Effect of Water Extract of Djulis (Chenopodium Formosaneum) and Its Bioactive Compounds on Alcohol-Induced Liver Damage in Rats
PinDer Duh1*, ShihYing Chen2, ChinChen Chu3, CharngCherng Chyau4, ZiHan Fu2
Affiliation
1 Department of Health and Nutrition, Chia Nan University of Pharmacy and Science, Tainan,Taiwan, ROC
2 Department of Anesthesiology, Chi-Mei Medical Center, Tainan,Taiwan, ROC
3 Research Institute of Biotechnology, Hungkuang University, Taichung, Taiwan, ROC
4 Department of Food Science and Technology, Chia Nan University of Pharmacy and Science,Tainan, Taiwan, ROC
†These authors contributed equally to this work
Corresponding Author
PinDer Duh, Chia Nan University of Pharmacy and Science, 60 Erh-Jen Road, Section 1, Pao-An, Jen-Te District,Tainan, Taiwan, R.O.C, Tel: 886-6-2660993; E-mail: ipdduh@mail.cnu.edu.tw
Citation
Duh, P., et al. Effect of Water Extract of Djulis (Chenopodium Formosaneum) and Its Bioactive Compounds on Alcohol-Induced Liver Damage in Rats. (2018) Int J Food Nutr Sci 5(1): 55- 63.
Copy rights
© 2018 Duh, P. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Alcohol intake; Djulis (Chenopodium Formosaneum); Oxidative stress; Glutathione (GSH); Lipid peroxidation; Hepatoprotection
Abstract
The effects of water extract of Djulis (Chenopodium Formosaneum) (WECF) and its bioactive compounds on alcohol-induced liver damage in rats was investigated. WECF and its bioactive compounds were co-supplemented with drinking water that contained alcohol (30 %) at 0.5, 1.0 and 2.5 mg/kg bw, respectively, for 90 days. WECF attenuated oxidative stress by lowering lipid peroxidation, reducing cytochrome P4502E1 (CYP2E1) activity, enhancing catalase activity, and improving histological architecture of rat liver. Rutin, kaempferol and betaninat 1.0 μg/ kg bw significantly inhibited lipid peroxidation in rat liver. Moreover, WECF at 1.0, 2.5 mg/kg bw and rutin and kaemferol at 1.0 μg/ kg bw significantly restored glutathione (GSH) in alcohol-treated rat liver. Rutin at 1.0 μg/kg bw significantly restored superoxide dismutase (SOD) activity. These results suggest that WECF has a beneficial effect in alleviating the adverse effect of alcohol and may be a potential anti-alcoholic agent for treating liver injury.
Introduction
Drinking alcoholic beverages plays an important social role in many cultures; however, chronic excessive alcohol intake will damage hepatic tissue and lead to liver diseases such as hepatic inflammation, fibrosis and cirrhosis[1]. Recent studies have shown that the negative effects of alcohol-induced liver injury involve inhibition of fatty acid oxidation, enhancement of lipogenesis, formation of reactive oxygen species (ROS) and production of inflammatory responses, subsequently leading to steatohepatitis, cirrhosis, and even liver cancer[2]. Therefore, liver damage resulting from chronic continuous alcohol intake is a major problem of worldwide proportions. However, drugs that can successfully treat liver injure remain few[3]. Considerable attention has recently been focused on complementary and alternative therapies for prevention of human diseases. The search for functional biomaterials from natural products capable of alleviating liver injury is of interest to scientists and researchers. Accumulating evidence has revealed that a diet rich in fruit, vegetables and herbs is a good source of phytochemicals, and is associated with a lower risk of chronic diseases[4]. It is, therefore, necessary to develop a scientific and logical strategy for attenuating alcohol-induced liver injury.
Djulis (Chenopodium formosanum) is a native cereal plant cultivated by aboriginal people of Taiwan. It is called “Hung Li” due to its bright red grain color and is also known as “ruby of cereals”. Djulis has recently been reported to have useful biological properties. For example, Djulis contains high protein, a balance amino-acid spectrum with high lysine and methionine contents, dietary fiber as well as phytochemicals[5,6]. In the previous works by the authors demonstrated that water extracts of Djulis were responsible for its protective effect against oxidative stress in vitro[7] and can effectively protect rat liver from CCl4 - induced liver injury[8]. In other words, Djulis is not only used as a novel source of coloring pigments in food industry but also demonstrates positive physiological benefits. However, there has been little research on the effectiveness of Djulis in protection against alcohol-induced liver injury in vivo. To make up for such deficiency, this study aims to evaluate the hepatoprotective effect of Dulis and its bioactive compounds against liver damage in vivo caused by alcohol intake.
Materials and Methods
The Djulis (Chenopodium formosaneum), purchased from Kullku Farm, Pingtung, Taiwan, were ground to a fine powder. The powder (100 g) was extracted with boiling water (1,000 mL) and stirred for 40 min. The extract was filtered and the residue was re-extracted under the same conditions. The combined filtrate was freeze-dried. The dehydrated powder was suspended in water and this water extract of Djulis were abbreviated to be WECF[7].
HPLC / ESI - MS - MS analysis of Djulis
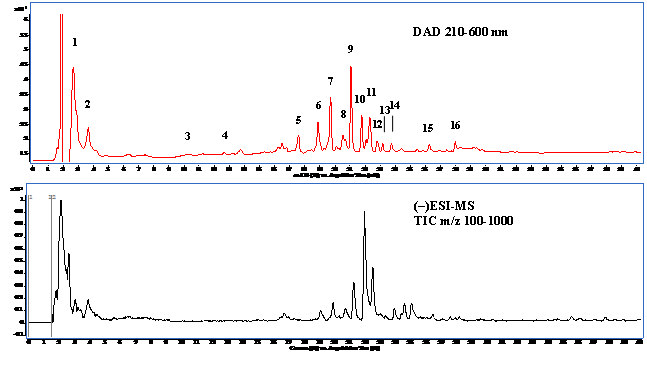
The HPLC/electro spray ionization (ESI) mass spectrometric analysis was performed on an Agilent 1260 Infinity HPLC system in connected with a 6420 mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) as our previous report[8]. In brief, water extracts (10 mg/mL) of Djulis was filtered through 0.45 µm membrane filter before being injected into the analysis column of Symmetry C-18 column (2.1 × 150 mm, 3.5 μm particle size, Waters Corporation, Milford, MA, USA), which was connected with a guard column (Security Guard C18 (ODS) 4 mm × 3.0 mm ID, Phenomenex Inc., Torrance, CA, USA) and was installed in a column oven set at 35°C. The mobile phase consisted of two solvents: Solvent A (water containing 0.1 % formic acid) and Solvent B (acetonitrile containing 0.1% formic acid). The flow rate during the elution process was set at 0.3 mL / min. A linear gradient elution was carried out with 20 - 30% B in 10 min, 30 - 95% B in 30 min and finally 95% B isocratic elution for 10 min. The absorption spectra of eluted compounds were scanned within 210 to 600 nm using the in-line PDA detector monitored at 280, 360 and 530 nm, respectively. The compounds having been eluted and separated were further identified with triple quadrupole mass spectrometer in the operating parameters as follows: nitrogen used both as a drying gas at a flow rate of 9 L / min and as a nebulising gas at a pressure of 35 psi, drying gas temperature 300 °C, and a potential of 3500 V applied across the capillary, fragment or voltage 90 V, and the collision voltage 15 V. Quadrupole 1 filtered the calculated m/z of each compound of interest, while quadrupole 2 scanned for ions produced by nitrogen collision of these ionized compounds in the range 100 – 1000 m/z at a scan time of 200 ms per cycle. Mass data were acquired in negative ionization mode. The identification of separated compounds was carried out by comparing their mass spectra provided by ESI-MS and ESI-MS/MS as described in literatures and remarked in the Table 1.
Table1: Retention time, UV-Vis and Mass spectral characteristics of the aqueous extract of Djulis (Chenopodiun formosaneum).
| Peak | Compound | tR min) | λmax (nm) | [M - H]- | fragments | Amount (mg/g)d |
References |
|---|---|---|---|---|---|---|---|
| 1 | Quinic acid b | 2.67 | < 210 | 191 | 128 | 366 | [15] |
| 2 | Quinic acid derivativeb | 3.69 | < 210 | 381 | 283 | 56 | [17] |
| 3 | Betanina | 10.21 | 534, 274 | 10 | |||
| 4 | Isobetaninb | 12.65 | 534, 236 | 5.6 | [6] | ||
| 5 | Unknownc | 17.59 | 220 | 34.6 | |||
| 6 | Quercetin-3-O-rutinoside- 7-O-rhamnosideb |
18.89 | 254, 354 | 755 | 609 | 53.4 | [18] |
| 7 | Camellianosideb | 19.71 | 268, 350 | 741 | 300, 179 | 83.6 | [13] |
| 8 | Quercetin-3-O- (6'''-p-coumaroyl-2"-glucosyl) rhamnoside |
20.54 | 254, 354 | 755 | 31.7 | [14] | |
| 9 | Rutina | 21.07 | 349, 267, 226 | 609 | 300, 301, 271 | 157.1 | |
| 10 | 20-Hydroxyecdysone | 21.78 | 246, 224sh | 525 | 58.1 | [16] | |
| 11 | Unknown | 22.31 | 222, 280 | 579 | 60.1 | ||
| 12 | Kaempferol-3-O-rutinosidea | 22.79 | 267, 350, 235sh | 593 | 285, 284, 255 | 26.6 | |
| 13 | Unknown | 23.18 | 224, 352 | 623 | 13.3 | ||
| 14 | Unknown | 23.75 | 224, 348 | 539 | 447 | 19.5 | |
| 15 | Unknown | 26.27 | 222, 314 | 677 | 9.9 | ||
| 16 | Unknown | 27.99 | 222, 312, 412 | 675 | 463 | 14.6 |
a The identification was confirmed further by authentic compound.
b Compounds were tentatively identified according to mass spectra and the matched data from literatures.
c Compounds were limitedly identified from mass spectra and UV-visible absorbance spectra.
d Peaks 3 was quantified as equivalent to betanin and all the others were quantified as quercetin (three replicates for all compounds) based on the amount of mg/g dried weight of extract.
Animal treatment: Male Wistar rats (7-week-old) were obtained from BioLASCO, Taiwan Co., Ltd. in this study. Animals were cared and used after the experimental protocols approved by institutional animal ethics committee (Chia-Nan University, Tainan, Taiwan, and ROC). The animals were all fed a commercial rodent chow diet in entire experiment. The rats were randomly assigned to one of ten groups of 6 rats each that were control, ethanol treatment (EtOH), silymarin,rutin, betanin, kaempferol with EtOH, and high dose, medium dose, low dose of WECF with EtOH, respectively. The last group was fed high dose (2.5 mg / kg bw) of WECF without EtOH. All animals were maintained in a controlled environment at 21 ± 2°C, 50 ± 5% relatively humidity and a cycle of 12 h dark/light and provided with food and water adlibitum. However, they were acclimatized for 1 week prior to use. Ethanol was introduced into the ethanol water starting at 20% (v/v) during days 1-14 and maintained at 30% (v/v) during days 15 - 90. Rutin (1.0 μg/kg bw), betanin (1.0 μg /kg bw), kaempferol (1.0 μg /kg bw), high dose (2.5 mg/kg bw), medium dose (1.0 mg/kg bw) and low dose (0.5 mg/kg bw) of WECF were gavage for 90 consecutive days. Furthermore, the positive control was oral-administered silymarin (100 mg / kg bw), the negative control was received ethanol water alone. However, the control group was treatment with water instead EtOH and the sample administration. At the end of the 90-day feeding period, the rats were fasted overnight and anesthetized with carbon dioxide. Blood samples were collected from abdominal vein into heparinized syringes. Immediately after killing, the liver was weighed and two portions were removed: one from the left lateral lobe and the other from the largest lobe. The samples were weighed separately, quickly frozen with liquid nitrogen, and stored at −80°C until used.
Serum biochemical parameters: Serum alanine amino transferase (ALT) and aspartate amino transferase (AST) were measured by a biochemical auto analyzer (Toshiba, TBA-200FR, Holliston, MA, USA), using the kits from Denka Seiken Co., LTD. (Tokyo, Japan). Blood urea nitrogen (BUN) and creatinine (CRE) levels in serum were determined by commercial kits from urea liquid and creatinine liquid, respectively (Sentinel Diagnostics, Milan, IT). The serum triglyceride (TG) concentration was measured using commercial kit from Triglycerides Liquid (Sentinel Diagnostics, Milan, Italy). Serum cholesterol (CHOL) concentration was determined using commercial enzymatic kit from T-CHO (Denka Seiken, Tokyo, Japan). Protein levels were determined by the Bradford Protein Assay Kit (Bio-Rad Laboratories)[9].
Assay for antioxidative status in liver: The activity of superoxide dismutase (SOD) and glutathione peroxidase (GPx) were, respectively, determined using commercial kits from Randox Laboratories Ltd., Crumlin, and Antrim, UK. The activity of catalase (CAT) and the content of reduced glutathione (GSH) in liver were, respectively, determined by commercial kits from Cayman Chemical Company, Ann Arbor, MI, USA. These assays were completed according to methodology recommended by the manufacturer and detected by a biochemical autoanalyzer (Toshiba, TBA-200FR, and Holliston, MA, USA).
Measurement of lipid peroxidation products: Liver tissues were homogenized in cold Tris - HCl (pH 7.4) (1:10, w/v) of 20 mmol/L. The homogenate was centrifuged for 30 min at 2,500 ×g and 4°C. The homogenate was stored at −80°C for the following experiments. Measurement of lipid peroxidation products was carried out by the method of Buege and Aust[10].
Assay for detoxification enzyme in liver: Cytochrome P450 2E1 (CYP2E1) concentrations were measured by ELISA kit E90988Ra, (Uscn, Life Science Inc., USA) according to the manufacturer’s protocol. Each set of experiments was carried out in a single lot of ELISA reagents. The concentration of CYP2E1 was calculated using the absorbance expressed as ng / g protein[8].
Comet assay was determined and modified by the methods of Szeto et al.[11] and Braz et al.[12] Tail moment and tail intensity were used to estimate DNA damage. As tail intensity (% DNA tail) gave similar results, only tail moment values were presented.
Liver tissues, trimmed into 2 mm thickness, were fixed with buffered formaldehyde for 24 h. The fixed tissues were embedded in paraffin, sectioned and rehydrated. The histological examination by the above conventional method was evaluated the index of ethanol-induced necrosis by assessing the morphological changes in the liver sections stained with hematoxylin and eosin (H&E). Sections were studied under light microscope (DIALUX 20 EB, Wetzlar, Germany) at 40 and 100 × magnifications.
The results are expressed as mean ± standard deviation (SD), and ANOVA was conducted by using the SPSS software (version 12.0; SPSS, Chicago, IL, USA). When a significant F ratio was obtained (p < 0.05) a post hoc analysis was conducted between groups by using a multiple comparison procedure with a LSD test. Statistical significance was accepted at a level of p < 0.05.
Results
Identification of bioactive compounds
The bioactive compounds present in WECF were identified using HPLC-DAD and HPLC-MS/MS analyses. Results of qualitative and quantitative analysis are indicated in Table 1 and Figure 1. The identification of ten compounds was based on the comparison of their retention times, MS / MS data with those of standard compounds and published data[6,13-18]. Among the compounds identified, quinic acid and rutin are the two most abundant compounds in WECF. Many studies reported the beneficial role of rutin which is acommon flavonol glycoside of vegetables in controlling various diseases[19]. Accumulating evidence indicates kaempferol which is abundant in fruits and vegetables showed significant and comparable biological activity[20]. In addition, betanin represents a group of pigments that are responsible for the red color in Djulis[21]. Silymarin, a known hepato protective drug, is widely used for the treatment of liver disease. Therefore, silymarin, rutin, kaempferol and betanin were selected as reference compounds for the series of experiments.
Figure 1: The total ion chromatogram of LC/MS (upper panel) and HPLC chromatogram (lower panel) of aqueous extract from Djulis (Chenopodiun formosaneum).
Note: The peak number was referred to Table 1.
Changes in body and organ weights: The gain in body and organ weights of rats in ten different groups was recorded during the experimental period (data not shown). The ethanol-treated rats gained significantly less weight than the control. No significant difference in body weight was observed among ethanol-consuming groups, indicating that co-administration of WECF at difference concentrations and its bioactive compounds, rutin, betanin, and kaempferol with ethanol has no effect on body weight gain.
Effect of WECF on ALT, AST, BUN, CRE, CHOL and TG: To assess ethanol-induced hepatic injury, the serum AST and ALT levels in ethanol-fed rat were examined (Table 2). Although there was no significant difference in ALT and AST levels between the ethanol-consuming and the ethanol-control, there were trends toward lower ALT and AST activities in groups treated with silymarin, WECF at different concentrations, rutin, kaempferol and betanin at 1.0 μg/kg bw, compared with the ethanol-control groups. In addition, there was no significant change in BUN and CRE levels among ethanol - consuming groups. After the rats were fed with ethanol for 90 days, the TG levels of all ethanol-consuming groups were higher than that of the control group. Of all ethanol - consuming groups, serum TG level in WECF at 2.5 mg/kg bw (110.2 mg/dL) were significantly lower than that of the ethanol-control group (153.0 mg/dL). There was no significant difference in CHOL levels between the ethanol-consuming group and the control group.
Table 2: Effects of water extracts of Djulis (Chenopodium formosaneum) (WECF) and its bioactive compounds on serum biochemical values in rats treated with ethanol (EtOH).
| Groups | AST (U/L) | ALT (U/L) | BUN (mg/dL) | CRE (mg/dL) | CHOL (mg/dL) | TG (mg/dL) |
|---|---|---|---|---|---|---|
| Control | 105 ± 15 | 47 ± 11 | 20 ± 2 | 0.75 ± 0.06 | 63.7 ± 1.2 | 82.3 ± 24.0 |
| EtOH | 110 ± 12 | 52 ± 10 | 22 ± 4 | 0.72 ± 0.02 | 71.5 ± 15.6 | 153.0 ± 17.3# |
| Silymarin (100 mg/kg bw)+ EtOH | 102 ± 14 | 47 ± 10 | 21 ± 3 | 0.72 ± 0.05 | 75.1 ± 11.5 | 153.2 ± 39.1 |
| Rutin (1.0 μg/kg bw)+ EtOH | 109 ± 3 | 48 ± 4 | 18 ± 1 | 0.68 ± 0.06 | 80.2 ± 20.4 | 128.8 ± 27.9 |
| Betanin (1.0 μg/kg bw)+ EtOH | 103 ± 13 | 50 ± 13 | 23 ± 3 | 0.73 ± 0.04 | 71.0 ± 10.9 | 139.8 ± 39.6 |
| Kaempferol (1.0 μg/kg bw)+ EtOH | 100 ± 12 | 42 ± 7 | 19 ± 1 | 0.67 ± 0.01 | 66.1 ± 8.2 | 121.5 ± 29.9 |
| WECF (0.5 mg/kg bw)+ EtOH | 100 ± 4 | 42 ± 9 | 20 ± 1 | 0.68 ± 0.04 | 62.7 ± 8.7 | 145.7 ± 5.7 |
| WECF (1.0 mg/kg bw)+ EtOH | 102 ± 10 | 52 ± 12 | 22 ± 4 | 0.68 ± 0.01 | 70.9 ± 8.7 | 130.2 ± 23.8 |
| WECF (2.5 mg/kg bw)+ EtOH | 100 ± 28 | 45 ± 12 | 20 ± 2 | 0.70 ± 0.06 | 69.0 ± 10.1 | 110.2 ± 14.6* |
| WECF (2.5 mg/kg bw) | 88 ± 5 | 45 ± 12 | 21 ± 2 | 0.74 ± 0.12 | 69.5 ± 2.1 | 119.0 ± 31.1 |
Values are means ± SD for six rats per group. Results were all statistically analyzed with LSD test.
#Significant difference from the control group (p < 0.05). *Significant difference from the EtOH group (p < 0.05). AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; CRE, creatinine; CHOL, cholesterol; TG, triglyceride.
Effect of WECF on GSH and antioxidant enzyme activities: The GSH levels and activities of hepatic antioxidant enzymes, such as SOD, GPx and CAT, are shown in Table 3. Alcohol treatment significantly raised GSH levels, compared with the control. GSH levels in groups treated respectively with WECF, rutin and kaempferol were significantly lower than that in the ethanol-consuming group. However, there was no significantly differences in GSH levels among the ethanol-control group, WECF at 2.5 mg / kg bw with ethanol-consumption and WECF at 2.5 mg/kg be without ethanol-consumption (P > 0.05). A notable decrease in SOD and CAT was observed in the ethanol control group, compared with the control group. Co-administration of WECF at different concentrations effectively recovered CAT activity. However, there was not significant change in SOD activity after co-administration of WECF at different concentrations with ethanol consumption. Of the bioactive compounds, administration of rutin (1.0 μg/kg bw) to ethanol-consuming rats significantly increased the GSH levels and SOD activity. There was no significant difference in GPx activity among the ten groups.
Table 3: Effects of water extracts of Djulis (Chenopodium formosaneum) (WECF) and its bioactive compounds on glutathione (GSH) and antioxidant enzymes activities in rats treated with ethanol (EtOH).
| Groups | GSH (nmole /gLiver) | SOD (U/g Liver) | GPx (U/g Liver) | CAT (nU/g Liver) |
|---|---|---|---|---|
| Control | 260 ± 57 | 1464 ± 367 | 118 ± 16 | 624 ± 37 |
| EtOH | 700 ± 63# | 918 ± 173# | 115 ± 16 | 461 ± 14# |
| Silymarin (100 mg/kg bw)+ EtOH | 493 ± 123* | 701 ± 106 | 130 ± 31 | 734 ± 119* |
| Rutin (1.0 μg/kg bw)+ EtOH | 522 ± 124* | 1210 ± 176* | 117 ± 17 | 467 ± 33 |
| Betanin (1.0 μg/kg bw)+ EtOH | 527 ± 125 | 860 ± 86 | 113 ± 9 | 660 ± 146 |
| Kaempferol (1.0 μg/kg bw)+ EtOH | 461 ± 119* | 985 ± 267 | 101 ± 8 | 504 ± 117 |
| WECF (0.5 mg/kg bw)+ EtOH | 645 ± 67 | 775 ± 142 | 120 ± 14 | 783 ± 57* |
| WECF (1.0 mg/kg bw)+ EtOH | 500 ± 134* | 607 ± 88 | 107 ± 15 | 811 ± 26* |
| WECF (2.5 mg/kg bw)+ EtOH | 461 ± 58* | 703 ± 171 | 97 ± 12 | 804 ± 91* |
| WECF (2.5 mg/kg bw) | 733 ± 70 | 1129 ± 216 | 124 ± 19 | 835 ± 60# |
Values are means ± SD for six rats per group.Results were all statistically analyzed with LSD test. # significant difference from the control group (p < 0.05). *Significant difference from the EtOH group (p < 0.05). GSH, glutathione, SOD, superoxide dismutase; GPx, glutathione peroxidase; CAT, catalase.
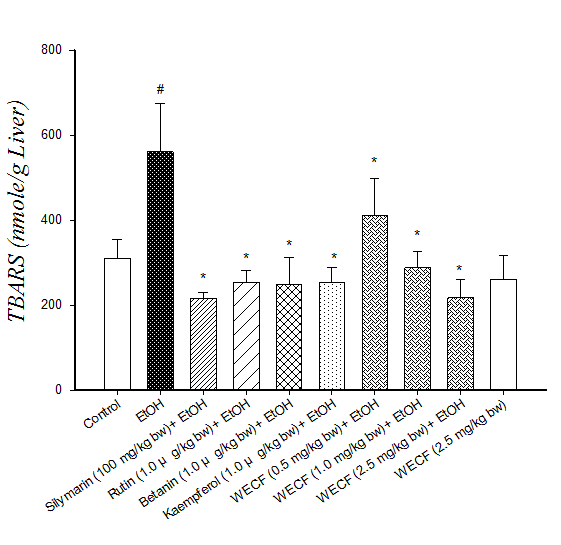
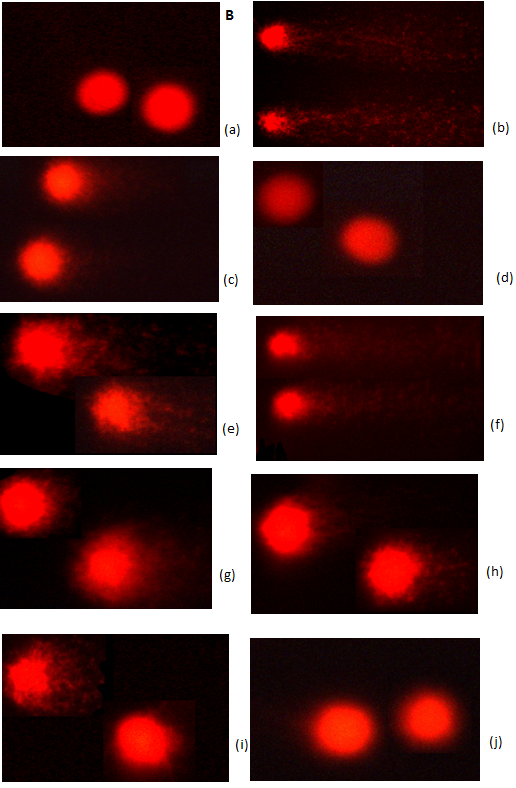
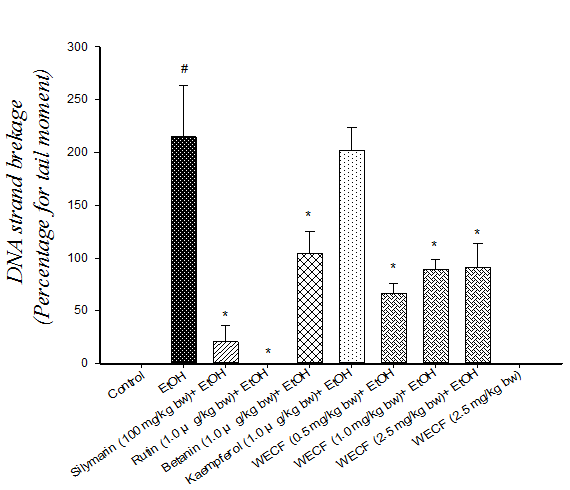
Effect of WECF on oxidative stress: The results of hepatic lipid peroxidation and oxidative damage to DNA in lymphocyte are shown in Figure 2. TBARS formation in the ethanol-consuming group was significantly higher than that in the control group (Figure 2A). Moreover, TBARS formation in groups treated respectively with WECF, rutin, kaemperol and betanin were significantly lower than that in the ethanol-consuming group. Oxidative damage to DNA in lymphocyte is shown in Figure 2B and Figure 2C. The results from the photomicrograph for comet (Figure 2B) and genotoxicity (Figure 2C) of blood lymphocyte reveal that co-administration of WECF at different concentrations, rutin, betanin, and silymarin at tested doses significantly decreased the ethanol-induced damage to the DNA strand.
Figure 2: Effects of water extracts of Djulis (Chenopodiun formosaneum) (WECF) and its bioactive compounds on thio barbituric acid reactive substances (TBARS) formation in the liver of rats treated with ethanol (EtOH).
Note: (A) and on DNA strand breakage (B). Photomicrographs of comets in lymphocyte stained with propidium iodide in different groups: (a) Control group; (b) ethanol (EtOH); (c) Rutin (1.0 μg/kg bw) + EtOH; (d) Betanin (1.0 μg/kg bw) + EtOH; (e) Kaempferol (1.0 μg/kg bw) + EtOH; (f) Silymarin (100 mg/kg bw) + EtOH; (g) WECF (0.5 mg/kg bw) + EtOH; (h) WECF (1.0 mg/kg bw) + EtOH; (i) WECF (2.5 mg/kg bw) + EtOH; (j) WECF (2.5 mg/kg bw) and genotoxicity of WECF on DNA strand breakage (C) in blood lymphocytes from rats treated with EtOH. Tail moment = percent of DNA in the tail × tail length (Tm). Data were presented as mean ± SEM. Results were all statistically analyzed with LSD test. #Significant difference from the control group (p < 0.05). * Significant difference from the EtOH group (p < 0.05). Values are means ± SD for six rats per group.
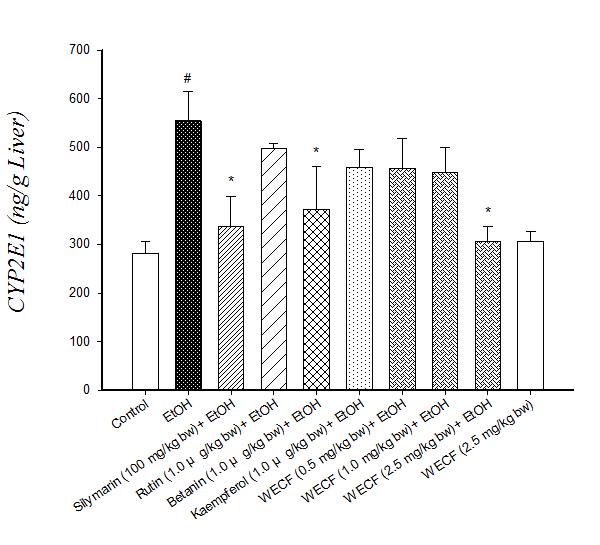
Effect of WECF on CYP2E1 activity: Figure 3 shows the effect of WECF on CYP2E1 activity in rats fed with ethanol. The results show that the ethanol group had higher CYP2E1 activity compared with the control group. Meanwhile, supplementation with WECF (2.5 mg/kg bw), silymarin (100 mg/kg bw) and betanin (1.0 μg/kg bw) significantly inhibited CYP2E1 activity in ethanol-consuming group.
Figure 3: Effects of water extracts of Djulis ( Chenopodium formosaneum) (WECF) and its bioactive compounds on CYP2E1 activity in the liver microsomes of rats treated with ethanol (EtOH).
Note: Values are means ± SD for six rats per group. #significant difference from the control group (p < 0.05). * Significant difference from the EtOH group (p < 0.05).
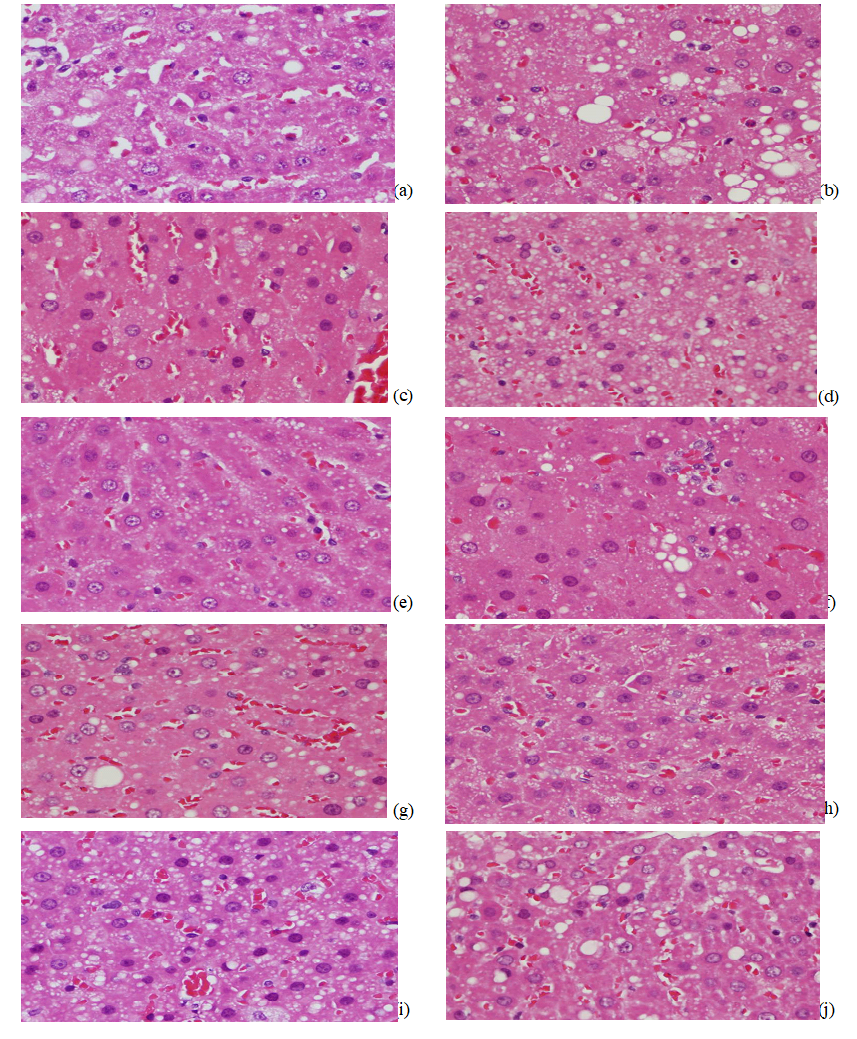
Effects on hepatic histology: Histological examination of liver provided supportive evidence for the biochemical analysis. Figure 4 shows the histology of the rat liver treated with ethanol. The histology of the liver sections of the control rat showed normal hepatic cells with well-preserved cytoplasm and slight fatty infiltration (Figure 4a). The liver sections of ethanol-consuming rat revealed severe fatty infiltration (Figure 4b). However, the histological hepatic lesions were significantly ameliorated by co-administration with WECF, silymarin, rutin, kaempferol and betanin (Figures 4c- 4i). Pretreatment of silymarin (Figure 4c), betanin (1.0 μg / kg bw, Figure 4e) and WECF at different concentrations (1.0 mg / kg bw, Figure 4h and 2.5 mg / kg bw, Figure 4i) noticeably attenuated fatty infiltration, showing only slight fatty infiltration almost similar to that in the control group. The results obtained suggested that WECF and its bioactive compounds, rutin, kaempferol and betanin might alleviate ethanol-induced liver damage.
Figure 4: Effects of water extracts of Djulis (Chenopodium formosaneum) (WECF) and its bioactive compounds on the liver histological damage after ethanol (EtOH) treatment in rats.
Note:(a) Control group; fatty infiltration, slight (b) EtOH group; fatty infiltration, diffuse, severe (c) Silymarin (100 mg/kg) + EtOH group; fatty infiltration, slight (d) Rutin (0.001 mg/kg) + EtOH group; fatty infiltration, diffuse, moderate (e) Betanin (0.001 mg/kg)) + EtOH group; fatty infiltration, slight (f) Kaempferol (0.001 mg/kg + EtOH group; fatty infiltration, moderate (g) WECF (0.5 mg/kg) + EtOH group; fatty infiltration, diffuse, moderate (h) WECF (1mg/kg) + EtOH group; fatty infiltration, diffuse, slight to moderate (i)WECF (2.5 mg/kg) + EtOH group; fatty infiltration, slight (j) WECF (2.5 mg/kg); fatty infiltration, slight. Magnification 400×.
Discussion
It is well known that alcohol consumption causes liver damage with the release of hepatic cellular enzymes such as AST and ALT into the circulatory system being a sign of hepatic injury. According to Table 2, there were no significant differences in AST and ALT activities in the serum of any group. However, there were trends toward lower AST and ALT activities in groups treated with silymarin, WECF at different concentrations and its bioactive compounds, rutin, kaempferol and betanin at 1.0 μg / kg bw compared with that in the ethanol-consuming group. These results suggest the occurrence of a compensatory hepatic response, which lessened ethanol-induced liver injury[21]. This probably explains why WECF and its bioactive compounds did not display any significant positive effect on AST and ALT activities. In addition, simple measurements of biochemical markers in serum or even urine cannot replace liver biopsy[22]. Therefore, in addition to AST and ALT activities in rats induced by ethanol, lipid peroxidation, GSH levels, antioxidant enzymes and CYP2E1 activity were measured and liver histopathology was observed.
GSH, an endogenous reductant and antioxidant, is well known to serve diverse biological function in cells. It has been reported that GSH is synthesized from glutamate, cysteine and glycine by γ-glutamylcysteine synthetase (γ-GCS) and glutathione synthetase (GS) taking place in the cytosol[23]. Many studies have revealed that some plant-derived compounds can increase γ-GCS activity and the consequent GSH levels[24]. As shown in Table 3, treatment with WECF at 2.5 mg / kg bw without ethanol consumption significantly increased GSH levels in rats, suggesting that WECF may up-regulate γ-GCS and GS activities, thus causing increase in GSH levels. This observation indicates that WECF may offer an indirect protection by regulating endogenous defense system. Several mechanisms resulting in a decrease in GSH levels as a consequence of ethanol intake have been reported[25]. However, according to the data in Table 3, ethanol consumption causes a marked increase in GSH levels. This result was in good agreement with the results of previous studies in showing that ethanol treatment increased liver GSH[26]. The possible results for increasing GSH levels in rats consumed with ethanol may be as a result of: (a) rebound synthesis of GSH: (b) conversion of homocysteine to GSH, or (c) mild cholestasis[26,27]. In addition, GSH is involved in the detoxication of many xenobiotics through the formation of S-conjugates with toxic metabolites in the second phase of biotransformation[28]. Apparently, the role of GSH as a reductant and a free radical scavenger may explain decrease of hepatic GSH levels in ethanol-consuming rats treated with WECF, rutin, kaempferol and beatnin, respectively[25]. These results show that an ethanol-consumption decrease in lipid peroxidation (Figure 2A) is associated with a decrease in GSH levels (Table 3).
CYP2E1 is responsible for alcoholic liver damage because it catalyzes alcohol and generates reactive oxygen species (ROS)[29]. Therefore, more rapid alcohol metabolism in hepatocytes results in less damage[30]. However, excessive alcohol consumption can lead to massive generation of ROS and creates a rapid increase in oxidative stress, thus causing liver dysfunction. Results obtained in this study reveal that ethanol consumption produces significant increase in CYP2E1 activity (Figure 3). Rats pretreated with WECF at 2.5 mg/kg bw and betanin at 1.0 μg / kg bw show similar effects produced by pretreatments with silymarin. Taken together, the results reveal that pretreatment of WECF prevents lipid peroxidation which may be attributed to suppression of CYP2E1 activity (Figure 3).
Alcohol dehydrogenase (ADH) in cytosol and catalase (CAT) in peroxisomes, in addition to CYP2E1, are the main enzymes that oxidize alcohol into acetaldehyde in hepatocytes, which is further metabolized into acetic acid by aldehyde dehydrogenase (ALDH)[30]. As shown in Table 3, ethanol consumption significantly decreased CAT activity while silymarin and WECF at different concentration increased CAT activity in ethanol-induced rats. WECF at 0.5, 1.0 and 2.5 mg / kg bw showed significant effects similar to that of silymarin, in enhancing CAT activity of rats with continuous ethanol consumption. When excessive ROS are produced due to lipid peroxidation and ethanol oxidation metabolized by CYP2E1 are produced, these ROS can be converted into H2O2 and O2, and H2O2 can subsequently be detoxified by CAT. This observation implies that WECF could provide protective effects against ethanol-induced liver damage through balancing CAT enzyme levels in the liver, reducing lipid peroxide formation and suppressing oxidative chain reaction[31]. Thus, the results evidenced that reduction of hepato toxicity due to ethanol exposure could be related to increased CAT activity.
Comet assay is a rapid and sensitive technique for demonstrating chemically induced DNA damage in eukaryotic cells. Comet assay results show that damage to the DNA strand in blood lymphocytes was significantly increased by ethanol consumption. However, there was also a significant decrease in ethanol-induced damage to the DNA strand after treatment with WECF, rutin, betanin, indicating a protective effect offered by the tested doses. Interestingly, pretreatment with WECF at 2.5 mg / kg bw without ethanol displays similar effect on the control group, suggesting WECF at the tested doses exerted no genotoxic damage on blood lymphocytes and no harmful effect on liver and kidney. That is to say, WECF ranging from 0.5 to 2.5 mg/kg bw was not toxic.
Many studies reported that rutin, kaempferol and betanin displayed considerable biological activities[6,32,33]. Results of the current study indicated of rutin, kaempferol and betanin are present in WECF. Rutin, kaempferol and betanin at a dose of 1.0 μg / kg bw inhibited TBARS formation in ethanol-induced rats. Moreover, rutin at 1.0 μg / kg bw restored SOD activity while betanin at 1.0 μg / kg bw suppressed of CYP2E1 activity in ethanol-induced rats. Kaempferol and betanin show no effect in restoring antioxidant enzymes back to the original levels in the control rats, although rutin at 1.0 μg / kg bw demonstrated marked restoration of SOD activity. These results suggest that 1.0 μg / kg bw of betanin and kaempferol did not significantly affect initial antioxidant enzymes. WECF contains biological compounds, such as betanin and other unidentified compounds, along with phenolics, which may contribute to the biological activity. In other words, these unidentified compounds in WECF could reduce hepato toxicity in ethanol-induced rat liver through a direct or a synergistic effect[9].
To the authors’ best knowledge, this study is the first report on protection offered by pretreatment with WECF against liver damage caused by chronic alcohol consumption. The protective effect of WECF against alcohol consumption may be associated with prevention of lipid peroxidation, restoration of CAT activity and inhibition of CYP2E1 activity. In addition, protection against liver damage caused by alcohol consumption may be partly associated with the bioactive WECF compounds. These observations may explain why WECF prevents hepato toxicity in rat liver treated with alcohol. According to the results obtained, WECF has potential to be developed as dietary supplement, used as functional food and applied in AFLD therapy. However, scientific trails in vivo are needed to confirm the results.
Acknowledgment: This research work was supported by research grants from the National Science Council of the Republic of China (NSC 102-2313-B-041-001-MY3)
References
- 1. Park, H.Y., Choi, H.D., Eom, H., et al. Enzymatic modification enhances the protective activity of citrus flavonoids against alcohol-induced liver disease. (2013) Food Chem 139(1-4): 231-240.
- 2. Chang, Y.Y., Lin, Y.L., Yang, D.J., et al. Hepatoprotection of noni juice against chronic alcohol consumption: lipid homeostasis, antioxidation, alcohol clearance, and anti-inflammation. (2013) J Agric Food Chem 61(46): 11016-11024.
- 3. Muriel, P., Rivera-Espinoza, Y. Beneficial drugs for liver diseases. (2008) J Appl Toxicol 28(2): 93-103.
- 4. Ness, A.R., Powles, J.W. Fruit and vegetables, and cardiovascular disease: a review. (1997) Int J Epidemiol 26(1): 1-13.
- 5. Bhargava, A., Shukla, S., Ohri, D. Chenopodium quinoa-An Indian perspective. (2006) Ind Crops Prod 23(1): 73-87.
- 6. Tsai, P.J., Sheu, C.H., Wu, P.H., et al. Thermal and pH stability of betacyanin pigment of Djulis (Chenopodium formosanum) in Taiwan and their relation to antioxidant activity. (2010) J Agric Food Chem 58(2): 1020-1025.
- 7. Chyau, C.C., Chu, C.C., Chen, S.Y., et al. Djulis (Chenopodiun formosaneum) and its bioactive compounds protect against oxidative stress in human HepG2 cells. (2015) J Funct Foods 18: 159-170.
- 8. Chu, C.C., Chen, S.Y., Chyau, C.C., et al. Protective effect of Djulis (Chenopodium formosanum) and its bioactive compounds against carbon tetrachloride-induced liver injury, in vivo. (2016) J Funct Foods 26: 585-597.
- 9. Chen, S.Y., Chyau, C.C., Chu, C.C., et al. Hepatoprotection using sweet orange peel and its bioactive compound, hesperidin, for CCl4-induced liver injury in vivo. (2013) J Funct Foods 5(4): 1591-1600.
- 10. Buege, J.A., Aust, S.D. Microsomal lipid peroxidation. (1978) Methods Enzymol 52: 302-310.
- 11. Szeto, Y.T., Chu, W.K., Benzie, I.F. Antioxidants in fruits and vegetables: a study of cellular availability and direct effects on human DNA. (2006) Biosci Biotechnol Biochem 70(10): 2551-2555.
- 12. Braz, M.G., Favero Salvadori, D.M. Influence of endogenous and synthetic female sex hormones on human blood cells in vitro studied with comet assay. (2007) Toxicol In Vitro 21(5): 972-976.
- 13. Onodera, K., Hanashiro, K., Yasumoto, T. Camellianoside, a novel antioxidant glycoside from the leaves of Camellia japonica. (2006) Biosci Biotechnol Biochem 70(8): 1995-1998.
- 14. Lin, L.Z., Chen, P., Ozcan, M., et al. Chromatographic profiles and identification of new phenolic components of Ginkgo bilobaleaves and selected products. (2008) J Agric Food Chem 56(15): 6671-6679.
- 15. Gouveia, S., Castilho, P.C. Characterisation of phenolic acid derivatives and flavonoids from different morphological parts of Helichrysum obconicum by a RP-HPLC–DAD-(−)–ESI-MSn method. (2011) Food Chem 129(2): 333-344.
- 16. Miyashita, M., Matsushita, K., Nakamura, S., et al. LC/MS/MS identification of 20-hydroxyecdysone in a scorpion (Liocheles australasiae) and its binding affinity to in vitro-translated molting hormone receptors. (2011) Insect Biochem Mol Biol 41(12): 932-937.
- 17. Simirgiotis, M.J. Antioxidant capacity and HPLC-DAD-MS profiling of Chilean peumo (Cryptocarya alba) fruits and comparison with German peumo (Crataegus monogyna) from southern Chile. (2013) Molecules 18(2): 2061-2080.
- 18. Elsadig Karar, M.G., Quiet, L., Ahmed, R., et al. Phenolic profile and in vitro assessment of cytotoxicity and antibacterial activity of Ziziphus spina-christi leaf extracts. (2016) Med Chem 6(3):143.
- 19. Kreft, I., Fabjan, N., Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products”. (2006) Food Chem 98(3): 508-512.
- 20. Chen, A.Y., Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. (2013) Food Chem 138(4): 2099-2107.
- 21. Chiu, W.C., Huang, Y.L., Chen, Y.L., et al. Synbiotics reduce ethanol-induced hepatic steatosis and inflammation by improving intestinal permeability and microbiota in rats. (2015) Food Funct 6(5): 1692-1700.
- 22. Gressner, O.A., Weiskirchen, R., Gressner, A.M. Biomarkers of liver fibrosis: Clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. (2007) Acta Chim Sinica 381(2): 107-113.
- 23. Shi, Y., Kovacs-Nolan, J., Jiang, B., et al. Peptides derived from eggshell membrane improve antioxidant enzyme activity and glutathione synthesis against oxidative damage in Caco-2 cells. (2014) J Funct Foods 11: 571-580.
- 24. Masella, R., Di Benedetto, R., Vari, R., et al. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. (2005) J Nutr Biochem 16(10): 577-586.
- 25. Assuncao, M., Santos-Marques, M.J., Monteiro, R., et al. Red wine protects against ethanol-induced oxidative stress in rat liver. (2009) J Agric Food Chem 57(14): 6066-6073.
- 26. Kerai, M.D.J., Waterfield, C.J., Kenyon, S.H., et al. Reversal of ethanol-induced hepatic steatosis and lipid peroxidation by taurine: a study in rats. (1999) Alcohol Alcohol 34(4): 529-541.
- 27. Yan, C.C., Bravo, E., Cantafora, A. Effect of taurine levels on liver lipid metabolism: an in vivo study in the rat. (1993) Proc Soc Exp Biol Med 202(1): 88-96.
- 28. Fernandez-Iglesias, A., Quesada, H., Diaz, S., et al. Combination of grape seed proanthocyanidin extract and docosahexaenoic acid-rich oil increases the hepatic detoxification by GST mediated GSH conjugation in a lipidic postprandial state. (2014) Food Chem 165: 14-20.
- 29. Huang, B., Ban, X., He, J., et al. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. (2010) Food Chem 120(3): 873-878.
- 30. Wu, M.T., Tzang, B.S., Chang, Y.Y., et al. Effects of Antrodia camphorata on alcohol clearance and antifibrosis in livers of rats continuously fed alcohol. (2011) J Agric Food Chem 59(8): 4248-4254.
- 31. Han, Y., Xu, Q., Hu, J.N., et al. Maltol, a food flavoring agent, attenuates acute alcohol-induced oxidative damage in mice. (2015) Nutrients 7(1): 682-696.
- 32. Vulić, J., Čanadanović-Brunet, J., Ćetković, G., et al. Antioxidant and cell growth activities of beet root pomace extracts. (2012) J Funct Foods 4(3): 670-678.
- 33. Wang, J.W., Chen, X.Y., Hu, P.Y., et al. Effects of Linderae radix extracts on a rat model of alcoholic liver injury. (2016) Exp Ther Med 11(6): 2185-2192.
Pubmed||Crossref||other