Functional and Symptomatic Improvement after Cellular Therapy in a Pediatric Case of Chronic Traumatic Incomplete Spinal Cord Injury
Alok Sharma1, Hemangi Sane2, Pooja Kulkarni2, Amruta Paranjape2, V.C Jacob3, Joji Joseph3, Sanket Inamdar3, Sarita Kalburgi3, Nandini Gokulchandran1, Prerna Badhe4, Samson Nivins2
Affiliation
- 1Department of Medical Services and Clinical research, NeuroGen Brain & Spine Institute, India
- 2Department of Research & Development, NeuroGen Brain & Spine Institute, India
- 3Department of Neurorehabilitation, NeuroGen Brain & Spine Institute, India
- 4Department of Regenerative Laboratory Services, NeuroGen Brain & Spine Institute, India
Corresponding Author
Suhasini Pai, NeuroGen Brain & Spine Institute, Stem Asia Hospital and Research Centre, Sector – 40, Plot No. 19, Palm Beach Road, Seawoods (W), New Mumbai-400706, Tel: 91-9920200400; E-mail: publications@neurogen.in
Citation
Sharma, A., et al. Functional and Symptomatic Improvement after Cellular Therapy in a Pediatric Case of Chronic Traumatic Incomplete Spinal Cord Injury. (2017) J Stem Cell Regen Biol 3(1): 115- 121.
Copy rights
© 2017 Sharma, A. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Spinal cord injury; Cellular therapy; Autologous transplantation; Mononuclear cells
Abstract
Spinal cord injury (SCI) in the pediatric population is a rare incidence and has devastating consequences. Cellular transplantation is one of the emerging strategies in the treatment of SCI. Here, we present a case report of an 8-year-old female who sustained traumatic incomplete SCI at the level of D10-D11 four years ago. Two years after the accident, she underwent 2 doses of cell transplantation with autologous bone marrow mononuclear stem cells (BMMNCs) administered intrathecally (injection into the sub-arachnoid space), followed by intensive neuro- rehabilitation. Over the span of 18 months’ post -cellular therapy, there was improvement in the functional status with FIM (Functional Independence Measure) score improving from 108 to 113. She improved in transfer mobility, static and dynamic balance in sitting and standing positions, ambulation and activities of daily living (ADLs). The bowel and bladder control improvement was significant. There was a shift from A to B on the American Spinal Injury Association (ASIA) scale. Spinal Cord Independence Measure (SCIM) score increased from 73 to 96 after the two cellular therapies. No adverse events related to the transplantation procedure were observed. This case is a “proof of concept study” based on the fact that transplantation of autologous bone marrow mononuclear cells along with rehabilitation may augment the recovery processes in patients with chronic traumatic spinal cord injuries. Further, randomized controlled clinical studies are warranted to prove it’s the therapeutic efficiency.
Introduction
Spinal cord injury (SCI) in pediatric population is a rare injury that can result in significant social and psychological consequences for the child and their family[1]. Spinal cord injury invariably results in the loss of neurons and axonal degeneration at the lesion site, leading to paralysis distal to the lesion, loss of sensation, neuropathic pain, and bowel/bladder dysfunction as a result of axonal damage[2]. Recovery from SCI is difficult because the injured spinal cord has a reduced ability to regenerate the damaged cells and re-establish functional neural connections[3].
In traumatic chronic spinal cord injuries immediate and comprehensive trauma care is important for survival. Long-term management of chronic SCI focuses on rehabilitation, pain relief, spasticity treatment and prevention of secondary complications[4]. Currently no effective treatment exists for the major neurological deficits of SCI. Cellular transplantation is one of the promising and pragmatic strategies in the treatment of chronic SCI that aims at reducing cell death, secondary injury and promoting regeneration and tissue repair at the site of injury[5]. We hereby present a case of an eight year old female, who suffered a road traffic accident(RTA) in December 2012, leading to chronic traumatic SCI at the level of dorsal vertebrae D10 – D11, injected with autologous ( from the same patient) bone marrow mononuclear cell (BMMNC) transplantation intrathecally followed by neuro-rehabilitation.
Case Report
An 8-year-old female presented with a history of RTA in December 2012, leading to SCI at the level of D10 – D11 (dorsal vertebrae). Post RTA, there was a loss of bowel and bladder control, muscle power and trunk control. She was treated conservatively with regular physiotherapy at a private hospital leading to complete recovery of her upper limbs. She achieved partial trunk balance. In June 2014, she had sensation of urine and bowel movements but there was no voluntary control. She developed a plateau in her recovery phase below the level of injury even with regular rehabilitation.
On detailed assessment prior to the cell therapy in October 2014, she exhibited weakness in the lower extremities with patchy sensations present on both feet including the soles. There was sensory loss below D10 level and in L1- L5 dermatomes. She was hypertonic with grade 1 spasticity in the plantar flexors. Hyperreflexia was present in bilateral ankles. Flexor and extensor spasms were also present. Babinski sign (reflex obtained by stimulating the outside of the sole of the foot, causing extension of the big toe while fanning the other toes) was positive. An ill-sustained type clonus was present bilaterally. Static balance in sitting was fair, whereas dynamic balance was poor. Static and dynamic balance while standing was poor. She was not able to walk or stand independently and had poor voluntary control in the lower extremity joints. She could walk with walker and KAFO support with assistance. Bowel and bladder sensations were present with minimum control for up to 1 minute. Functionally, she was partially dependent in most activities of daily living (ADL).
Thoraco-lumbar spine MRI revealed post traumatic myelomalacia of spinal cord at D10-D11 level. Functional MRI (fMRI) study revealed mild activation of the left parafalcine motor cortex and few secondary association areas in the right temporo-parietal lobes. Fibre tractography of spinal cord was suggestive of increased extracellular edema with reduction in the number of fibers due to increased extracellular space. She scored 108 on Functional Independence Measure (FIM) and 73 on Spinal Cord Independence Measure (SCIM). On American Spinal Injury Association (ASIA) scale, she was at level A (no sensory or motor function is preserved in the sacral segments S4-5).
Procedure
She underwent first cellular transplantation in October 2014. The selection of the patient was based on the World Medical Associations Helsinki declaration[6]. The protocol was reviewed and ethically approved by Institutional Committee for Stem Cell Research and Therapy (IC-SCRT). The procedure of cellular therapy was explained in detail to her parents and a duly filled informed consent was obtained prior to the therapy.
Before the intervention, the patient underwent a comcomplete evaluation consisting of neurological, psychological and pre-operative assessment to assess the pre-anesthesia fitness. Granulocyte-Colony Stimulating Factor (G-CSF) (300 mcg) injections were administered subcutaneously, 72 hours and 24 hours prior to bone marrow aspiration. On the day of transplantation, 100 ml bone marrow was aspirated from the left anterior superior iliac spine under local anesthesia, using bone marrow aspiration needle and was collected in heparinized tubes. The BMMNCs were separated from the aspirate using density gradient method. The purified MNCs were tested for total cell count, viability and CD34+ cell content by Fluorescence Activated Cell sorting (FACS). CD34+ count was found to be 3.16 %. The separated cells were then injected intrathecally at the level between L4 and L5. Simultaneous intravenous administration of 1 gm methyl prednisolone in 500 ml of Ringer Lactate solution was carried out to decrease immediate inflammation and to enhance the survival of the injected cells. Total number of cell injected were 9.6 × 107 with 94% viability.
Following the autologous transplantation, she underwent multidisciplinary neuro-rehabilitation. Physiotherapy consisted of rolling, trunk rotation, assisted bridging, trunk strengthening exercises, push-ups, bed mobility exercises and suspension exercises. These were aimed at increasing the trunk control, strength of the preserved muscles and improving balance and gait. Occupational therapy aimed at improving the pelvic control, bed mobility, transfer techniques, lower extremity control and balance. Counseling was provided by a psychologist to cope better with the disease.
The patient was discharged at one week post-transplantation and was advised to continue the rehabilitation at home. The follow-up assessment was conducted at three and seven months after the intervention. In view of the improvements observed after the treatment, the patient underwent second cellular transplantation in May 2015 i.e. 7 months after the first transplantation. The transplantation procedure was replicated in the second intervention. Total numbers of mononuclear cells injected were 9.8 × 107 with 96% viability.
Results
Within one week of cellular transplantation, the patient showed improved sensation in L1 and L2 dermatomes which was totally absent before. Four months after the 1st cellular transplantation, the static and dynamic standing balance improved with the help of calipers. She was able perform ADLs independently including walking with elbow crutches and putting the orthoses. She could transfer from chair to bed independently. Bed mobilities, rolling, supine to sit and sit to stand activities were evident that was completely absent before. Urinary control improved. She could now hold urine for up to two minutes and voluntarily control the urine stream. She was able to ambulate with minimum support on the parallel bars. The scores on SCIM increased from 73 to 92 and on FIM from 108 to 113.
At seven months after 1st cellular transplantation, she was able to climb up the stairs with support. Further improvement in the sitting and standing balance was observed. Transfers from bed to chair to floor (higher to lower levels) were comparatively faster than before. Bladder and bowel control further improved up to 5 minutes. The static and dynamic balance in sitting and standing positions were maintained. She was now able to do tasks like bending down and picking up objects from floor in standing and sitting position, which was difficult for her earlier. Participation in sports and cultural activities had increased. There were no reports of any falls. fMRI studies showed an increase in the areas of activation in the bilateral parafalcine motor cortex. There was a shift from A to B on the ASIA scale. The SCIM score increased to 96. On the Manual Muscle Testing (MMT) scale, the strength in back extensor and lower abdominals increased from 1 to 2++ and 1++ respectively. (Table 1) In our clinical experience, the scoring of the muscle strength using mMRC-MMT was not sensitive to consider subtle changes in the strength. Therefore, we further subdivided the scale (“Appendix 1 ”), which has been standardized for all the patients[7].
Table 1: Table showing improvement in the muscle strength through MMT( Manual Muscle Testing) grading before and after the cellular transplantation.
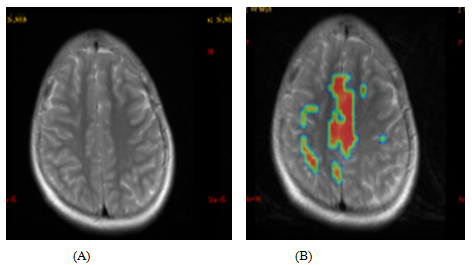
Based on the above improvements, the patient underwent 2nd cellular transplantation. The follow-up was conducted after 10 months of the second cellular transplantation. She could stand with calipers without an external support and do activities like drinking, washing hands and upper body dressing. Bilateral abduction and adduction in the lower limb improved which was more evident in the right side than the left. Spasticity of grade 2 was observed in glutei, quadriceps (knee extensor), adductors and plantar flexors. All the other improvements were preserved. Functional MRI (fMRI) of the brain revealed activation of bilateral motor cortices and increased activation of multiple secondary association areas in both cerebral hemispheres as compared to prior fMRI done. (Figure 1)
Comparative study of pre and post cell therapy fMRI scan shows increased activation of bilateral motor cortices and multiple secondary association areas in both the cerebral hemispheres.
Figure 1: Pre (A) and Post (B) second cellular transplantation fMRI scan images.
Discussion
Spinal cord injury in the pediatric population is very rare and has devastating consequences[8]. The incidence of spinal injuries in children is reported to be 2 to 5% of all spine injuries with motor vehicle accidents being the most common cause[9]. Loss of cellular components and myelination that occurs as a post injury inflammatory process impedes the functional recovery, and adds to regenerative complexity of spinal cord. So far, medical treatment and rehabilitation have focused on preventing complications and maximizing residual functional capacities[10]. Cell therapy has emerged as the most promising treatment strategies because it focuses on replacing the lost or damaged cells with progenitor or stem cells, leading to further axonal growth, re-myelination of axons, and reduction of neuronal degeneration[11-13]. Moreover, pediatric stem cells have been found to have greater plasticity with high reprogramming efficiency to differentiate into different cell lineages[12]. Through this property cellular therapy attempts to regain maximum functional recovery which might help the child to lead a better life.
In this case, we administered the patient with autologous BMMNCs intrathecally. The bone marrow consists of a heterogeneous population of stem cells, including hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs)[13]. Safety of bone marrow MNCs has been well studied in various clinical trials[14,15]. The enormous heterogenous population of stem cells derived from the bone marrow also offers a great variety of effects from different cell lineage[16]. The administration of autologous cells reduces the risk of rejection by the immune system and hence is more effective than the allogenic cells[17,18]. Intrathecal route was used for administration of cells as it is considered to be a more targeted way of transmission than intravenous[19]. It was found that magnetically labeled CD34+ cells were well tolerated and migrated at the site of injury when administered intrathecally in SCI subject[20].
A multitude of cells have been tested pre-clinically and clinically to address the multifactorial pathophysiology of SCI. The postulated mechanism of action is by secretion of anti-inflammatory, anti-apoptotic, anti-gliotic molecules and trophic factors that promote neuronal regeneration, angiogenesis, immune-modulation and protection from apoptotic cell death[21]. Bone marrow stromal cells have been found to replace white and gray matter and act as cellular scaffolds for growing axons and protect them through neuro-protective immune-modulatory effect, leading to functional improvements in animal models[22,23]. One of the recent studies showed that human neuronal stem cells when transplanted into damaged spinal cord of a mouse, generated new neurons and oligodendrocytes, leading to locomotor recovery[24]. There are strong evidences in humans concluding that autologous BMMNCs improve the functional and symptomatic outcomes in patients with chronic cervical and thoraco-lumbar spinal cord injuries[25,26]. One of the clinical findings also provides a correlation observed between the implantation of bone marrow stromal cells, the amount of myelin, enhanced conduction velocity and the extent of functional recovery[27].
Spinal cord injury (SCI) affects conduction of sensory and motor signals at and around the site of lesion(s). ASIA score provides a systematic examination of the dermatomes and myotomes, to determine the affected cord segments by spinal cord injury that may help to assess the extent of damage and recovery[28]. The SCIM scale has high clinical relevance for the rehabilitation for individuals with either traumatic/non-traumatic and complete/incomplete SCI[29]. Meta-analysis and systematic review regarding bladder recovery after cellular therapy demonstrated significant improvements in voiding pressure, residual urine and non- voiding contraction (NVC) in total 224 SCI patients[30]. Improvement in the sensations at the affected dermatomes and bladder, as seen in the patient, implies repair and regeneration process of the damaged myelin sheath. The strengthening of the abdominals and back extensors improved balance and lower limb control. A fMRI study of the brain demonstrated changes to cortical activation patterns following SCI[31]. The study provided evidence that the sensory-motor network undergoes dynamic reorganization following SCI, which was also observed in our patient. In this case the individual effect of cellular therapy and rehabilitation cannot be assessed. Many pre-clinical and clinical studies have proved that voluntary physical exercise induces precursor cell proliferation thereby expanding the pool and enhancing the mobilization of progenitor cells that are available for neuro-regeneration[32,33]. The clinical improvements observed in the subject can be attributed to the cumulative effect of two cellular therapies and multidisciplinary neuro-rehabilitation, supporting the observation that a combi¬nation of cellular therapy and exercise training results in significant functional improvement through neuro-facilitation. There were no adverse events observed in the patient.
The limitation of this case study is that it is a solitary case and the results cannot be generalised for the population. But taking into account the chronicity, the patient could serve as a self- control.
Conclusion
In this particular case study, the functional recovery had reached a plateau phase with two years of standard rehabilitation treatment since the accident in December 2012. But with cellular therapy along with regular rehabilitation, improvements were observed even at the chronic stage which can be attributed to the plasticity of stem cells in the developing spinal cord. The various mechanism of action of BMMNCs promotes repair and regeneration in the damaged spinal cord which was evident from the improvement in the patient. This case is a “proof of concept study” based on the fact that transplantation of autologous bone marrow mononuclear cells along with rehabilitation may augment the recovery in patients with chronic traumatic spinal cord injuries. To effectively prove the therapeutic benefits of cellular therapy in paediatric SCI, a multicentre randomized controlled trial with larger sample size is warranted.
Conflict of interest:
The authors declare that there is no conflict of interests regarding the publication of this paper.
Appendix 1: Comparison of the grades of the scales mMRC-MMT and mMRC-MMT (I).
| m-MRC MMT grade | Description | mMRC-MMT (I) grade | Description |
| 0 | No Movement | 0 | No Movement |
| 1 | A flicker of movement is seen or felt in the muscle | 1 | Flicker of contraction |
| 1+ | Muscle moves the joint through up to 1/3rd of the ROM when gravity is eliminated | ||
| 1++ | Muscle moves the joint more than 1/3rd less than 2/3rd of the ROM when gravity is eliminated | ||
| 2 | Muscle moves the joint when gravity is eliminated | 2- | Muscle moves the joint more than 2/3rd to less than the full ROM |
| 2 | Muscle moves the joint through complete ROM when gravity is eliminated | ||
| 2+ | Muscle moves the joint up to 1/3rd ROM against gravity | ||
| 3- | Muscle moves the joint against gravity, but not through full mechanical range of motion | 2++ | Muscle moves the joint > 1/3rd, < 2/3rd of ROM against gravity |
| 3- | Muscle moves the joint more than 2/3rd to less than complete ROM | ||
| 3 | Muscle cannot hold the joint against resistance but moved the joint fully against gravity | 3 | Muscle moves the joint through complete ROM against gravity |
| 3+ | Muscle moves the joint against combination of gravity and moderate resistance up to 1/3rd of ROM | ||
| 3+ | Muscle moves the joint fully against gravity and is capable of transient resistance, but collapses abruptly | 3++ | Muscle moves the joint against combination of gravity and moderate resistance from 1/3rd to 2/3rd of ROM |
| 4- | Same as grade 4, but muscle holds the joint only against minimal resistance | 4- | Muscle moves the joint more than 2/3rd to less than complete ROM against combination of gravity and moderate resistance |
| 4 | Muscle holds the joint against a combination of gravity and moderate resistance | 4 | Muscle moves the joint against combination of gravity and moderate resistance though complete ROM |
| 4+ | Same as grade 4 but muscle holds the joints against moderate to maximal resistance | 4+ | Muscle moves the joint against combination of gravity and moderate to maximal resistance up to 1/3rd of ROM |
| 5- | Barely detectable weakness | 4++ | Muscle moves the joint against combination of gravity and moderate to maximal resistance from 1/3rd to 2/3rd of ROM (Barely detectable weakness) |
| 5 | Normal strength | 5 | Muscle moves the joint against combination of gravity and moderate to maximal resistance though complete ROM (Normal Strength) |
References
- 1. Dhal, A., Roy, K., Ghosh, S., et al. A Study on Pediatric Spinal Injury: An IPGMER, Kolkata Experience. (2006) Indian Journal of Neurotrauma 3(1): 41-48.
Crossref - 2. Lee?Liu, D., Edwards-Faret, G., Tapia, V.S., et al. Spinal cord regeneration: Lessons for mammals from non?mammalian vertebrates. (2013) Genesis 51(8): 529-544.
Pubmed || Crossref - 3. Anderson, K.D. Targeting recovery: priorities of the spinal cord-injured population. (2004) J Neurotrauma 21(10): 1371-1383.
Pubmed || Crossref - 4. Jones, D.G., Anderson, E.R., Galvin, K.A. Spinal cord regeneration: moving tentatively towards new perspectives. (2003) NeuroRehabilitation 18(4): 339-351.
Pubmed - 5. Tator, C.H., Hashimoto, R., Raich, A., et al. Translational potential of preclinical trials of neuroprotection through pharmacotherapy for spinal cord injury. (2012) J Neurosurg Spine 17(Suppl1): 157-229.
Pubmed || Crossref - 6. Carlson, R.V., Boyd, K.M., Webb, D.J. The revision of the Declaration of Helsinki: past, present and future. (2004) Br J Clin Pharmacol 57(6): 695-713.
Pubmed || Crossref - 7. Lue, Y.J., Jong, Y.J., Lin, Y.T., et al. [The strength and functional performance of patients with Duchenne muscular dystrophy based on natural history]. (1992) The Gaoxiong Yi Xue Ke Xue Za Zhi 8(11): 597-604.
Pubmed - 8. Greenberg, J.S., Ruutiainen, A.T., Kim, H. Rehabilitation of pediatric spinal cord injury: From acute medical care to rehabilitation and beyond. (2009) J Pediatr Rehabil Med 2(1): 13-27.
Pubmed - 9. Reynolds, R. Pediatric spinal injury. (2000) Curr Opin Pediatr 12(1): 67-71.
Pubmed - 10. Zhang, N., Yin, Y., Xu, S.J., et al. Inflammation & apoptosis in spinal cord injury. (2012) The Indian journal of medical research 135(3): 287.
Pubmed - 11. Moviglia, G.A., Varela, G., Gaeta, C.A., et al. Autoreactive T cells induce in vitro BM mesenchymal stem cell transdifferentiation to neural stem cells. (2006) Cytotherapy 8(3): 196-201.
Pubmed || Crossref - 12. Guasti, L., Prasongchean, W., Kleftouris, G., et al. High Plasticity of Pediatric Adipose Tissue-Derived Stem Cells: Too Much for Selective Skeletogenic Differentiation? (2012) Stem Cells Transl Med 1(5): 384-395.
Pubmed || Crossref - 13. Ozdemir, M., Attar, A., Kuzu, I. Regenerative treatment in spinal cord injury. (2012) Curr Stem Cell Res Ther 7(5): 364-369.
Pubmed || Crossref - 14. Geffner, L.F., Santacruz, P., Izurieta, M., et al. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: comprehensive case studies. (2008) Cell transplantation 17(12): 1277-1293.
Pubmed || Crossref - 15. Sharma, A., Gokulchandran, N., Chopra, G., et al. Administration of autologous bone marrow-derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. (2012) Cell transplantation 21(1): S79-90.
Pubmed || Crossref - 16. Glover, L.E., Tajiri, N., Weinbren, N.L., et al. A step-up approach for cell therapy in stroke: translational hurdles of bone marrow-derived stem cells. (2012) Transl stroke res (1): 90-98.
Pubmed || Crossref - 17. Prasongchean, W., Ferretti, P. Autologous stem cells for personalised medicine. (2012) New Biotechnology 29(6): 641-650.
Pubmed || Crossref - 18. Jung, D.I., Ha, J., Kang, B.T., et al. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. (2009) J Neurol Sci 285(1): 67-77.
Pubmed || Crossref - 19. Sharma, A., Sane, H., Khopkar, D., et al. Functional recovery in chronic stage of spinal cord injury by neurorestorative approach: A case report. (2014) Case reports in surgery 2014.
Crossref - 20. Callera, F., de Melo, C.M. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells' migration into the injured site. (2007) Stem Cells and Development. 16(3): 461-466.
Pubmed || Crossref - 21. Hsu, Y.C., Chen, S.L., Wang, D.Y., et al. Stem cell-based therapy in neural repair. (2013) Biomed J 36(3): 98.
Pubmed || Crossref - 22. Bottai, D., Cigognini, D., Madaschi, L., et al. Embryonic stem cells promote motor recovery and affect inflammatory cell infiltration in spinal cord injured mice. Experimental neurology. (2010) Jun 223(2): 452-463.
Pubmed || Crossref - 23. Ohta, M., Suzuki, Y., Noda, T., et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. (2004) Experimental neurology 187(2): 266-278.
Pubmed || Crossref - 24. Ogawa, Y., Sawamoto, K., Miyata, T., et al. Transplantation of in vitro?expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. (2002) J Neurosci Res 69(6): 925-933.
Pubmed || Crossref - 25. Sharma, A., Gokulchandran, N., Sane, H., et al. Detailed analysis of the clinical effects of cell therapy for thoracolumbar spinal cord injury: an original study. (2013)Journal of Neurorestoratology 1: 13-22.
Crossref - 26. Sharma, A., Sane, H., Gokulchandran, N., et al. Role of autologous bone marrow mononuclear cells in chronic cervical spinal cord injury-a longterm follow up study. (2013) Journal of Neurological Disorders 2013.
- 27. Akiyama, Y., Radtke, C., Kocsis, J.D. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. (2002) The journal of neuroscience 22(15): 6623-6630.
Pubmed - 28. Maynard, F.M., Bracken, M.B., Creasey, G.J., Ditunno., et al . International standards for neurological and functional classification of spinal cord injury. (1997) Spinal cord 35(5): 266-274.
Pubmed || Crossref - 29. Catz, A., Tamir, A., Itzkovich, M., et al. Spinal Cord Independence Measure: a new disability scale for patients with spinal cord lesions. (1998) Spinal cord 36(734): 735.
Pubmed - 30. Kim, J.H., Shim, S.R., Doo, S.W., et al. Bladder recovery by stem cell based cell therapy in the bladder dysfunction induced by spinal cord injury: systematic review and meta-analysis. (2015) PloS one. 10(3): e0113491.
Pubmed || Crossref - 31. Oni-Orisan, A., Kaushal, M., Li, W., et al. Alterations in Cortical Sensorimotor Connectivity following Complete Cervical Spinal Cord Injury: A Prospective Resting-State fMRI Study. (2016) PloS one 11(3): e0150351.
Pubmed || Crossref - 32. Carvalho, K.A., Vialle, E.N., Moreira, G.H., et al. Functional Outcome of Bone Marrow Stem Cells (CD45+/CD34−) After Cell Therapy in Chronic Spinal Cord Injury in Wistar Rats. (2008) In Transplant Proc 40(3): 845-846.
Pubmed || Crossref - 33. Fabel, K., Wolf, S., Ehninger, D., et al. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. (2009) Front Neurosci 3: 2.
Pubmed || Crossref