Evaluation of the Physicochemical and Thermal Properties of the Biofield Energy Healing Treated Ofloxacin
Alice Branton, Mahendra Kumar Trivedi, Dahryn Trivedi, Gopal Nayak,
Affiliation
1Trivedi Global, Inc., Henderson, USA
2Trivedi Science Research Laboratory Pvt Ltd., Bhopal, India
Corresponding Author
Snehasis Jana, Trivedi Science Research Laboratory Pvt Ltd, Bhopal, India, Tel: +91-022-25811234; Email: publication@trivedieffect.com
Citation
Alice, B., et al. Evaluation of the Physicochemical and Thermal Properties of the Biofield Energy Healing Treated Ofloxacin. (2018) J Pharm Pharmaceutics 5(2): 80- 87.
Copy rights
© 2018 Alice, B. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Ofloxacin; The Trivedi Effect®; Consciousness energy healing treatment; Complementary and alternative medicine; PXRD; Particle size; DSC; TGA / DTG
Abstract
Ofloxacin is a broad-spectrum antibiotic useful for the treatment of many Gram-positive and Gram-negative bacterial infections. The objective of this research work was to evaluate the impact of the Trivedi Effect® on the physicochemical and thermal properties of ofloxacin using modern analytical techniques. The sample was divided into two parts. One part of ofloxacin was considered as the control sample (no biofield energy treatment was provided), whereas the second part was received the Biofield Treatment remotely by a famous Biofield Energy Healer, Alice Branton and was termed as the treated sample. The PXRD peak intensities and crystallite sizes were significantly altered ranging from 7.45% to 73.06% and -44.1% to 77.95%, respectively in the treated sample compared to the control sample. The particle size values were significantly altered at d10 (-19.47%), d50 (1.14%), d90 (-8.70%), and D (4, 3) (-10.20%); whereas, the specific surface area was significantly increased by 15.66% in the treated sample compared to the control sample. The latent heat of fusion and latent heat of decomposition of the treated ofloxacin were significantly increased by 16.24% and 88.03%, respectively compared with the control sample. The total weight loss was significantly decreased by 6.21%; additionally, the residue amount was significantly increased by 47.16% in the treated ofloxacin compared with the control sample. Thus, the Trivedi Effect® might generate a new polymorphic form of ofloxacin which would be more soluble, bioavailable, and be thermally more stable compared with the untreated sample. The Biofield Treated ofloxacin would be more efficacious against urinary tract infections, infections of the urethra and cervix, infectious diarrhoea, pneumonia, cellulitis, chronic bronchitis, prostatitis, plague, etc.
Introduction
Ofloxacin is a broad-spectrum antibiotic useful for the treatment of a number of both Gram-positive and Gram-negative bacterial infections[1]. It acts by inhibiting DNA gyrase (a type II topoisomerase and topoisomerase IV) which separate the replicated DNA, thereby inhibiting bacterial cell division[2]. It is used for the treatment of urinary tract infections, infections of the urethra and cervix (i.e., gonorrhea), infectious diarrhoea, pneumonia, cellulitis, chronic bronchitis, prostatitis, plague, multidrug-resistant tuberculosis, bacterial infection of the eye and ear, otitis media when there is a hole in the eardrum, etc[1,3,4]. Ofloxacin is the fluoroquinolone family of medications, and the common side effects of it include a headache, vomiting, diarrhoea, tendon rupture, numbness, skin rash, seizures, psychosis, etc[1]. Ofloxacin may inhibit drug metabolizing enzymes and thereby increase blood levels of other drugs such as theophylline, cyclosporine, warfarin, etc. The fluoroquinolones have shown increasing the anticoagulant, cardiotoxicity, and arrhythmias effect when co-administered with drugs such as acenocoumarol, dihydroquinidine, barbiturate, etc[4,5]. Ofloxacin has a short biological half-life, and its bioavailability is strongly dependent on the physiological condition of the gastrointestinal tract. It is highly soluble in acidic media and precipitates in alkaline media thus losing its solubility[4].
Since, the physicochemical properties of a pharmaceutical compound play a crucial role in its dissolution, absorption, and bioavailability profile in the body[6], many research works are carrying out throughout the globe by the researchers to improve these parameters of the pharmaceuticals or nutraceuticals in the formulations. In this scenario, it has been observed that the Trivedi Effect®- Biofield Energy Healing Treatment has a significant impact on various properties such as particle size, surface area, and other physicochemical properties of pharmaceutical/nutraceutical compounds[7-10]. The Trivedi Effect® is a natural and is the only scientifically proven phenomenon in which a person can harness this inherently intelligent energy and transmit it anywhere on the planet through the possible mediation of neutrinos[11]. Every living organism possesses this unique energy field surrounding the body called the “Biofield”, which is infinite and para-dimensional electromagnetic field. The “Biofield” (Putative Energy Fields) based Energy Healing Therapies have been reported to have significant beneficial outcomes against various disease conditions[12]. The National Institutes of Health / National Center for Complementary and Alternative Medicine (NIH / NCCAM) recommend and included the Energy therapy under the Complementary and Alternative Medicine (CAM) category along with naturopathy, homeopathy, Ayurvedic medicine, essential oils, traditional Chinese herbs and medicines, massage, acupuncture, acupressure, yoga, meditation, healing touch, Reiki, hypnotherapy, Tai Chi, Qi Gong, deep breathing, special diets, relaxation techniques, aromatherapy, guided imagery, chiropractic/osteopathic manipulation, movement therapy, pilates, Rolfing structural integration, mindfulness, cranial sacral therapy, and applied prayer. The most of the U.S. population has accepted the CAM with several advantages[13,14]. The Trivedi Effect® (Biofield Energy Healing Treatment) has been widely reported with astounding capability to alter the characteristic properties of the several living and non-living object(s), i.e., agricultural plants[15], microorganisms[16], live stocks[17], metals and ceramics[18], and organic compounds[19]. The Trivedi Effect® has also enhanced the bioavailability of pharmaceutical / nutraceutical compounds[20,21]. This study the impact of the Trivedi Effect®- Consciousness Energy Healing Treatment on the physicochemical, and thermal properties of ofloxacin using particle size analysis (PSA), powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC), and thermo gravimetric analysis (TGA) / Differential thermo gravimetric analysis (DTG).
Materials and Methods
Chemicals and Reagents
The ofloxacin was purchased from Sigma Aldrich, USA. All other chemicals substances used during the experiments were of the analytical standard available in India.
Consciousness energy healing treatment strategies: The test sample (ofloxacin) was divided into two parts. One part of ofloxacin was considered as a control sample (no Biofield Energy Treatment was provided). However, the second part of ofloxacin was treated with the Consciousness Energy Healing Treatment remotely under standard laboratory conditions for 3 minutes. This sample known as the Trivedi Effect® Treated or the Biofield Energy Treated ofloxacin sample. The Biofield Treatment was provided through the healer’s unique energy transmission process by the famous Biofield Energy Healer, Alice Branton, USA, to one part of the test sample. Further, the control sample was treated with a “sham” healer. The “sham” healer did not have any information about the Biofield Energy Treatment. After treatment, the Biofield Energy Treated and untreated samples were kept in sealed conditions and characterized using modern analytical techniques.
Characterization
Powder X-ray diffraction (PXRD) analysis: The PXRD analysis of ofloxacin was performed with the help of Rigaku MiniFlex-II Desktop X-ray diffractometer (Japan)[22,23]. The average size of individual crystallites was calculated from XRD data using the Scherrer’s formula (1)
G = kλ / βcosθ (1)
Where k is the equipment constant (0.94), G is the crystallite size in nm, λ is the radiation wavelength (0.154056 nm for Kα1 emission), β is the full-width at half maximum (FWHM), and θ is the Bragg angle[24].
The percent change in crystallite size (G) of ofloxacin was calculated using the following equation 2:
Where GControl and GTreated are the crystallite size of the control and the Biofield Energy Treated samples, respectively.
Particle size analysis (PSA): The particle size analysis of ofloxacin was conducted on Malvern Mastersizer 2000, from the UK with a detection range between 0.01 μm to 3000 μm using wet method[25,26].
The percent change in particle size (d) for ofloxacin at below 10% level (d10), 50% level (d50), 90% level (d90), and D (4,3) was calculated using the following equation 3:
Where dControl and dTreated are the particle size (μm) for at below 10% level (d10), 50% level (d50), and 90% level (d90) of the control and the Biofield Energy Treated samples, respectively.
The percent change in surface area (S) was calculated using the following equation 4:
Where SControl and STreated are the surface area of the control and the Biofield Energy Treated ofloxacin, respectively.
Differential scanning calorimetry (DSC): The DSC analysis of ofloxacin was performed with the help of DSC Q200, TA instruments. The sample of ~1 - 2 mg was loaded into the aluminium sample pan at a heating rate of 10°C / min from 30°C to 350°C[25,26]. The % change in melting point (T) was calculated using the following equation 5:
Where, TControl and TTreated is the melting point of the control and treated samples, respectively.
Percent change in the latent heat of fusion (ΔH) was calculated using the following equation 6:
Where, ΔHControl and ΔHTreated are the latent heat of fusion of the control and treated ofloxacin, respectively.
Thermal gravimetric analysis (TGA) / Differential thermogravimetric analysis (DTG): TGA / DTG thermograms of ofloxacin were obtained with the help of TGA Q50 TA instruments. A sample of ~3 - 6 mg was loaded to the platinum crucible at a heating rate of 10°C / min from 25°C to 1000°C with the recent
literature[25,26]. The % change in weight loss (W) was calculated using the following equation 7:
Where WControl and WTreated are the weight loss of the control and the Biofield Energy Treated ofloxacin, respectively.
The % change in maximum thermal degradation temperature (Tmax) (M) was calculated using the following equation 8:
Where MControl and MTreated are the Tmax values of the control and the Biofield Energy Treated ofloxacin, respectively.
Results and Discussion
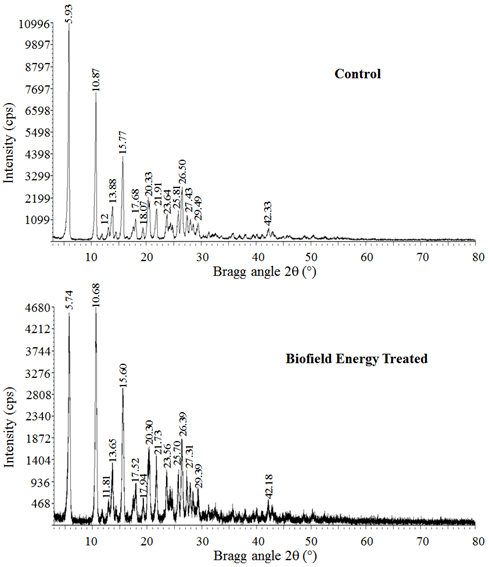
Powder X-ray diffraction (PXRD) analysis : The PXRD diffractograms of the control ofloxacin shown sharp and intense peaks at Bragg’s angle (2q) equal to 5.93°, 10.87°, 12°, 13.88°, 14.52°, 15.77°, 17.68°, 18.07°, 19.44°, 20.33°, 21.91°, 23.67°, 25.81°, 26.5°, 27.43°, 28.52°, 29.49°, 32.74°, 35.88°, 38.06°, 42.33°, 42.91°, 48.79°, and 50.35° (Figure 1). Similarly, the biofield energy treated ofloxacin also shown sharp and intense peaks at Bragg’s angle (2q) equal to 5.74°, 10.68°, 11.81°, 13.65°, 14.29°, 15.6°, 17.52°, 17.94°, 19.16°, 20.3°, 21.73°, 23.56°, 25.7°, 26.39°, 27.31°, 28.31°, 29.39°, 32.4°, 35.63°, 37.88°, 42.18°, 42.77°, 48.53°, and 50.22° (Figure 1). The sharp and intense peaks indicated that both the samples were crystalline. The PXRD diffractograms of the control and the biofield energy treated samples shown the highest peak intensity at 2θ equal to 5.93° and 5.74°, respectively (Table 1, entry 1). All the peak intensities of the Biofield Energy Treated ofloxacin were significantly decreased from 7.45% to 73.06% compared to the control sample. The crystallite sizes of the biofield energy treated ofloxacin sample were significantly altered ranging from - 44.1% to 77.95% compared to the control sample. Overall, the average crystallite size of the biofield energy treated ofloxacin (264.8 nm) was decreased by 0.45% compared with the control sample (266 nm).
The variations in the crystallite sizes and intensities indicated the modification of the crystal morphology of the biofield energy treated ofloxacin compared to the control sample. The peak intensity of each diffraction face on the crystalline compound changes according to the crystal morphology[27] and alterations in the PXRD pattern provide the proof of polymorphic transitions[28,29]. The Trivedi Effect®- Consciousness Energy Healing Treatment probably produced a new polymorphic form of ofloxacin through the Biofield Energy via neutrino oscillations[11]. Different polymorphic forms of pharmaceuticals/nutraceuticals have the significant effects on the drug performance, i.e., therapeutic efficacy, bioavailability, and toxicity, because of their thermodynamic and physicochemical properties like energy, melting point, stability, and especially solubility, are different (probably the improvement) from the original form[30,31]. Thus, it can be anticipated that the Trivedi Effect® Treated ofloxacin would be more efficacious in the pharmaceutical formulations containing ofloxacin.
Figure 1: PXRD diffractograms of the control and the Biofield Energy Treated ofloxacin.
Table 1: PXRD data for the control and the Biofield Energy Treated ofloxacin.
| Entry No. | Bragg angle (°2q) | Peak Intensity (%) | Crystallite size (G, nm) | |||||
|---|---|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | % change a | Control | Treated | % change b | |
| 1 | 5.93 | 5.74 | 2244 | 1195 | -46.75 | 460 | 277 | -39.78 |
| 2 | 10.87 | 10.68 | 1494 | 1037 | -30.59 | 305 | 238 | -21.97 |
| 3 | 12.00 | 11.81 | 41 | 27 | -34.15 | 431 | 345 | -19.95 |
| 4 | 13.88 | 13.65 | 382 | 330 | -13.61 | 227 | 237 | 4.41 |
| 5 | 14.52 | 14.29 | 59 | 39 | -33.90 | 306 | 361 | 17.97 |
| 6 | 15.77 | 15.60 | 953 | 669 | -29.80 | 255 | 241 | -5.49 |
| 7 | 17.68 | 17.52 | 149 | 77 | -48.32 | 228 | 286 | 25.44 |
| 8 | 18.07 | 17.94 | 243 | 161 | -33.74 | 257 | 245 | -4.67 |
| 9 | 19.44 | 19.16 | 115 | 68 | -40.87 | 288 | 161 | -44.10 |
| 10 | 20.33 | 20.30 | 673 | 472 | -29.87 | 180 | 177 | -1.67 |
| 11 | 21.91 | 21.73 | 400 | 276 | -31.00 | 258 | 264 | 2.33 |
| 12 | 23.67 | 23.56 | 516 | 139 | -73.06 | 242 | 358 | 47.93 |
| 13 | 25.81 | 25.70 | 210 | 192 | -8.57 | 346 | 295 | -14.74 |
| 14 | 26.50 | 26.39 | 579 | 398 | -31.26 | 253 | 260 | 2.77 |
| 15 | 27.43 | 27.31 | 201 | 154 | -23.38 | 301 | 324 | 7.64 |
| 16 | 28.52 | 28.31 | 132 | 109 | -17.42 | 250 | 240 | -4.00 |
| 17 | 29.49 | 29.39 | 165 | 112 | -32.12 | 228 | 256 | 12.28 |
| 18 | 32.74 | 32.40 | 94 | 87 | -7.45 | 96 | 120 | 25.00 |
| 19 | 35.88 | 35.63 | 75 | 41 | -45.33 | 195 | 347 | 77.95 |
| 20 | 38.06 | 37.88 | 46 | 25 | -45.65 | 303 | 322 | 6.27 |
| 21 | 42.33 | 42.18 | 125 | 69 | -44.80 | 223 | 288 | 29.15 |
| 22 | 42.91 | 42.77 | 101 | 54 | -46.53 | 164 | 201 | 22.56 |
| 23 | 48.79 | 48.53 | 38 | 19.8 | -47.89 | 372 | 323 | -13.17 |
| 24 | 50.35 | 50.22 | 46 | 32 | -30.43 | 216 | 189 | -12.50 |
| 25 | Average crystallite size | 266 | 264.8 | -0.45 | ||||
adenotes the percentage change in the intensity of the Biofield Energy Treated sample with respect to the control sample;
bdenotes the percentage change in the crystallite size of the Biofield Energy Treated sample with respect to the control sample.
Particle size analysis (PSA): The PSD analysis of both the control and the Biofield Energy Treated ofloxacin were performed, and the data are presented in Table 2. The particle size values of the control ofloxacin at d10, d50, d90 and D (4, 3) were 2.62 μm, 16.61 μm, 179.68 μm, and 60.70 μm, respectively. Similarly, the particle sizes of the biofield energy treated ofloxacin at d10, d50, d90 and D(4,3) were 2.11 μm, 16.80 μm, 164.05 μm, and 54.51 μm, respectively. Therefore, the particle size values in Alice’s Biofield Energy Treated ofloxacin were significantly decreased at d10, d90, and D (4, 3) by 19.47%, 8.70%, and 10.20%, respectively, whereas slightly increased at d50 by 1.14 compared to the control sample. The specific surface area of the biofield energy treated ofloxacin (1.13 m2 / g) was significantly increased by 15.66% compared with the control sample (0.977 m2/g). Hence, it can be assumed that due to the Trivedi Effect® - Consciousness Energy Healing Treatment the particle sizes of ofloxacin were reduced. As per the literature report pharmaceutical compounds on reducing the particle size increase the surface area and improve the solubility, dissolution rate, and bioavailability in the physiological system[6,32]. Ofloxacin slightly soluble in water, alcohol, dichloromethane, and methyl alcohol; sparingly soluble in chloroform[33]. Thus, it is anticipated that the Trivedi Effect® Treated ofloxacin might offer better solubility and bioavailability compared with the untreated sample.
Table 2: Particle size distribution of the control and the Biofield Energy Treated ofloxacin.
| Parameter | d10 (μm) | d50 (μm) | d90 (μm) | D(4,3) (μm) | SSA (m2/g) |
|---|---|---|---|---|---|
| Control | 2.62 | 16.61 | 179.68 | 60.70 | 0.977 |
| Biofield Treated | 2.11 | 16.80 | 164.05 | 54.51 | 1.13 |
| Percent change* (%) | -19.47 | 1.14 | -8.70 | -10.20 | 15.66 |
d10, d50, and d90: particle diameter corresponding to 10%, 50%, and 90% of the cumulative distribution, D (4, 3): the average mass-volume diameter, and SSA: the specific surface area. *denotes the percentage change in the Particle size distribution of the biofield energy treated sample with respect to the control sample.
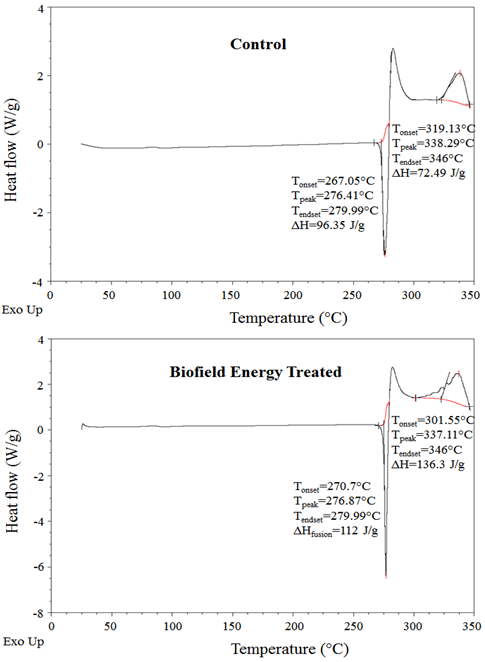
Differential scanning calorimetry (DSC) analysis: DSC analysis of both control and the biofield energy treated ofloxacin were performed, and the thermograms are presented in Figure 2. The DSC thermograms of the control and the biofield energy treated ofloxacin showed a sharp endothermic peak at 276.41°C and 276.87°C, respectively. The thermogram pattern and melting point closely matched to the reported data[34]. The melting point of the biofield energy treated ofloxacin was slightly increased by 0.17% compared with the control sample (Table 3). Similarly, the control and the biofield energy treated samples showed exothermic peaks at 338.29°C and 337.11°C, respectively (Figure 2). The decomposition temperature of the biofield energy treated ofloxacin was slightly decreased by 0.35% compared with the control sample (Table 3). The melting and decomposition temperatures did not alter significantly.
Figure 2: DSC thermograms of the control and the Biofield Energy Treated ofloxacin.
Table 3: DSC data for both control and the Biofield Energy Treated samples of ofloxacin.
| Sample | Melting Temp (°C) | Decomposition Temp (°C) | ΔH (J/g) | |
|---|---|---|---|---|
| Melting | Decomposition | |||
| Control Sample | 276.41 | 338.29 | 96.35 | 72.49 |
| Biofield Energy Treated | 276.87 | 337.11 | 112.00 | 136.30 |
| % Change* | 0.17 | -0.35 | 16.24 | 88.03 |
ΔH: Latent heat of fusion / decomposition, *denotes the percentage change of the Biofield Energy Treated ofloxacin with respect to the control sample.
The latent heat of fusion (ΔHfusion) of the biofield energy treated ofloxacin (112 J/g) was significantly increased by 16.24% compared with the control sample (96.35 J/g) (Table 3). Similarly, the latent heat of decomposition (ΔHdecomposition) of the biofield energy treated ofloxacin (136.3 J/g) was significantly increased by 88.03% compared with the control sample (72.49 J/g) (Table 3). The change in the latent heat of fusion can be attributed to the disrupted molecule chains and the crystal structure[35]. Thus, it can be predicted that the Trivedi Effect®- consciousness energy healing treatment might be responsible for the disruption the molecular chains and crystal structure of ofloxacin which was the cause of improved thermal stability of the treated sample compared with the control sample.
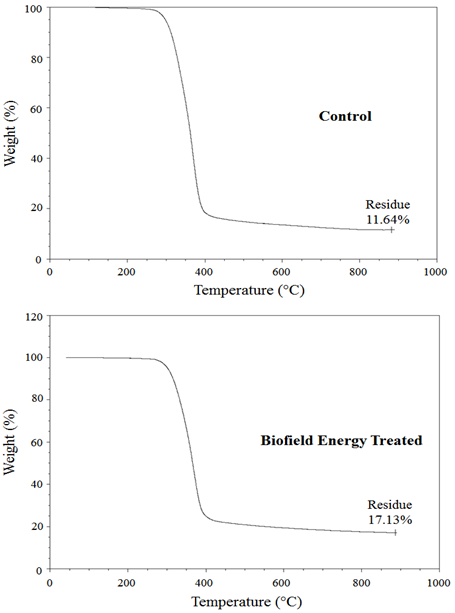
Thermal gravimetric analysis (TGA) / Differential thermogravimetric analysis (DTG): The TGA thermograms of the control and the biofield energy treated samples displayed one step of thermal degradation (Figure 3). The total weight loss in the biofield energy treated ofloxacin was significantly decreased by 6.21% compared with the control sample (Table 4). Therefore, the residue amount was significantly increased by 47.16% in the Biofield Energy Treated ofloxacin compared to the control sample (Table 4).
Table 4: TGA / DTG data of the control and the Biofield Energy Treated samples of ofloxacin.
| Sample | TGA | DTG | |
|---|---|---|---|
| Total weight loss (%) | Residue % | Tmax (°C) | |
| Control | 88.36 | 11.64 | 368.93 |
| Biofield Energy Treated | 82.87 | 17.13 | 368.91 |
| % Change* | -6.21 | 47.16 | -0.01 |
*denotes the percentage change of the Biofield Energy Treated sample with respect to the control sample, Tmax = the temperature at which maximum weight loss takes place in TG or peak temperature in DTG.
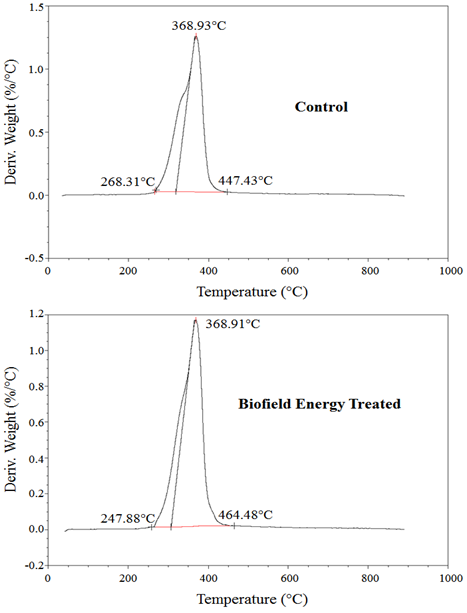
The DTG thermograms of the control and the biofield energy treated ofloxacin similarly shown only one peak (Figure 4). The Tmax of the biofield energy treated sample did not alter compared with the control sample (Table 4). Overall, TGA / DTG revealed that the thermal stability of the biofield energy treated ofloxacin was improved compared with the control sample.
Figure 3: TGA thermograms of the control and the Biofield Energy Treated ofloxacin.
Figure 4: DTG thermograms of the control and the Biofield Energy Treated ofloxacin.
Conclusion
The Trivedi Effect®-Consciousness Energy Healing Treatment has shown a significant effect on the crystallite size, particle size, surface area, and thermal properties of the treated ofloxacin. The PXRD data reported that all the peak intensities of the Biofield Energy Treated ofloxacin were significantly decreased from 7.45% to 73.06% compared to the control sample. Similarly, the crystallite sizes of the Biofield Energy Treated sample were significantly altered ranging from -44.1% to 77.95% compared to the control sample. The particle size values in the biofield energy treated ofloxacin were significantly decreased at d10, d90, and D (4, 3) by 19.47%, 8.70%, and 10.20% respectively, whereas slightly increased at d50 by 1.14 compared to the control sample. The specific surface area of the biofield energy treated ofloxacin was significantly increased by 15.66% compared to the control sample. The DSC data revealed that the ΔHfusion and ΔHdecomposition in the biofield energy treated ofloxacin were significantly increased by 16.24% and 88.03%, respectively compared with the control sample. The total weight loss was significantly decreased by 6.21%; therefore the residue amount was significantly increased by 47.16% in the biofield energy treated ofloxacin compared with the control sample. Thus, the Trivedi Effect®-Consciousness Energy Healing Treatment might lead to generate a new polymorphic form of ofloxacin which would be more soluble, bioavailable, and thermally stable compared with the untreated sample. The Trivedi Effect®- Consciousness Energy Healing Treated ofloxacin would be very useful in designing better pharmaceutical formulations that might offer better therapeutic response against urinary tract infections, infections of the urethra and cervix (i.e., gonorrhea), infectious diarrhoea, pneumonia, cellulitis, chronic bronchitis, prostatitis, plague, multidrug-resistant tuberculosis, bacterial infection of the eye and ear, otitis media when there is a hole in the eardrum, etc.
Acknowledgements
The authors are grateful to Central Leather Research Institute, SIPRA Lab. Ltd., Trivedi Science, Trivedi Global, Inc., Trivedi Testimonials, and Trivedi Master Wellness for their assistance and support during this work.
References
- 1. Ofloxacin. The American Society of Health-System Pharmacists. (2018).
Pubmed|| Crossref|| Others
- 2. Drlica, K., Zhao, X. DNA gyrase, topoisomerase IV, and the 4-quinolones. (1997) Microbiol Mol Biol Rev 61(3): 377-392.
- 3. national formulary (69 Ed.). (2015) British Med Asso 757-782.
Pubmed|| Crossref|| Others
- 4. Monk, J.P., Campoli-Richards, D.M. Ofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. (1987) Drugs 33: 346-391.
Pubmed|| Crossref|| Others
- 5. Van der, L., Sturkenboom, M.C., Herings, R.M., et al. Increased risk of achilles tendon rupture with quinolone antibacterial use, especially in elderly patients taking oral corticosteroids. (2003) Arch Intern Med 163(15): 1801-1807.
- 6. Chereson, R. Bioavailability, bioequivalence, and drug selection. (2009) (1st Edn) Pharmaceutical Press London.
Pubmed|| Crossref|| Others
- 7. Trivedi, M.K., Tallapragada, R.M., Branton, A., et al. Physicochemical characterization of biofield energy treated calcium carbonate powder. (2015) American J Heal Res 3(6): 368-375.
- 8. Trivedi, M.K., Tallapragada, R.M., Branton, A., et al. Evaluation of biofield energy treatment on physical and thermal characteristics of selenium powder. (2015) J Food Nut Sci 3(6): 223-228.
- 9. Trivedi, M.K., Patil, S., Shettigar, H., et al. An impact of biofield treatment on spectroscopic characterization of pharmaceutical compounds. (2015) Mod Chem Appl 3: 159.
- 10. Trivedi, M.K., Branton, A., Trivedi, D., et al. Fourier transform infrared and ultraviolet-visible spectroscopic characterization of biofield treated salicylic acid and sparfloxacin. (2015) Nat Prod Chem Res 3: 186.
- 11. Trivedi, M.K., Mohan, T.R.R. Biofield energy signals, energy transmission and neutrinos. (2016) Amer J Mod Phy 5(6): 172-176.
- 12. Rubik, B., Muehsam, D., Hammerschlag, R., et al. Biofield science and healing: history, terminology, and concepts. (2015) Glob Adv Health Med 4: 8 -14.
- 13. Barnes, P.M., Bloom, B., Nahin, R.L. Complementary and alternative medicine use among adults and children: United States, 2007. (2008) Natl Health Stat Report 12: 1-23.
Pubmed|| Crossref|| Others
- 14. Koithan, M. Introducing complementary and alternative therapies. (2009) J Nurse Pract. 5(1): 18-20.
- 15. Trivedi, M.K., Branton, A., Trivedi, D., et al. Effect of biofield energy treatment on chlorophyll content, pathological study, and molecular analysis of cashew plant (Anacardium occidentale L.). (2015) J Plant Sci 3(6): 372-382.
Pubmed|| Crossref|| Others
- 16. Trivedi, M.K., Patil, S., Shettigar, H., et al. In vitro evaluation of biofield treatment on Enterobacter cloacae: Impact on antimicrobial susceptibility and biotype. (2015) J Bacteriol Parasitol 6: 241.
- 17. Trivedi, M.K., Branton, A., Trivedi, D., et al. Effect of biofield treated energized water on the growth and health status in chicken (Gallus gallus domesticus). (2015) Poult Fish Wildl Sci 3: 140.
Pubmed|| Crossref|| Others
- 18. Trivedi, M.K., Nayak, G., Patil, S., et al. Characterization of physical and structural properties of brass powder after biofield treatment. (2015) J Powder Metall Min 4: 134.
- 19. Trivedi, M.K., Branton, A., Trivedi, D., et al. Impact of biofield treatment on spectroscopic and physicochemical properties of p-nitroaniline. (2015) Insights Anal Electrochem 1: 1-8.
- 20. Branton, A., Jana, S. Effect of the biofield energy healing treatment on the pharmacokinetics of 25-hydroxyvitamin D3 [25(OH)D3] in rats after a single oral dose of vitamin D3. (2017) American J Phar Phy 2(1): 11-18.
Pubmed|| Crossref|| Others
- 21. Branton, A., Jana, S. The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male Sprague Dawley rats. (2017) Int J Clin Devel Ana 3(3): 9-15.
Pubmed|| Crossref|| Others
- 22. Desktop X-ray Diffractometer “MiniFlex+”. (1997) The Rigaku J 14: 29-36.
Pubmed|| Crossref|| Others
- 23. Zhang, T., Paluch, K., Scalabrino, G., et al. Molecular structure studies of (1S,2S)-2-benzyl-2,3-dihydro-2-(1Hinden-2-yl)-1H-inden-1-ol. (2015) J Mol Struct 1083: 286-299.
Pubmed|| Crossref|| Others
- 24. Langford, J.I., Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. (1978) J Appl Cryst 11(2): 102-113.
- 25. Trivedi, M.K., Sethi, K.K., Panda, P., et al. A comprehensive physicochemical, thermal, and spectroscopic characterization of zinc (II) chloride using Xray diffraction, particle size distribution, differential scanning calorimetry, thermogravimetric analysis/differential thermogravimetric analysis, ultraviolet-visible, and Fourier transforminfrared spectroscopy. (2017) Int J Pharm Investig 7(1): 33-40.
- 26. , M.K., Sethi, K.K., Panda, P., et al. Physicochemical, thermal and spectroscopic characterization of sodium selenate using XRD, PSD, DSC, TGA/DTG, UV-vis, and FT-IR. (2017) Mar Pharmal J 21: 311-318.
- 27. Inoue, M., Hirasawa, I. The relationship between crystal morphology and XRD peak intensity on CaSO4.2H2O. (2013) J Crystal Growth 380: 169-175.
- 28. Raza, K., Kumar, P., Ratan, S., et al. Polymorphism: The phenomenon affecting the performance of drugs. (2014) SOJ Pharm Pharm Sci 1(2): 10.
- 29. Brittain, H.G. Polymorphism in pharmaceutical solids. (2009) Drugs and Pharmaceutical Sciences 2nd edt 192.
Pubmed|| Crossref|| Others
- 30. Censi, R., Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. (2015) Molecules 20(10): 18759-18776.
- 31. Blagden, N., de Matas, M., Gavan, P.T., et al. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. (2007) Adv Drug Deliv Rev 59(7): 617-630.
- 32. Zhao, Z., Xie, M., Li, Y., et al. Formation of curcumin nanoparticles via solution-enhanced dispersion by supercritical CO2. (2015) Int J Nanomedicine 10: 3171-3181.
- 33. Al-Omar, M.A., Ofloxacin In Harry G. Brittain editor: Profiles of Drug Substances, Excipients, and Related Methodology. (2009) Burlington: Academic Press 34: 265-298.
Pubmed|| Crossref|| Others
- 34. Gulkari, V.D., Bakhle, S.S., Yelane, L.S. Development and evaluation of ofloxacin floating tablets using natural polymer: Sterculia foetida linn Gum. (2016) Int J Pharm Pharm Sci. 8(5): 356-360.
Pubmed|| Crossref|| Others
- 35. Zhao, Z., Xie, M., Li, Y., et al. Formation of curcumin nanoparticles via solution-enhanced dispersion by supercritical CO2. (2015) Int J Nanomedicine 10: 3171-3181.