Congenital Transmission of Erythema Migrans or Lyme Disease, Myth or Reality?
Lapenta, JM
Affiliation
1Lapenta, J. Medic Surgeon, Specialty Dermatology, 24 years of exercise. University of Carabobo, Venezuela. Ceo Dermagic Express
2 Lapenta, J.M. Medic Surgeon. University of Carabobo. Diplomat in Facial Aesthetics Occupational Medicine and Prehospital Auxiliary. Resident Doctor Ambulatorio Del Norte Maracay Aragua State. Coo Dermagic Express.
Corresponding Author
Lapenta J, Medic Surgeon, Specialty Dermatology, 24 years of exercise. University of Carabobo, Venezuela, Email: www.dermagicexpress.blogspot.com
Citation
Lapenta, J., et al. Congenital Transmission of Erythema Migrans Or Lyme Disease, Myth Or Reality? (2018) Invest Demerol and Venereol Res 4(1): 16- 21.
Copy rights
© 2018 Lapenta, J. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Lyme disease; Borrelia burgdorferi; congenital lyme; fetal damage; pregnancy
Abstract
Lyme disease or Erythema Migrans, described many years ago, and previously known under the name of Lyme Juvenile Arthritis, is produced by a spirochete transmitted by the bite of a family tick. Ixodidade, Ixodes scapularis and many others; discovered by the scientist Willy Burgdorfer in the year of 1981, being named Borrelia Burgorferi, in honor of its discoverer. Apart from the numerous cutaneous and organic manifestations attributed to Borrelia, it is nowadays discussed in the scientific field if it is capable of cross the placenta and cause fetal damage. In this review we will show you that this biological agent, as in syphilis, produced by Treponema Pallidum, another spirochete, crosses the placenta and can produce serious consequences to the fetus, including death.
Introduction
Main objective
The main objective of this work is to demonstrate that Lyme disease and their biological agent, spirochete Borrelia Burgdorferi is not only an illness with cutaneous manifestations. It can cross the placenta during pregnancy and produce fetal damage that in severe cases can cause death in newborns.
Secondary objectives
• Describe the clinical manifestations in children born from positive Lyme mothers who did not receive treatment in pregnancy, or in those who received treatment with resistance to it.
• Alert the World community that there is indeed transplacental transmission of Borrelia Burgdorferi in pregnant Lyme positive women and if there is not adequate treated in time, both the mother and fetus can present clinical symptoms ranging from mild, to severe, even stillbirth.
• To call attention to the World Health Organization so that in the revision of the international codes of diseases (ICD-11), this year 2018, the code “Congenital Lyme” be included in them.
Introduction
The Center for Disease Control and Prevention (CDC) affirms in its website that the pregnant woman Lyme positive when making her treatment, the child will be born healthy and recommends for this, the use of the antibiotic amoxicillin or cefuroxime, because doxycycline, which is the antibiotic of choice, can cause damage to the developing fetus[1].
Other antibiotics recommended by the CDC are the macrolides azithromycin, clarithromycin or erythromycin in case of allergy or intolerance to those previously mentioned[2].
The CDC itself recognizes that Lyme disease and its causative agent Borrelia Burgdorferi can cross the placenta and cause stillbirths[1,2].
The question here is what would happen if the Borrelia species, as in some cases, is resistant to amoxicillin or another antibiotic, or the antibiotic to which Borrelia is sensitive cannot be indicated because it would harm the fetus? And beyond, if the patient does not receive the treatment by omission or carelessness?[3].
As we said previously the Borrelia Burgdorferi was discovered by Willy Burgdorfer in the year 1981, and just two (2) year later, in 1983 the first study was published by Shirts SR, and Brown MS, Bobitt Jr, where it is suspected that this spirochete can cross the placenta[4].
After this study began to appear others, who definitely showed that this spirochete is able to cross the placenta and cause fetal damage, of which we will present in chronological order the most important and confirm the above said.
Chronological Evolution
1983: The first suspicion described that ante partum fever may be caused by the Borrelia Burgdorferi species, was made in 1983 in two febrile pregnant women in the third-trimester. The two newborn survived, but the scientists suggested the establishment of early laboratory tests to identify the causative agent and the establishment of a rapid treatment to avoid future complications in the pregnant and the fetus[4].
1985: Really, the first study describing the maternal-fetal transmission of Lyme disease, Borrelia Burgdorferi was published in 1985 by Schlesinger PA, Duray PH, Burke BA, Steere AC, and Stillman MT., Where they describe a case of a pregnant woman who acquired Lyme Borreliosis and did not receive treatment with antibiotics. The child was born at 35 weeks of pregnancy and died of congenital heart disease the first week of life. The autopsy revealed the Borrelia Burgdorferi spirochete in the spleen, kidneys and bone marrow[5].
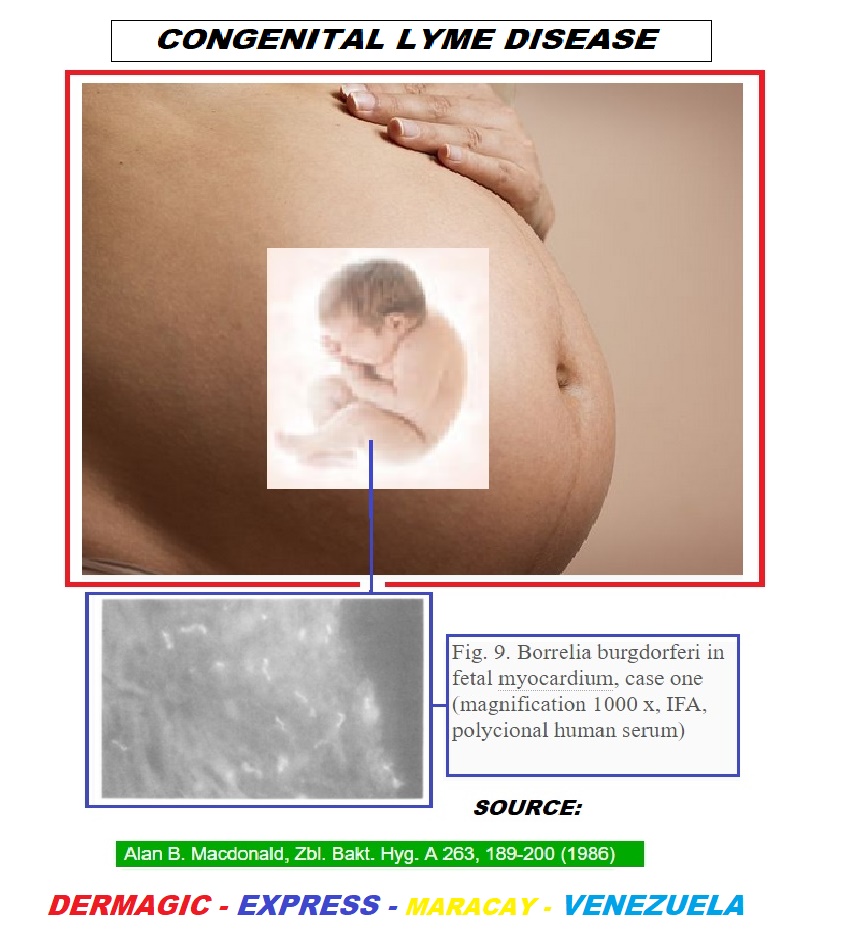
1986-1989: In the year 1986, MacDonald A.B, describes 4 cases of abortions in pregnant women who tested positive for Lyme, and in whose fetuses the Borrelia Burgdorferi was found in their tissues. This same author does another work in 1989 about Lyme disease and its implications for the fetus and describes side effects such as fetal death, hydrocephalus, cardiovascular abnormalities, neonatal respiratory distress, hyperbilirubinemia, intrauterine growth retardation, cortical blindness, sudden death syndrome of the infant and maternal toxemia of pregnancy, and raises the similarity of these with neonatal syphilis[6,7].
1987: Later, the same Willy Burgdorfer the discoverer of the Borrelia Burgdorferi, together with Dr. Alan Mc Donald and Jorge Benach PhD, published in year 1987 (31 years ago) a work where they relate this disease in children born dead associated in pregnant Lyme positive.
Among the highlights are description of congenital malformations, fetal death, and cardiac anomalies; also alert the scientific community to investigate exposure during the first trimester of pregnancy in the presence of Borrelia Burgdorferi; and in these cases to determine if cardiac organogenesis is complete by the end of the first trimester of pregnancy. They also recommend starting treatment with Penicillin at the same dose of syphilis in those cases of pregnant women who show signs and early symptoms of the disease[8,9].
1988-2012: In these 24 years a total of more than 80 papers were published where Lyme disease is effectively related to pregnancy with fetal damage, studies done in the countries: United States, Canada, Hungary, Germany, Italy, Switzerland, Africa, Turkey, Czech Republic, Poland and Belgrade former Yugoslavia, and others[10-52].
Another study that is worth noting is that carried out by the updated MEDLINE database for the year of July 2012, the last revision of November 2012 of 88 journal articles from the PUBMED database, which we summarize in this way.
Maternal-Fetal transmission of lyme disease (Results):
Mothers with active Lyme disease, treated: 14.6% of pregnancies resulted in sequel.
Mothers with active Lyme disease not treated: 66.7% of pregnancies resulted in sequel.
Positive Lyme mothers, the treatment is unknown: 30.3% resulted in sequel.
Specific adverse results included:
Cardiac 22.7%,
Neurological 15.2%,
Orthopedic 12.1%,
Ophthalmologic 4.5%,
Genitourinary 10.6%,
Miscellaneous anomalies 12.1%[53].
Now we will put a summary of the most frequent clinical manifestations described in a study of more than 100 children born to mothers with LYME positive disease, conducted in the year 2005.
Common signs and symptoms in lyme positive children: Low grade fever: 59% -60%; Fatigue and lack of resistance: 72%; Nocturnal sweating: 23%; Pale, dark circle under the eyes: 42%; Abdominal pain: 20-29%; Diarrhea or constipation: 32%; Nausea: 23%; Cardiac anomalies: 23%: palpitations / PVC, heart murmur, mitral valve prolapse. ; Orthopedic disorders: sensitivity (55%); pain (69%) spasms and generalized muscle pain (69%); rigidity and / or retarded motion (23%); Respiratory infections of the superior tract and otitis: 40%; Arthritic disorders and painful joints: 6% -50-%; Neurological disorders: Headaches: 50%, Irritability: 54%, Bad memory: 39%; Delay in development: 18%; Seizure disorder: 11%; Vertigo: 30%; Tic disorders: 14%; Involuntary athetoid movements: 9%.; Earning disorders and humor changes: 80%: Cognitive speaking: 27%, Speech delay: 21%, Reading-writing problems: 19%, Problems of vocal articulation: 17%, Auditory / visual processing problems: 13%, Word selection problems: 12%, Dyslexia: 8%; Suicidal thoughts: 7%; Anxiety: 21%; Anger or rage: 23%; Aggression or; violence: 13%; Irritability: 54% - 80%; Emotional disorders: 13%; Depression: 13%; Hyperactivity: 36%; Photophobia: 40-43%; Gastro esophageal reflux with vomiting and coughing: 40%; Secondary eruptions: 23%; other eruptions: 45%; Cavernous haemangioma: 30%; Ocular problems: posterior cataracts, myopia, stigmatism, conjunctive erythema (Lyme eyes), optical nerve atrophy and / or uveitis: 30%; Sensitivity of skin and noise (hyperacuity): 36 - 40%; Autism: (9%)[41].
2013-2018: In recent years the number of cases of Lyme disease has increased notably in North America, Europe, Asia and Africa; in the United States and Canada, it is the most commonly transmitted vector-borne disease reported today[54-62].
For the year 2017 the World Health Organization (WHO) in charge of “coding” diseases through the ICD-10 (International Classification of Diseases year 2017), had not yet included and recognized the code “Congenital Lyme”. It is expected for this year 2018 that all codes of Lyme disease will be recognized by this organization[63-68].
You can read this classification and codes here:
Understanding Lyme disease classification and codes[69].
We must remember that Borrelia has been studied phylogenetically and numerous variations of it have been described, finding 21 genospecies of Borrelia Burgdorferi according to human sensitivity.
10 species of them with pathogenicity known to humans whose group includes:
B. afzelii, B. bavariensis, B. bissettii, B. burgdorferi sensu stricto, B. garinii, B. kurtenbachii, B. lusitaniae, B. mayonii, B. spielmanii and B. valaisiana.
11 identified species that have not yet been detected or isolated from humans. This group includes B. americana, B. andersonii, B. californiensis, B. carolinensis, B. chilensis, B. finlandensis, B. japonica, B. tanukii, B. turdi, B. sinica and B. yangtze.
Borrelia Burgorferi circulates predominantly in the United States, and Borrelia Afzelli and Borrelia garinii in Europe and Asia.
Regardless of the genospecies involved with pathogenicity demonstrated in humans, all the studies presented show that Borrelia, being a spirochete like the Treponema Pallidum causal agent of syphilis, is able to cross the placenta and conquer the fetus, if the positive Lyme pregnant woman is not treated in time[70-75].
Endosymbiosis
A new aspect to consider in the transmission of bacteria to its hosts is the term Endosymbiosis, wich comes from the Greek endon “within”, syn “together”, and biosis “living”. Any organism that lives inside the body or cells of another organism in a symbiotic relationship with the host’s body or cell is defined as an endosymbiont organism, often, but not always, for the benefit of both.
This event has been demonstrated in the year 2018 in the female Ixodes Ricinus tick which present the Gram-Negative bacteria Mitochondrium Midichloria present in the mitochondria in various ovarian cells and they have a high rate of seroprevalence in individuals exposed to ticks. This fact raises the probability that non-pathogenic endosymbionts can play an important role in the immune response and transmission of the spirochete Borrrelia by ticks.
This fact would explain why some species of ticks have greater power of infestation than others, the presence of endosymbiont organisms in their cells that predispose them to be more pathogenic than others, more likely to reach the fetus in some instances.
Conclusion
• Lyme disease is caused by the bite of a tick transmitted by the Borrelia Burgdorferi spirochete, discovered by Willy Burgdorfer in the year 1981. Initially, skin, joint and cardiac manifestations were described in those affected, but not in pregnant women or the fetus.
• Two years later, in 1983, was described the suspicion of infection in pregnant women, and in 1985 it was found the first clinical manifestations in pregnant women and fetuses, highlighting congenital malformations and fetal death.
• We demonstrate scientifically that definitely Lyme disease, in addition to its multiple organic manifestations, its causative agent Borrelia Burgdorferi, crosses the placenta and reaches the fetus in pregnant women, producing the side effects already described, regardless of the species involved, with proven pathogenicity in humans.
• All pregnant women living in endemic areas of Lyme disease should take the tests to rule out Borreliosis of pregnancy, to establish immediate treatment in case of being positive.
In addition to establishing adequate treatment, we alert the population to defend against the bite of possibly infected ticks.
• We urge the World Health Organization to recognize all the codes and classification of Lyme disease in ICD-11 (International Classification of Diseases year 2018).
Acknowledgments:
To the community of patients affected by Lyme disease.
To the organizations that fight for the recognition of this pathology as a public health problem World.
To the Ommega team who has suggested specific and interesting aspects in the present investigation.
References
- 1. Center for Disease Control and Prevention. (2018) Lyme diseae. (FAQ)
Pubmed||Crossref||Others
- 2. Center for Disease Control and Prevention. (2017) Lyme diseaeTreatment.
Pubmed||Crossref||Others
- 3. Weber, K., Bratzke, H.J., Neubert, U., et al. Borrelia burgdorferi in a newborn despite oral penicillin for Lyme borreliosis during pregnancy. (1988) Pediatr Infect Dis J 7(4): 286-289.
- 4. Shirts, S.R., Brown, M.S., Bobitt, J.R. Listeriosis and borreliosis as causes of antepartum fever. (1983) Obstet Gynecol 62(2): 256-261.
- 5. Schlesinger, P.A., Duray, P.H., Burke, B.A., et al. Maternal fetal transmission of the Lyme disease spirochete, Borrelia burgdorferi. (1985) Ann Intern Med 103(1): 67-68.
Pubmed||Crossref||Others
- 6. MacDonald, A.B. Human fetal borreliosis, toxemia of pregnancy, and fetal death. (1986) Zentr Bakteriol Mikrobiol 263(1-2): 189-200.
- 7. MacDonald, A.B. Gestational Lyme borreliosis. Implications for the fetus (1989). Rheum Dis Clin North Am 15(4): 657-677.
- 8. MacDonald, A.B., Benach, J.L., Burgdorfer, W. Stillbirth following maternal Lyme disease. (1987) N Y State J Med 87(11): 615-616.
Pubmed||Crossref||Others
- 9. Carlomagno, G., Luksa, V., Candussi, G., et al. Lyme Borrelia positive serology associated with spontaneous abortion in an endemic Italian area. (1988) Acta Eur Fertil 19(5): 279-281.
- 10. Medici, F., Benach, J., Williams, C., et al. Lyme disease during Pregnancy A Cord Blood Serosurvey. (2006) Annals New York Aca Sci 539(1): 504-506.
Pubmed||Crossref||Others
- 11. Health and Welfare Canada. (1988) Canada Dis Weekly Report
Pubmed||Crossref||Others
- 12. Lyme disease in Canada. Epidemiologic Report. (1988) CMAJ 139.
Pubmed||Crossref||Others
- 13. Halperin, J.J., Dattwyler, R.J., David, J.V., et al. A Perspective on the treatment of Lyme Borreliosis. (1989) Rev inf dis 11(6): 1518-1525.
Pubmed||Crossref||Others
- 14. Nadal, D., Hunziker, U.A., Bucher, H.U., et al. Infants born to mothers with antibodies against Borrelia burgdorferi at delivery. (1989) Eur J Pediatr 148(5): 426-427.
- 15. Steere. Lyme Seropositivity and pregnancy outcome in the absence of symptoms of Lyme disease. (1989) Annual Meeting of American Col Rheu.
Pubmed||Crossref||Others
- 16. Lakos, A. Lyme Borreliosis in Hungary in the years 1984 through 1989. (1991) Parasit hung 24: 5-51.
Pubmed||Crossref||Others
- 17. ACOG Committee Opinion. Lyme disease during pregnancy. (1992) Int J Gynecol Obstet 39(1): 59-60.
Pubmed||Crossref||Others
- 18. Bracero, L.A., Wormser, G.P., Leikin, E., et al. Prevalence of seropositivity to the Lyme disease spirochetes during pregnancy in an epidemic area: A preliminary report. (1992) J Matern Fetal Investig 2(2): 265-268.
Pubmed||Crossref||Others
- 19. Hercogova, J., Tomankova, M., Frosslova, D., et al. Early-stage lyme borreliosis during pregnancy: treatment in 15 women with erythema migrans. (1993) Cesk Gynekol 58(5): 229-232.
- 20. Strobino, B.A., Williams, C.L., Abid, S., et al. Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. (1993) Am J Obstet Gynecol 169(2 Pt 1) 367-374.
- 21. Gasser, R., A Most Unusual case of a whole family suffering from late Lyme Borreliosis for Over 20 years. Angiology 45(1): 85-86.
Pubmed||Crossref||Others
- 22. Trevisan, G., Cinco, M. Lyme Borreliosis; A general survey. (1990) Int J Dermatol 29(1): 1-8.
Pubmed||Crossref||Others
- 23. Elsukova, L.V., Korenberg, E.I., Kozin, G.A., [Pathology of pregnancy and the fetus in Lyme disease. (1994) Med Parazitol 4: 59-62.
Pubmed||Crossref||Others
- 24. Gardner, T. Infectious Diseases of the Fetus and Newborn, 4th edition, New York, NY. W.B. Saunders Company. (1995) Lyme disease 447-528.
Pubmed||Crossref||Others
- 25. Williams, C.L., Strobino, B., Weinstein, A., et al. Maternal Lyme disease and congenital malformations: a cord blood serosurvey in endemic and control areas. (1995) Paediatr Perinat Epidemiol 9(3): 320-330.
- 26. Schmidt, B., Aberer, E., Klade, H., et al. Detection of Borrelia burgdorferi DNA by Polymerase Chain Reaction in the Urine and Breast Milk of Patients with Lyme Borreliosis. (1995) Diagn Microbiol Infect Dis 21(3): 121-128.
- 27. Alexander, J. M., Cox, S. Lyme disease and Pregnancy. (1995) Inf Dis Obstet Gynecol 3(6): 256-261
- 28. Figueroa, R., Bracero, L.A., Aguero-Rosenfeld, M., et al. Confirmation of Borrelia burgdorferi Spirochetes by Polymerase Chain Reaction in Placentas of Women with Reactive Serology for Lyme Antibodies. (1996) GynecolObstet Invest 41(4): 240-243.
- 29. Maraspin, V., Cimperman, J., Lotric-Furlan, S., et al. Treatment of Erythema Migrans during Pregnancy. (1996) Clin Infect Dis 22(5): 788-793.
Pubmed||Cross0072ef||Others
- 30. Mhalu, F.S., Matre, R., Serological evidence of Lyme borreliosis in Africa: results from studies in Dar es Salaam, Tanzania. (1996) East Afr Med J 73(9): 583-585.
- 31. Silver, H.M. Lyme Disease During Pregnancy. (1997) Inf Dis Clinics of North Am 11(1): 93-97.
Pubmed||Crossref||Others
- 32. Trevisan, G., Stinco, G., Cinco, M. Neonatal skin lesions due to a spirochetal infection; a case of congenital lyme borreliosis? (1997) Int J Dermatol 36(9): 677- 680.
- 33. Norris, C., Gardner, T.D., Danis, P.G. Aseptic Meningitis in the Newborn and Young Infant. (1999) Am Fam Physician 59(10): 2761-2770.
Pubmed||Crossref||Others
- 34. Elliot, D., Eppes, S., Klein, J. Terratogen Update; Lyme disease. (2001) Teratology 64(5): 276-281.
- 35. Gardner, T. Remington and Klein: Infectious diseases of the Fetus and Newborn, Fifth edition. New York, NY. W.B. Saunders Company (2001) Lymph dis 519-641.
Pubmed||Crossref||Others
- 36. Gardner, T. Lyme disease in pregnancy. Program and abstracts of the 14th International Scientific Conference on Lyme disease and Other Tick-Borne Disorders (2001) Hart Connecticut.
Pubmed||Crossref||Others
- 37. Goldenberg, R. L., Thompson, CThe infectious origins of stillbirth. (2003) Am J Obstet Gynecol 189(3): 861-873.
- 38. Harvey, W.T., Salvato, P. Lyme disease: ancient engine of an unrecognized borreliosis pandemic? (2003) Med Hypotheses 60(5): 742-759.
- 39. Onk, G., Acun, C., Kalayci, M., et al. Gestational Lyme disease as a rare cause of congenital hydrocephalus. (2005) J Turkish German Gynecol 6(2): 156-157.
Pubmed||Crossref||Others
- 40. Jones, C.R., Smith, H., Gibb, E. et al. Gestational Lyme Disease: Case Studies of 102 Live Births. (2005) Lyme Times. Gestational Lyme Studies 36-38.
Pubmed||Crossref||Others
- 41. Goldenberg, R.L., Culhane, J.F., Johnson, D.C. Maternal Infection and Adverse Fetal and Neonatal Outcomes (2005) Clin Perinatol 32(3): 523-559.
Pubmed||Crossref||Others
- 42. Walsh, C.A., Mayer, E.W., Baxi, L.V. Lyme disease in pregnancy. (2007) Obstet Gynecol Surv 62(1): 41-50.
- 43. Bransfield, R.C., Wulfman, J.S., Harvey, W.T., et al. The association between tick-borne infection, lyme borreliosis and autism spectrum disorder. (2008) Med hypotheses 70(5): 967-974.
- 44. Hercogova, J., Vanousova, D. Syphilis and borreliosis during pregnancy. (2008) Dermatol Ther 21(3): 205-209.
- 45. Theiler, R.N., Rasmussen, S., Treadwell, T.A., et al. Emerging and Zoonotic infections in women. (2008) Infect Dis Clin North Am 22(4): 755-772.
- 46. Lakos, A., Solymosi, N. Maternal Lyme borreliosis and pregnancy outcome. (2010) International Journal of Infectious Diseases 14(6): 494-498.
- 47. Hulinska, D., Votypka, J., Vanousova, D., et al. Identification of Anaplasma phagocytophilum and Borrelia Burgdorferi sensu lato in Patients with Erythema Migrans. (2009) Folia Microbiol 54(3): 246-256.
- 48. Mylonas, I. Borreliosis during Pregnancy: A risk for the Unborn Child? (2011) Vector Borne Zoonotic Dis 11(7): 891-898.
- 49. Sliwa, Leopold. Teratogenic effects of the bacteria Borrelia sp. on the fetuses of pregnant women with Lyme disease. (2011) Nowa Medycyna.
Pubmed||Crossref||Others
- 50. Relic, M., Goran, R., Lyme borreliosis and pregnancy. (2012) Vojnosanit Pregl 69(11): 994-998.
Pubmed||Crossref||Others
- 51. MEDLINE results for: borrelia pregnancy AND human. 88 journal articles in the PubMed database BDH, July (2012) Latest Revision.
Pubmed||Crossref||Others
- 52. Onyett, H., Lyme disease in Canada: Focus on Children. (2014) Paediatr Child Health 19 (7): 379-383.
- 53. O’Brien, J.M., Martens, M.G. Lyme disease in pregnancy; a New Jersey medical advisory. (2014) MD advisory 24-27.
Pubmed||Crossref||Others
- 54. Jasik, K. P., Okła, H., Słodki, J., et al. Congenital tick bore Diseases: Is this an alternative route of transmission of tick borne pathogens in Mammals? (2015) Vector Borne Zoonotic Dis 15(11): 637-644.
- 55. Hu, L.T., Tsibris, A.M., Branda, J.A. Case Records of the Massachusetts General Hospital: Case 24-2015; A 28 year-old pregnant woman with fever, chills, headache and fatigue. (2015) N Engl J Med 373(5): 468 -475.
- 56. Maldonato, Y, Nizet, V, Klein, J, Remington, J, Wilson, C. Current concepts of Infections of the Fetus and Newborn Infant. Chapter 1. page 6. Infectious Diseases of the Fetus and Newborn Infant. 8th Edition. 2016
Pubmed||Crossref||Others
- 57. O’Brien, J.M., Baum, J. D. Updated and printed by JC on November (2017) 66(8) : 9-10.
Pubmed||Crossref||Others
- 58. Lyme disease and Pregnancy. March of Dimes.
Pubmed||Crossref||Others
- 59. Eddens, T., Kaplan, D.J., Andrew, J. N., et al. Insights from the Geographic Spread of the Lyme Disease Epidemic. (2018) Clin Infect Dis 16.
- 60. Pajoro, M., Pistone, D., Varotto, B.I., et al. Molecular screening for bacterial pathogens in ticks (Ixodes ricinus) collected on migratory birds captured in northern Italy. (2018) Folia Parasitol (Praha) 15: 65.
- 61. Data and Statistics. Cent Dis Contr USA. Lyme disease.
Pubmed||Crossref||Others
- 62. ICD9/ICD9CM codes.
Pubmed||Crossref||Others
- 63. ICD-10 Version 2016.
Pubmed||Crossref||Others
- 64. New ICD-10-CM Codes in 2018.
Pubmed||Crossref||Others
- 65. Information from World Health Organization (WHO): List of Official ICD-10 Updates. For the ICD-11 revision: The ICD 11th Revision is due by 2018.
Pubmed||Crossref||Others
- 66. International Statistical Classification of Diseases and Related Health Problems. Wikipedia
Pubmed||Crossref||Others
- 67. Lapenta, J., Lapenta, J.M. Understanding The Lyme Disease, Classification and Codes (.2018) Ommega Publisher 4(1): 1-11.
Pubmed||Crossref||Others
- 68. Rudenko, N., Golovchenko, M., Grubhoffer, L., et al. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. (2011) Ticks and Tick-borne Diseases 2(3): 123-128.
- 69. Cutler, S.J., Rudenko, N., Golovchenko, M., et al. Diagnosing Borreliosis. (2017) Vector Borne Zoonotic Dis 17(1): 2-11.
- 70. Qiu, W.G., Martin, C.L. Evolutionary genomics of borrelia burgdorferi sensu lato: findings, hypotheses, and the rise of hybrids. (2014) Infect Genet Evol 27: 576-593
- 71. Qiu, W.G., Schutzer, S.E., Bruno, J.F., et al. Genetic exchange and plasmid transfers in Borrelia burgdorferi sensu stricto revealed by three-way genome comparisons and multilocus sequence typing. (2004) Proc Natl Acad Sci 101(39): 14150-14155.
- 72. Theisen, M., Borre, M., Mathiesen, M. J., et al. Evolution of the Borrelia burgdorferi outer surface protein OspC. (1995) J Bacteriol 177 (11): 3036-3044.
Pubmed||Crossref||Others
- 73. Margulis, L., Chapman, M.J. Kingdoms & domains an illustrated guide to the phyla of life on Earth. (2009) Amst :Aca Press/Elsevier4thed.
Pubmed||Crossref||Others
- 74. Kubiak, K., Sielawa, H., Chen, W., et al. Endosymbiosis and its significance in dermatology. (2018) J Eur Acad Dermatol Venereol 32(3): 347-354.
- 75. Kubiak, K., Sielawa, H., Chen, W., et al. Endosymbiosis and its significance in dermatology. (2018) J Eur Acad Dermatol Venereol 32(3): 347-354.