Flavonoids as Potential Wnt/β-Catenin Signaling Modulators for Colorectal Cancer Prevention
Omnia E. Hussein
Affiliation
Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt
Corresponding Author
Ayman M. Mahmoud, Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, 62514, Beni-Suef, Egypt, Tel.: +2011-4416-8280; E-mail: ayman.mahmoud@science.bsu.edu.eg
Citation
Mahmoud, A.M., et al. Flavonoids as Potential Wnt/β-Catenin Signaling Modulators for Colorectal Cancer Prevention. (2016) Int J Food Nutr Sci 3(1): 210-212.
Copy rights
© 2016 Mahmoud, A.M. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Flavonoids; Signaling modulators; Colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the widespread cancers accounting for approximately one million of new cancer cases worldwide[1]. It is the third most common cancer and number one cause of nonsmoking cancer-related deaths[2]. CRC progresses from aberrant crypt foci (ACF) to adenocarcinoma over long periods of time. Current treatment of CRC consists mostly of conventional chemotherapy and surgery, which confers limited benefits[3]. Therefore, finding strategies to counteract CRC becomes a necessity.
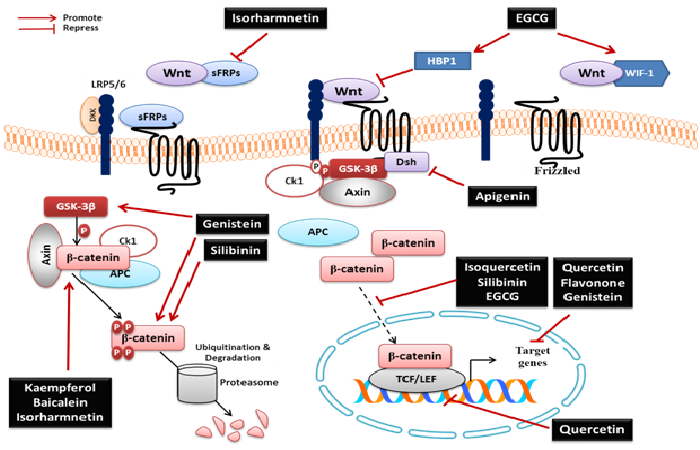
To date, several signaling pathway alterations in CRC have been characterized. The most commonly altered signaling pathway in CRC is the Wnt/β-catenin, where adenomatous polyposis coli (APC) mutation is the most prevalent[4]. In the canonical pathway, Wnt binds to Frizzled/low-density lipoprotein-related protein 5/6 (LRP5/6) receptor complexes leading to stabilization of β-catenin. Stabilized β-catenin translocates into the nucleus, complexes with the T-cell factor (TCF)/lymphoid enhancer factor 1 (LEF-1) and modulates the expression of specific target genes controlling development and cell cycle proteins. Activation of the Wnt signaling is controlled by regulation in disassembly of a destruction complex, composed of APC, Axin1, Glycogen synthase kinase 3-β (GSK-3β) and Casein kinase 1 (CK1). This complex functions to phosphorylate serine residues in β-catenin leading to its ubiquitination by F-box/WD repeat-containing protein 1A (β-Trcp) and degradation by the proteasomes (Figure 1). With dysfunctional/mutated APC, β-catenin translocates into the nucleus and inappropriately promotes the transcription of genes leading to uncontrolled cell proliferation[5,6]. Since Wnt signaling is critical for CRC development, many studies have been searching novel agents capable of modulating this signaling pathway[7].
It is now well documented that flavonoids have played an important role in anticancer therapy. Flavonoids are non-nutritive dietary substances that are widely distributed in plants[8]. There are more than 6000 known flavonoids and the number is certain to increase[9]. Flavonoids have attracted appreciable interest because of their health benefits. Several studies focus on the ability of these natural compounds to modulate tumor-related signaling pathways. Flavonoids may represent a potential source for the discovery of novel agents modulating the Wnt/β-catenin signaling pathway. As some flavonoids are capable of modulating the Wnt/β-catenin signaling pathway, they can play pivotal role in counteracting CRC. Here, a selection of flavonoids well characterized as Wnt pathway modulators is presented (Figure 1).
Figure 1: Flavonoids regulate different components of the Wnt/β-catenin signaling pathway
Apigenin was the first characterized flavonoid as regulator of the Wnt/β-catenin signaling pathway because of its ability to selectively inhibit CK2. It has been reported to reduce the levels of β-catenin and dishevelled (Dsh) proteins, promoting cell cycle arrest in breast cancer cells[10]. Thereafter, the modulatory effect of many flavonoids on Wnt/β-catenin signaling has been demonstrated.
Epigallocatechin-3-gallate (EGCG), a vastly studied anti-cancer flavonoid, has been found to inhibit Wnt/β-catenin signaling in CRC cells[11]. The mechanism by which EGCG acts on Wnt/β-catenin signaling involves other proteins that regulate this pathway. Through its demethylation, EGCG promotes Wnt inhibitory factor 1 (wif-1)gene expression[12]. In addition, EGCG reduced tumor multiplicity in the APC−/+ mouse, a model for intestinal tumorigenesis, by inhibiting the translocation of Wnt mediator β-catenin to the nucleus[13]. EGCG has also been demonstrated to induce expression of the Wnt inhibitor SFRP1 gene in hepatoblastoma[14]. In addition, EGCG treatment induced expression of HBP1, a suppressor of Wnt signaling, in breast cancer cells[15].
In 2010, Park and Choi showed that genistein, kaempferol, isorharmnetin and baicalein were able to inhibit Wnt/β-catenin signaling pathway[16]. In a dose-dependent manner, genistein suppressed β-catenin/TCF transcriptional activity in the CRC cell line SW480. Genistein suppressed AKT phosphorylation, thus inhibiting GSK-3β dephosphorylation. Phosphorylated GSK-3β phosphorylates β-catenin, thus facilitating its ubiquitylation and degradation. On the other hand, kaempferol, isorharmnetin and baicalein affect Wnt upstream to β-catenin[16].
Quercetin is one of the most studied flavonoids and has been proposed as a potential anti-cancer drug in CRC[17]. Quercetin has been considered a Wnt/β-catenin signaling pathway inhibitor. Treatment of SW480 cells with quercetinfor 24 h decreased the amount of β-catenin/TCF complex[18,19]. Recently, it has been reported that quercetin inhibits GSK-3β which is a constitutively acting multi-functional serine–threonine kinase involved in Wnt/β-catenin signaling pathway[6]. More recently, Amado et al. showed that quercetin and its glycosylated derivative isoquercetin are able to inhibit Wnt signaling pathway in Xenopusembryos as model system[20] and glioblastoma cells[21], respectively.
Wogonin is a mono-flavonoid isolated from Scutellaria radix and has been shown to inhibit the Wnt/β-catenin signaling through down-regulation of Wnt3A, Cyclin D1, LRP6, c-Myc gene expression. It has also been reported to increase expression of Axin1[22]. Another flavonoid, flavanone, is able to modulate Wnt signaling on transcriptional levels, but not interferes with β-catenin levels[19].
The effect of flavonoids in CRC cells has been found in specific cell lines rather than in every cell types. In this context, the flavonoid silibinin induced cell death, inhibited cell growth and decreased nuclear and cytoplasmic levels of β-catenin in SW480 cells, which harbor mutation in the APC gene. In HCT-116 CRC cell line, which harbors wild-type APC but mutant β-catenin, silibinin treatment has no effect. These findings suggest selective effects of silibinin on Wnt/β-catenin pathway[23].
Overall, these findings demonstrate the importance of flavonoids as potent modulators of Wnt/β-catenin signaling and highlight their potential in fighting and preventing CRC. Since case-control as well as in vitro and in vivo animal studies have reported a plethora of bona fide flavonoid compounds with protective effects against CRC malignancy, special attention has been given to flavonoids as a promising candidate for CRC prevention. Flavonoids interact with different components of the Wnt/β-catenin signaling pathway, thereby modulating cell proliferation and tumor growth. In addition, great efforts have been made to understand the molecular mechanism of action by which flavonoids are acting to inhibit tumor growth. However, the molecular mechanisms of flavonoid compounds need further development and better identification. Many flavonoids are so far not thoroughly investigated as Wnt/β-catenin signaling modulators. The identification of their ability to target Wnt signaling may shed light on future CRC therapies.
Conflict of Interest: The author declared no conflicts of interest.
References
- 1. Jemal, A., Bray, F., Center, M.M., et al. Global cancer statistics. (2011) CA Cancer J Clin 61(2): 69-90.
- 2. Ferlay, J., Shin, H.R., Bray, F., et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. (2010) Int J Cancer 127(12): 2893-2917.
- 3. Banerjee, S., Wang, Z., Mohammad, M., et al. Efficacy of selected natural products as therapeutic agents against cancer. (2008) J Nat Prod 71(3): 492-496.
- 4. Peignon, G., Durand, A., Cacheux, W., et al. Complex interplay between beta-catenin signalling and Notch effectors in intestinal tumorigenesis. (2011) Gut 60(2): 166-176.
- 5. Logan, C.Y., Nusse, R. The Wnt signaling pathway in development and disease. (2004) Annu Rev Cell DevBiol 20: 781-810.
- 6. MacDonald, B.T., Tamai, K., He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. (2009) Dev Cell 17(1): 9-26
- 7. Voronkov, A., Krauss, S. Wnt/β-catenin signaling and small molecule inhibitors. (2013) Curr Pharm Des 19(4): 634-664.
- 8. Mahmoud, A.M. Influence of rutin on biochemical alterations in hyperammonemia in rats. (2012) Exp Toxicol Pathol 64(7-8): 783-789
- 9. Ferrer, J.L., Austin, M.B., Stewart, C., et al. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. (2008) Plant PhysiolBiochem 46(3): 356-3570.
- 10. Landesman-Bollag, E., Song, D.H., Romieu-Mourez, R., et al. Protein kinase CK2: signaling and tumorigenesis in the mammary gland. (2001) Mol Cell Biochem 227(1–2): 153-165.
- 11. Pahlke, G., Ngiewih, Y., Kern, M., et al. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. (2006) J Agric Food Chem 54(19): 7075-7082.
- 12. Gao, Z., Xu, Z., Hung, M.S., et al. Promoter demethylation of WIF-1 by epigallocatechin-3-gallate in lung cancer cells. (2009) Anticancer Res 29(6): 2025-2030.
- 13. Bose, M., Hao, X., Ju, J., et al. Inhibition of tumorigenesis in ApcMin/+ mice by a combination of (–)-epigallocatechin-3-gallate and fish oil. (2007) J Agric Food Chem 55(19): 7695-7700.
- 14. Gödeke, J., Maier, S., Eichenmüller, M., et al. Epigallocatechin-3-gallate inhibits hepatoblastoma growth by reactivating the Wnt inhibitor SFRP1. (2013) Nutr Cancer 65(8): 1200-1207.
- 15. Kim, J., Zhang, X., Rieger-Christ, K.M., et al. Suppression of Wnt signaling by the green tea compound (–)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells requirement of the transcriptional repressor HBP1. (2006) J BiolChem 281(16): 10865-10875.
- 16. Park, S., Choi, J. Inhibition of β-catenin/Tcf signaling by flavonoids. (2010) J Cell Biochem 110(6): 1376-1385.
- 17. Temraz, S., Mukherji, D., Shamseddine, A. Potential targets for colorectal cancer prevention. (2013) Int J MolSci 14(9): 17279-17303.
- 18. Park, C.H., Chang, J.Y., Hahm, E.R., et al. Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. (2005) Biochem Biophys Res Commun 328(1): 227-234.
- 19. Park, C.H., Hahm, E.R., Lee, J.H., et al. Inhibition of beta-catenin-mediated transactivation by flavanone in AGS gastric cancer cells. (2005) Biochem Biophys Res Commun 331(4): 1222-1228.
- 20. Amado, N.G., Fonseca, B.F., Cerqueira, D.M., et al. Effects of natural compounds on xenopus embryogenesis: A potential read out for functional drug discovery targeting Wnt/β-catenin signaling. (2012) Curr Top Med Chem 12(19): 2103-2113.
- 21. Amado, N.G., Cerqueira, D.M., Menezes, F.S., et al. Isoquercitrin isolated from Hyptisfasciculata reduces glioblastoma cell proliferation and changes β-catenin cellular localization. (2009) Anticancer Drugs 20(7): 543-552.
- 22. He, L., Lu, N., Dai, Q., et al. Wogonin induced G1 cell cycle arrest by regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in human colorectal cancer carcinoma cells. (2013) Toxicology 312: 36-47.
- 23. Kaur, M., Velmurugan, B., Tyagi, A., et al. Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of β-catenin-dependent signaling. (2010) Neoplasia 12(5): 415-424.