Detection of Specific Antibody Levels against Photobacterium damselae subsp. piscicida in Sea Bass during Experimental Challenge
Zaccone R
Affiliation
Institute for Coastal Marine Environment (I.A.M.C.) Spianata San Raineri 86, 98122 Messina, Italy
Corresponding Author
Mancuso M, Department of Section of Messina Spianata, Istituto per l’Ambiente Marino Costiero (I.A.M.C.) – S. Raineri 86, 98122 Messina, Italy, Tel: +090/669003; Fax: 090/669007; E-mail: monique.mancuso@iamc.cnr.it
Citation
Mancuso, M., et al. Detection of Specific Antibody Levels against Photobacterium damselae subsp. piscicida in Sea Bass during Experimental Challenge. (2016) J Marine Biol Aquacult 2(2): 1- 5.
Copy rights
© 2016 Mancuso, M. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Photo bacterium damselae subsp; Piscicida; Indirect ELISA; Immune response; Sea bass; Antibody
Abstract
The detection of specific antibodies in fish serum is an important assay for disease diagnosis and may furnish information on the real status of fish immunization to infectious disease.
To evaluate the immune response of sea bass (Dicentrarchus labrax) against photo bacterium damselae subsp. piscicida, during an experimental challenge specific antibodies were on serum samples detected by an indirect ELISA protocol. Results showed the presence of antibodies against - P. d. p. in fish sera ten days after challenge, indicating that the specimens began to react to the pathogen.
Introduction
The halophilic, gram-negative, non-capsulated, coccobacillus, Photobacterium damselae subsp. piscicida is the aetiological agent of the Photobacteriosis.
Regardless of the geographic origin and source of isolation, all strains of this pathogen are biochemically and serologically homogeneous (Magariños et al., 1996; Bakopoulos et al., 1997; Mancuso et al. 2007).
However, DNA fingerprinting methods detected two clear separate clonal lineages: one European and one Japanese and American (Magariños et al., 2003; Hawke et al., 2003; Mancuso et al. 2007).
Photobacteriosis also known as Pseudo tuberculosis is a bacterial disease affecting sea bass (dicentrarchus labrax) and sea bream (Sparus aurata) in the Mediterranean area (Baudin Laurencin et al., 1991; Toranzo et al., 1991; Baptista et al., 1996; Candan et al., 1996). In Italy, caused severe mortalities and problems to the local economies (Ceschia G., 1992).
The pathology is characterized by haemorrhagic septicaemia, the body presenting slight haemorrhagic areas in gills and head; the internal exam shows whitish tubercles in the target organs (spleen and kidney) (Kubota et al. 1970; Kusuda and Miura 1972; Robohm 1979; Thune et al. 1993; Mancuso et al. 2005).
It known that the stress linked to biotics and abiotics factors is the primary cause of the decrease of immune defences and the consequent outbreaks of diseases (Scapigliati et al., 2002).
The disease is influenced by environmental changes (overall high temperatures) and depends on the age of the fish (Scapigliati et al. 1999); generally, in young specimens, developed the hyper-acute or acute form of disease with septicemia, cutaneous hyper-pigmentation, respiratory problems, uncoordinated swimming movements, anorexia, bleeding at the base of fins and skin, bleeding of internal organs, splenomegaly, whitish nodules on spleen, liver and kidney. The nodules are granules that corresponded to necrotic areas with bacterial colonies surrounded by macrophages.
In sea bass were observed haemorragic suffusions on the opercula, the jaw, the fins base and anus.
Generally adults presents a chronic form that depend also from the species and was reported in several fish (Kubota et al. 1970; Wolke 1975; Egusa 1983; Toranzo et al. 1991; Thune et al. 1993; Mancuso et al., 2005). Ghittino et al., in the 1993 reported a sub-clinic form, with no external lesion but frequent mortalities, this kind of form was reported by Magariños et al. 2001 when the temperature is lower than 18°C .
Fish immune system is based on humoral and cellular responses operating in a way that is similar to the other vertebrates and producing antibodies that allow the survival and the maintenance of the homeostasis in adverse environmental conditions (Manning 1994).
The production of antibodies, as a consequence of natural or experimental outbreaks of disease, was studied in several fish species such as the rainbow-trout (Cossarini-Dunier, 1985; Thuvander et al. 1987), the Atlantic salmon (Harverstein et al, 1990; Maggnadottir, Gudmundsdottir, 1992; Estevez et al. 1994), atlantic cod (Lund 2006) and Greater amberjack (Zaccone, Mancuso, 2008) through immuno-enzymatic techniques.
Inside of a research project “Monitoring of pasteurellosis and vibriosis in fish species of new interest for aquaculture: testing rapid diagnostic methods and possible correlations with challenges in microcosm”, studies on humoral immune response of teleosts were carried out using a new indirect ELISA protocol (Zaccone, Mancuso, 2008).
The aim of this study was to evaluate the immune response of sea bass (D. labrax) specimens by the production of antibodies against Photo bacterium damselae subsp piscicida during an experimental challenge.
Materials and Methods
Fish and experimental design
• The challenge was performed on juvenile sea bass specimens coming from a Sicilian aquaculture farm were carried out.
• Fish were infected with a wild virulent strain P. damselae subsp. piscicida (Italian strain L7C) isolated by (Mancuso et al., 2005).
• Before challenge different tests were conducted to evaluate the sensitivity of sea bass to P. damselae subsp. piscicida at different incubation temperatures.
• D. labrax specimens were kept into 4 tanks (30 l volume) (10 fish for each tank) and were inoculated intraperitoneally with 0,1 ml of bacterial suspension; each tank was maintained at different temperatures:18, 20, 22 and 24°C (respectively A, B, C and D), the experiment was 2 weeks long.
LD50: LD50 was performed, to establish the challenge dose; 4 bacterial suspensions ranging from 105 to 108 cell/ml were tested. 75 fish were kept in five PVC indoor tanks (500 l volume), each containing 15 sea bass specimens (a. w. 25 g) inoculated with 0,1 ml of each dilution. One tank was used as control (sterile solution).
During the experiment, which lasted 10 days, temperature was maintained at 24°C; salinity (38%) and pH (7.8) were monitored daily; tanks were filled with through-flow sea water and placed under natural photoperiod.
Challenge
After LD50 determination (3.1 × 107 cell/ml), we used 125 sea bass (D. labrax) specimens (m.w. 25 g) for the challenge, divided in 4 replicates and 0.1 ml of bacterial suspension inoculated intraperitoneally, one tank was used as control and fish inoculated with 0.1 ml of sterile saline solution.
The duration of the experiment was 49 days, during which the water temperature was maintained at 24°C; salinity (38%) and pH (7.8) were always monitored.
Fish sera samples: At 24 h, 48 h, 10, 21, 31, 37 and 49 days (survived fish) after challenge, blood was collected from caudal vein of infected fish, previously anaesthetised in a tricaine metansulfonate bath (MS-222) (conc. 0.1 g/l), using a syringe (1 ml), blood (a pool of at least 3 fish) was placed into vials incubated at room temperature to clot at room temperature (22 - 24°C) for 2 h and then centrifuged at 1500 × g for 10 min at 4°C; obtained sera were stored at -20°C till further use.
Bacteriological assays: Blood, kidney and spleen samples were collected from moribund or infected specimens and spread on B.A. (Blood Agar) (Oxoid) and T.S.A. (Triptic Soy Agar – Oxoid) added with NaCl (1.5% final concentration) and incubated at room temperature (24°C) for 24 - 48 h.
Suspected strains grown on B.A. and that presented greyish-white colour, opaque, 1 mm in diameter, smooth with an entire border, non haemolytic were isolated in pure culture and identified by API 20 E and confirmed by latex agglutination test BIONOR-MONO-Pp.
Antigen extraction
P. damselae subsp. piscicida. colonies spread on T.S.A. (1.5 % NaCl final concentration) were incubated for 48 h at 24°C.
Antigen extraction was performed suspending the colonies into 4 ml of sterile PBS (108 cell/ml) and was boiled in a water bath for 90 min at 100°C, then cooled in ice and stored at 4°C until the ELISA test.
Indirect ELISA protocol
Wells of flat-bottomed micro plates (96-wells Nunc) were coated with 50 μl of antigen solution, the last one was used as blank, having added sterile PBS instead of serum; micro plate optical density (O.D.) was read at 450 nm with an automatic plate reader (Titertek-multiscan plus MKII) and then incubated to 4°C overnight.
The ELISA procedure was already reported by Zaccone and Mancuso (2008). 50 μl of a monoclonal antibody Anti- European sea bass IgM (Aquatic diagnostics) diluted with TTBS + 3% of skimmed milk was used as specific antibody and incubated for 30 min at 37°C. Following a further series of washes, 50 °l of Goat Anti Mouse IgG (H + L) peroxidase conjugate (Biorad) was added and incubated for 30 min at 37°C.
After the last washing, 100 μl of TMB peroxidise substrate (Biorad) were added in each well and incubated at 377° C in the dark with a gentle shaking for 30′. The reaction was stopped with 50 μl of H2SO4 1 N, and the O.D. of the micro plate was read at 450 nm.
One-way ANOVA test was carried out among different sampling times and control.
Results
Sensitivity assays
Results of challenge tests at different temperatures showed that the disease didn’t develop at low temperature (A and B tanks); at 22°C (C tank) the outbreak was 5 days after the pathogen inoculation and the mortality registered was 20% while in D tank (24°C) the disease out broke after 24 h (50% mortality).
All bacterial isolates were identified as P. damselae subsp. piscicida.
Challenge: During the challenge the outbreak of the disease occurred 24 h after the inoculum of the bacterial suspension. All infected and moribund fish presented cutaneous hyperpigmentation, haemorrhages in the mouth, pectoral and anal fins; internally, they showed intraperitoneal haemorrhages; the liver and the spleen were characterized by typical whitish nodules. The presumptive identification of the pathogen was based on standard biochemical tests and with API-20 E that not include the codex for Ph. damselae subsp. piscicida but is useful for a rapid presumptive diagnosis of the disease because all strains display a characteristic profile (2005004) (Kent 1982). In addition to confirm the identification we use latex agglutination test BIONOR-MONO-Pp.
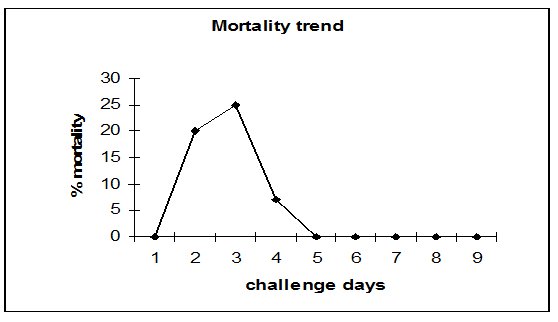
The mortality was registered between 24 and 72 h, reaching a peak at 48 h (25 %) after pathogen injection. Cumulative mortality was 52% (Figure 1).
Figure 1: Mortality trend 1: sera before challnge; 2: 24 h; 3: 48 h; 4: 72 h; 5: 10 d; 6:20 d; 7: 30 d; 8: 35 d; 9: 40 d.
ELISA: Obtained results on preimmune sera, showed antibody against-Photobacterium damselae subsp. piscicida (O.D. mean values were 0.086 ± 0.015).
The PBS value was 0.049 ± 0.003 and was used as background. The positive values were set at the mean optical density value of fish control sera plus three standard deviations of the mean (Crowther, 1995; Cecchini, Saroglia 2002).
As a consequence the OD value of 0.131 was taken as a cut-off for validation of data.
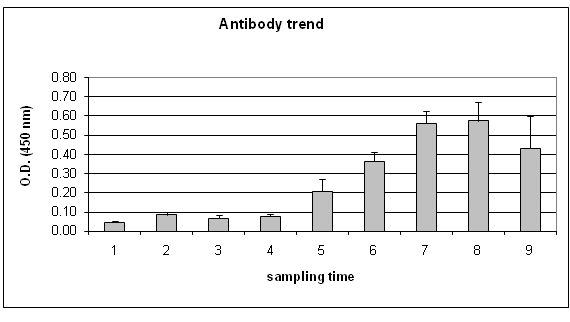
The positive samples were recorded on the 10th day after injection of bacterial suspension (O.D. = 0.210). The antibody concentration increased with the challenge time (days), showing the highest values 37 days after the challenge (O.D. = 0.575). No elevated levels of antibodies were detected in the control group (Figure 2).
Figure 2: Specific antibody trend. 1:PBS; 2: sera before challenge; 3: 24 h; 4: 48 h; 5: 10 d; 6:21 d; 7: 31 d; 8: 37 d; 9: 49 d.
ANOVA test showed significant variation (P < 0.01) between preimmune serum and sampling times starting from 10th day. After the 31st day no significant increase was observed
The antibodies level observed in the fish that survived (49 days) gradually declined compared to that observed on previous days, following a natural decreasing curve.
Conclusion
An efficient immune system is a fundamental prerequisite for maintaining the health of marine species, both in the wild that farmed fish (Scapigliati et al. 2006).
Sea bass is one of the main fish species cultured and was usually used as model for study host-pathogens interactions. Increase of knowledge on mechanisms of resistance to pathogens and specific features of immune response against various infectious agents should benefit the development of effective vaccines. Fish are in intimate contact with the environment, which can contain vary high concentrations of bacteria and viruses (Ellis, 2001).
The importance of environmental condition on resistance to the infection was confirmed by our experiment at different temperature indicating that this bacterial species need high temperature typical of summer period ( > 20°C) to develop of the disease, as also observed in mariculture plant by (Mancuso, et al., 2005).
While low temperatures may provoke a sub-clinic form of disease as reported by (Magariños et al. 2001). In our experiment at 18 and 20°C the disease didn’t develop; at 22°C there was a slowdown in the development of the disease; while the optimum temperature was observed at 24°C.
Moreover some authors confirmed that the best immune response of sea bass was obtained at the water summer temperature (Manning, Nakanishi, 1996) and the immunoglobulin levels related to the temperature showed the optimum at 24°C; lower temperature reduce the magnitude and delay the timing of the antibody response (Scapigliati, et al., 1999; Cecchini, Saroglia et al., 2002).
In challenged sea bass specific humoral immune response was evaluated trough the detection of specific antibodies against P. damselae subsp. piscicida, by ELISA.
In this study obtained results showed that there was a significant increase (P < 0.01) of antibodies 10 days after challenge, suggesting that the subjects suddenly reacted to the pathogen. Our findings are in agreement with the previous studies conducted by (Bakopoulos, et al., 1997) that registered an increase of antibody levels in Dicentrarchus labrax serum infected with Photobacterium damselae subsp. piscicida 8 days after the challenge recording O.D. values similar to our results.
Also (Arijo, et al., 2004) demonstrated a significant increase of serum antibodies in sea bream 4 weeks after vaccination with formalin killed cells.
Similar results were obtained by (Satoh, et al., 1995) during a Photobacteriosis outbreak in Japanese Amberjack (Seriola quinqueradiata), that detected a production of specific antibodies against-P.d.p.
(Esteve-Gassent, et al., 2003) studying the kinetics of antibody production in serum of European eel (Anguilla anguilla), detected by ELISA, after vaccination against Vibrio vulnificus, reported a different immune response in mucus (peak at 3 - 4 days) and in serum (peak at 7 days) with a different duration times respectively titres significantly elevated up to day 5 and 11 for skin and mucus and elevated for more than 25 days for serum.
During the following days O.D. mean values increased reaching the highest values at 31 days after challenge subsequently at 49 days after challenge there was a decrease of the antibody levels.
(Zaccone, Mancuso, 2008) showed that in greater amberjack (S. dumerilii) specimens infected with Listonella anguillarum antibody level increased 5 days after challenge reaching the peak 20 days after challenge.
The detection of a disease-causing organism or its antibody in the host is the pre-requisite for the diagnosis of any infectious diseases and could provides to ascertain a pre-exposure to the pathogen (Swain, Nayak, 2003); the immune status of fish is not only useful in an epizootiological survey, but it also helps in evaluating vaccination programmes (Waterstrat, et al., 1989).
The use of this method simple, sensitive and economically as reported by (Hsu, et al., 1991; Pasho, et al., 1987) is important because it allow to test many samples simultaneously using small amounts of serum, is a valuable aid in the study of specific immune response for seabass during the challenge and also can be used for a positive control on successful vaccination (Shelby et al., 2004 and Pedro P. Garcia-Pereira – personal communication).
Thanks to the information’s obtained from survivors fish is considered important to continue the experiments by extending the period to have more detailing the antibodies trend, at the moment we know that after 49 days from the challenge there is a decrease of the antibodies in the blood of sea bass.
Further studies elongating the experiment duration could furnish new information’s to better understand the antibody trend of this species.
Acknowledge:
This work was supported by funds of the Italian Ministry of Agriculture and Forestry (MIPAF), VI three-year plan, “Monitoring of pasteurellosis and vibriosis in fish species, new candidates for aquaculture: test of rapid diagnostic methods and relationships with experimental infection in microcosm”.
References
- 1. Arijo, S., Balebona, C., Martinez-Manzanares, E.,et al. Immune response of gilt-head seabream (Sparus aurata) to antigens from Photobacterium damselae subsp. piscicida. (2004) Fish & Shellfish Immunol 16(1): 65-70.
- 2. Bakopoulos, V., Volpatti, D., Adams, A., et al., Qualitative differences in the immune response of rabbit, mouse and seabass, D. labrax, to Photobacterium damsela subsp piscicida, the causative agent of fish pasteurellosis”. (1995) Fish and Shellfish Immunology 7(3): 161-174.
- 3. Bakopoulos V., Volpatti D., Papapanagiotou E., Richards R. , Galeotti M., Adams A. (1997b) - Development of an ELISA to detect Pasteurella piscicida in culture and in “spiked” fish tissue. Aquaculture, 156: 359-366.
- 4. Baptista, T., Romalde, J.L., Toranzo, A.E. First epizootic of pausterellosis in Portugal affecting culture gilthead seabream (Sparus aurata). (1996) Ass Fish Pathol 16: 92-95.
- 5. Baudin-Laurencin, F., Pepin, J.F., Raymond, J.C. First observation of an epizootic pausterellosis in farmed and wild fish of the French Mediterranean coasts. (1991) Ass Fish Pathol 17.
- 6. Candan A., Ang Kuker M., Kataras S. Pasteurellosis in cultured sea bass. (1996) (Dicentrarchus labrax) 16 (5):150-153.
- 7. Cecchini, S., Saroglia, M. Antibody response in sea bass (Dicentrarchus labrax, L.) in relation to water temperature and oxygenation. (2002) Aquaculture Research 33: 607-613.
- 8. Ceschia G. Principali patologie batteriche nell’acquicoltura italiana.” (1992)Boll. Soc It. 10: 21-29.
- 9. Ceschia, G., Giorgetti, G., Bovo, G., Grave epizoozia da Pasteurella piscicida in specie eurialine del Nord Adriatico. (1990) Boll Soc It. 4: 11-16.
- 10. Coeurdacier, J.L., Pepin, J.F., Fauvel, C., et al. Alteration in total protein, IgM and specific antibody activity of male and female sea bass (Dicentrarchus labrax L., 1758) sera following injection with killed Vibrio anguillarum. (1997) Fish and Shellfish Immunology 7:151-160.
- 11. Colorni , (1993) comunicazione personale.
- 12. Cossarini-Dunier, M. Indirect enzyme-linked immunosorbent assay (ELISA) to titrate rainbow trout serum antibodies against two pathogens: Yersinia ruckeri and EGTVED virus. (1985) Aquaculture 49(3-4): 197-208.
- 13. Crowther, J.R. ELISA Theory and Practice (1995) Methods in Mol Biol 42:1-218.
- 14. Egusa, S.) Disease problems in Japanese yellowtail, Seriola quinqueradiata culture: a review. In: Stewart J.E.(ed) Diseases of Commercially Important Marine Fish and shellfish. (1983Conseil Intarnational pour l’Exploration de la Mer, Copenhagen 10-18.
- 15. Ellis, A.E. Innate hoste defense mechanisms of fish against viruses and bacteria. (2001) Dev comp immunol 25(8-9)8: 827-839.
- 16. Ercolini, C., Pasini, G. C., Fisichella, S. Et al. Su di un focolaio di Pasteurellosi in alcune specie ittiche della zona di estuario di un fiume della provincia di La Spezia. (1991) Boll Soc It Patol Ittica 5: 43-49.
- 17. Esteve-Gassent M.D., Nielsen M.E., Amaro, C. The kinetics of antibody production in mucus and serum of European eel (Anguilla anguilla L) after vaccination against Vibrio vulnificus: development of a new method for antibody quantification in skin mucus. (2003) Fish and Shellfish Immunol. 15(1): 51-61.
- 18. Estevez, J., Leiro, J., Toranzo, A.E., et al. Kinetics of antibody production against Vibrio anguillarum antigens in turbot. (1994) Aquaculture. 123(3-4): 191-196.
- 19. Garcia-Pereira P.P. Evaluation of the humoral immune response in vaccinated animals against vibriosis, furuncolosis, flexibacteriosis and streptococcosis (Personal communication).
- 20. Ghittino C., Arfarà S. e Prearo M. (1993) - La Pasteurellosi ittica in Grecia. Boll. Soc. It. Patol. Ittica, 11: 11-20.
- 21. Harvestein, L.S., Endresen, C., Hjeltnes B., et al. Specific immunoglobulins in serum from Atlantic salmon, Salmo salar L., immunized with Vibrio salmonicida and infectious pancreatic necrosis virus. (1990) J. Fish Dis 13(2): 101-111.
- 22. Hsu H.M., Bowser P.R., Schanchte J.H. Jr. (1991) - Development and evaluation of a monoclonal antibody based enzyme linked immunosorbent assay for the diagnosis of Renibacterium salmoninarum infection. J. Aquat. Health, 3: 168-175.
- 23. Kent M.L. Characteristics and identification of Pasteurella and Vibrio pathogenic to fishes using API-20E (Analytab Products) multitube test strips. (1982) Canadian J Fisheries and Aqua Sci 39(12): 1725-1729.
- 24. Klesius, P., Johnson, K., Durborow, R., et al. - Development and evaluation of enzyme-linked immunosorbent assay for catfish serum antibody to Edwardsiella ictaluri. (1991) J Aquat Anim Health 3: 94-99.
- 25. Kubota, S., Kimura, M, Egusa, S. Studies on “bacterial tuberculosis” in cultured yellowtail. I. Symptomatology and histopathology. (1970) Fish Pathol 4(2): 1111-1118.
- 26. Kusuda, R., Miura, W. Characterization of Pasteurella sp. Pathogenic for pond cultured ayu. (1972) Fish Pathol 7: 51-57.
- 27. Magariños, B., Couso, N., Noya, M., et al. - Effect of temperature on the development of pasteurellosis in carrier gilthead seabream (Sparus aurata). (2001) Aquaculture 195: 17—21.
- 28. Mancuso, M., Basile V., Innella G., et al. Mugil cephalus: un campanello d’allarme della comparsa di focolai di Pseudotubercolosi in Spigole allevate in gabbie off-shore cages. (2005) Biol Marina Mediterranea, 12 (1): 195-197.
- 29. Manning, M. J.. Immunology: a comparative approach ( R.J- Turner ed.) (1994) Fishes 69-100.
- 30. Nousias H. (1994) Comunicazione personale.
- 31. Pasho R.J., Elliot D.G., Mallett R.W., Mulcahy D. (1987) - Comparison of five techniques for the detection of Renibacterium salmoninarum in adult Coho salmon. Trans. Am. Fish Soc., 116: 882-890.
- 32. Pickering, A.D., Pottinger, T.G. Cortisol can increase the susceptibility of brown trout, Salmo trutta L., to disease without reducing the white blood cell count. (1989). J Fish Biol 27: 611-619.
- 33. Robohm R.A.) – Pasteurella piscicida, the etiologic agent of an epizootic in striped bass (Morone saxatilis) in Long Island Sound. 4th Annual Eastern Fish Health Workshop, (1979) Halifax, N.S. Canada.
- 34. Satoh, K.I., Fukuda, Y., Nakano S - Changes in agglutinin titre against Pasteurella piscicida in cultured yellowtail during the epizootics of Pseudotubercolosis in 1993 and 1994. (1995) Fish Pathol., 30(1995): 291-292.
- 35. Scapigliati, G., Mazzini, M., Buonocore F. Il sistema immunitario della spigola Dicentrarchus labrax. (2006) Biol Mar Medit 13(2): 24-25.
- 36. Scapigliati G., Scalia D., Marras A., et al. Immunoglobulin levels in the teleost sea bass (Dicentrarchus labrax (L. )) in relation to age, season and water oxigenation. (1999) Aquaculture 174 : 207-212.
- 37. Shelby, R., Shoemaker, C.A., Klesius ,P.H. , Development of an ELISA to measure the humoral immune response of hybrid striped bass Morone chrysops x M. saxatilis to Streptococcus iniae. (2004) Aquacult Res 35(10): 997-1001.
- 38. Sitjà-Bobadilla, A., Redondo, M.J., Macias, M.A., (2004) Development of immunohistochemistry and enzyme-linked immunosorbent assays for the detection of circulating antibodies against Enteromyxum scophtalmi (Mixozoa) in turbot (Scophtalmus maximus L.) (2004) Fish Shellfish Immunol. 17(4): 335-345.
- 39. Swain, P., Nayak, S.K. Comparative sensitivity of different serological tests for seromonitoring and surveillance of Edwardsiella tarda infection of Indian major carps. (2003) Fish Shellfish Immun 15(4): 333-340.
- 40. Thune, R. L., Stanley, L.A., Cooper, R.K. Pathogenesis of gram-negative bacterial infections in warmwater fish. (1993) Annu rev Fish Dis 3 : 37-68
- 41. Toranzo, A.E., Barreiro, S., Casal, J.L., et al.(1991) – Pausterellosis in cultured gilthead seabream (Sparus aurata): first report in Spain. Aquaculture 99: 1-15.
- 42. Volpatti, D. Pathogenetic study of fish pasteurellosis in sea bass (1995) (Dicentrarchus labrax L.).
- 43. Waterstrat P.R., Ainsworth A.J., Capley G.Use of an indirect enzyme-linked immunosorbent assay (ELISA) in the detection of channel catfish ,Ictalurus punctatus (Rafinesque), antibodies to Edwardsiella ictaluri. (1989) J Fish Dis 12(2): 87-94.
- 44. Wolke, R.E. Pathology of bacterial and fungal diseases affecting fish. (1975) The pathol fishes 33-116.
- 45. Zaccone, R., Mancuso, M. First report on antibody response of Seriola dumerilli (Risso 1810) challenged with Listonella anguillarum. (2008) Fish Shellfish Immun 25: 689-692.