections of the control group showed a normal histological structure of seminiferous tubules with complete spermatogenesis and sperm production. Likewise, rats received TAU showed no histopathological changes. But CF administration showed degeneration of spermatogoneal cells lining seminiferous tubule and congestion of interstitial blood vessels. In addition administration of PCM to rats revealed degeneration of spermatogoneal cells lining seminiferous tubules with formation of spermatic giant cells. However testis of TAU+CF-treated group showed that no histopathological changes. Rats received TAU+PCM showed that congestion of interstitial blood vessel. Besides administration of PCM+CF showed marked degeneration of spermatogoneal cells lining seminiferous tubules, interstitial edema and congestion of interstitial blood vessel. On the other hand, administration of TAU+CF+PCM to rats revealed marked degeneration of spermatogoneal cells lining seminiferous tubules and interstitial edema.
Figure (5): Effects of tested treatments on the histopathological examination in rat testes as compared with control rats. The 1st group was kept as a normal control received 1 ml / kg distilled water. The 2nd, 3rd and 4th groups received 250 mg / kg, 40 mg / kg, and 270 mg/kg; orally of TAU, CF and PCM, respectively. Additionally, the 5th, 6th, 7th and 8thgroups received TAU+PCM, TAU + CF, CF+PCM and TAU + CF + PCM, respectively, for 45th consecutive days.
Photomicrographs showed that, the histopathological examination of sections of the control group showed a normal histological structure of seminiferous tubules with complete spermatogenesis and sperm production. Likewise, rats received TAU showed no histopathological changes. But CF administration showed degeneration of spermatogoneal cells lining seminiferous tubule and congestion of interstitial blood vessels. In addition administration of PCM to rats revealed degeneration of spermatogoneal cells lining seminiferous tubules with formation of spermatic giant cells. However testes of TAU + CF-treated group showed that no histopathological changes. Rats received TAU+PCM showed that congestion of interstitial blood vessel. Besides administration of PCM + CF showed marked degeneration of spermatogoneal cells lining seminiferous tubules, interstitial edema and congestion of interstitial blood vessel. On the other hand, administration of TAU + CF + PCM to rats revealed marked degeneration of spermatogoneal cells lining seminiferous tubules and interstitial edema. Whereas; Taurine, CF; Caffeine, PCM; Paracetamol.
Discussion
In the current study, evaluation of the effects of CF, TAU and /or PCM on male fertility in rat and the development of their fetuses was estimated. Actually, the andrological results showed that PCM induced remarkable reduction in sperm count and motility, markers of testicular function, alone or in CF+PCM-treated group and TAU+CF+PCM-treated group. As well as degeneration in testicular tissue showed in histopathological examination. This suggests that PCM was able to permeate the blood-testis barrier with resultant alteration in the microenvironment of the seminiferous tubules, and thus, creating a different microenvironment in the inner part of the wall of the seminiferous tubules from that in the outer part according suggestion of[22] who studied PCM at dose 7.5 mg/kg in rats for 42 days, so even low dose of PCM with long duration may be capable to penetrate the barrier. Related information was given by[23]. So it is preferable that continuing administration of PCM daily for a long period of time should not occur.
Noteworthy, abnormalities of head and tail of sperm formation, which were recorded, are appeared clearly. Generally, impairment to the sperm cell by substances could happen by one of three mechanisms: physiological, cytotoxic and genetic. The morphological abnormalities might have been caused by alterations in testicular DNA that in turn disrupts the process of differentiation of spermatozoa as well exposure to chemicals that could produce pituitary-hypothalamic or sex hormonal effects which in turn could affect spermatogenesis[24,25]. Similar report was given by[26].
Moreover, the percentage of pregnancy was diminished significantly in PCM and CF +PCM treated groups.The ant- reproductive effect induced by PCM, alone or in combination with CF may be due to an increase in pre-implantation losses or from oligospermia (low concentration of sperms in the seminal fluid), azoospermia, impairments sperm motility, and reduction in the fertilizing potential of spermatozoa[27]. However, TAU didn’t affect the percentage of pregnancy as compared with control group.
What is more, fetus malformation was intense after administration of PCM alone and it was at a lower extent in CF-treated group. We recommend the proposed mechanisms by which CF might encourage this malformation, which may involve down-regulation of adenosine A2 receptors, leading to reduce cerebral blood flow in premature infants. But both treatments in combination with TAU revealed notable improvement. These results were in harmony with[28].
Additionally, PCM and/or CF increased testicular oxidative stress, which was revealed by augment of MDA content in all PCM treated groups along with increase in NOx content. These effects might be attributed to the activation of the enzyme, xanthine oxidase, which catalyzes the oxidation of xanthenes to uric acid, which generates superoxides, hydrogen peroxides, and free radicals[29]. Besides, albeit high doses of PCM instituted a conjugated bond with glucuronic acid or sulphate, a major portion is metabolized by the cytochrome P450 system. This probably leads to the production of reactive toxic metabolites such as N– acetyl-p-benzoquinone imine (NAPQI) interacting with sulfhydryl groups in the glutathione molecule. Consequently, PCM induced a depletion of cellular GSH stores. Binding cellular proteins, the remaining portion of NAPQI begins lipid peroxidation and ultimately induces damage[30].
In fact, the presence of TAU with PCM or/and CF reduced the toxicity to a large extent, while the presence of PCM almost increases the toxicity. Our study has provided some data and information that may be useful for public health. There is the need for regular public health checks on the consumption of some socially acceptable analgesics compounds such as PCM or/and CF. Furthermore, the presence of PCM and CF at the same time of administration had a synergistic effect that significantly increased harmness of sexual health state.
Acknowledgment: The authors are grateful to Dr. Kawkab Abdel-Aziz, Professor of Pathology, Faculty of Veterinary Medicine, Cairo University, for her helpful guide in histopathological examination.
Disclosure: The authors declare no conflicts of interest.
References
1. Ohta, A., Lukashev, D., Jackson, E. K., et al. 1,3,7-Trimethylxanthine (Caffeine) May Exacerbate Acute Inflammatory Liver Injury by Weakening the Physiological Immunosuppressive Mechanism. [2007] J Immunol 179(11):7431-7438.
Pubmed||Crossref||Others
2. Ross, G.W., Abbott,R.D., Petrovitch,H., et al. Association of coffee and caffeine intake with the risk of Parkinson disease. [2000] JAMA; 283 (20):2674 –2679.
3. Benedetti, M.D., Bower, J.H., Maraganore, D.M., et al. Smoking, alcohol, and coffee consumption preceding Parkinson’s disease: a case-control study. [2000] Neurology 55(9):1350 –1357.
4. Fredholm, B.B., Battig, K., Holmen, J., et al. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. [1999] Pharmacol Rev 51(1): 83-133.
5. Fouka, L.C., Daniele, N.C., Ktori, K.E., et al. Direct effects of caffeine and theophylline on p110 ? and other phosphoinositide 3-kinases: differential effects on lipid kinase and protein kinase activities. [2002] J Biol Chem 277(40): 37124 – 37130.
Pubmed||Crossref||Others
6. Harris, R,Wen, S. Review: Taurine: A “very essential” amino acid. Molecular Vision. [2012] 18:2673-2686.
7. Niittynen, L., Nurminen, M.L., Korpela,R., et al. Role of arginine, taurine and homocysteine in cardiovascular diseases.[1999] Ann Med 31(5): 318-326.
8. Abbasoglul, S., Kanbagli, O., Balkan, J., et al. The protective effect of taurine against thioacetamide hepatotoxicity of rats.[2001] Human & Experi Toxico 20(1):23-27.
9. Bhavsar, T.M., Patel, S.N., Lau-Cam, C.A. Protective action of taurine, given as a pre-treatment or as a post treatment, against endotoxin-induced acute lung inflammation in hamsters. [2010] J Biomed Sci 17: 1-19.
10. Milenkovi?, D., ?orovi?, J., Jeremi?, S., et al. Free radical scavenging potency of dihydroxybenzoic acids. [2017] Journal of Chemistry: 1-9.
11. Gunnell, D., Hawton, K., Murray, V., et al. Use of Paracetamol for suicide and non-fatal poisoning in the UK and France: are restrictions on availability justified? [1997] J Epidemiol Community Health 51(2):175-179.
12. Sheen, C.L., Dillon, J.F., Bateman, D.N., et al. Paracetamol toxicity: epidemiology, prevention and costs to the health–care system. [2002] QJM 95(9):609-619.
13. Smith, D.G., Aronson, J. Oxford Textbook of clinical pharmacology and drug therapy. (3rdEd). [2002] Oxford University press, New York.
Pubmed||Crossref||Others
14. Mahmood, N.D., Mamat, S.S., Kamisan, F.H., et al. Amelioration of paracetamol-induced hepatotoxicity in rat by the administration of methanol extract of Muntingia calabura L. leaves. [2014] BioMed research international: 1-10.
15. Gibson JD, Pumford NR, Samokyszyn VM, Hinson JA. Chem Res Toxicol 1996; 9:580-585.
Pubmed||Crossref||Others
16. Wassef, B., Kohansieh, M., Makaryus, A.N. Effects of energy drinks on the cardiovascular system. [2017] World J Cardiol 9(11): 796-806.
17. Zulli,A., Smith,R.M., Kubatka, P., et al. Caffeine and cardiovascular diseases: critical review of current research. [2016] Eur J Nutr 55(4):1331–1343.
18. Bearden,H.J., Fuquay, J.W. Applied animal reproduction. 1980.
Pubmed||Crossref||Others
19. Erel, O. A new automated colorimetric method for measuring total oxidant status. [2005] Clin Biochem 38(12):1103–1111.
20. Snell, G.D. Biology of the Laboratory Mouse. New York: Blakiston Co. 1956.
21. McClain, R.M., Becker, B.A. Teratogenicity, fetal toxicity, and placental transfer of lead nitrate in rats. [1975] Toxicology and applied pharmacology 31(1): 72-82.
22. Oyedeji, K.O., Bolarinwa, A.F., Ojeniran, S.S. Effect of paracetamol (acetaminophen) on haematological and reproductive parameters in male albino rats. [2013] Research Journal of pharmacology 7(2): 21-25.
23. Verma, P.K., Sharma, A., Annu, M., et al. Effect of Sarcostemmaacidum stem extract on spermatogenesis in male albino rats. [2002] AsianJ Androl4(1): 43 – 47.
24. Bowman WC, Rand MJ. The reproductive system and drugs affecting the reproductive systems. [1985] Textbook of pharmacology, 2nd edition, 20:1-8.
Pubmed||Crossref||Others
25. Ekaluo, U.B., Ikpeme, E.V., Udokpoh, A.E., Sperm head abnormality and mutagenic effects of aspirin, paracetamol and caffeine containing analgesics in rats. [2009] The Internet Journal of Toxicology 7(1): 1-6.
Pubmed||Crossref||Others
26. Krishnamoorthy, P., Viathinathan, S., Rani, V., et al. Effect of Terminalia chebula fruit extract on lipid peroxidation and antioxidative system of testis of albino rats. [2007] African Journal of Biotechnology 6 (16): 1888–1891.
27. Ratnasooriya, W.D., Jayakody, J.R., Long-term administration of large doses of paracetamol impairs the reproductive competence of male rats. [2000] Asian J androl 2(4): 247-255.
28. Burdan,F., Siezieniewska, Z., Ki?, G., et al. Embryofetotoxicity of acetaminophen (paracetamol) in experimental in vivo model. [2001] Ann Univ Mariae Curie Sklodowska 56:89-94.
Pubmed||Crossref||Others
29. Obochi, G.O., Amali, O.O., Ochalefu, D.O. Effect of melatonin and caffeine interaction on caffeine induced oxidative stress and sleep disorders. [2010] Niger J Physiol Sci 25(1): 17-24.
Pubmed||Crossref||Others
30. Kandemir, F.M., Kucukler, S., Eldutar, E., et al. Chrysin protects rat kidney from paracetamol-induced oxidative stress, inflammation, apoptosis, and autophagy: A multi-biomarker approach. [2017] Sci pharm 85(1): 4.