Functionalized Cellulose Nanofiber –Composite Membranes for Wastewater Treatment -a Review
Lingayya Hiremath
Affiliation
1Department of Biotechnology, R.V. College of Engineering, Bangalore, Karnataka, India
1Department of Electronics and Communication, R.V. College of Engineering, Bangalore, Karnataka, India
1Department of Mathematics R.V. College of Engineering, Bangalore, Karnataka, India
Corresponding Author
Lingayya Hiremath, Department of Biotechnology, R.V. College of Engineering, Bangalore, Karnataka, India-560 059, Tel: +91-9886699277; E-mail: lingayah@rvce.edu.in
Citation
Hiremath, L., et al. Functionalized Cellulose Nanofiber – Composite Membranes for Waste Water Treatment -a Review. (2018) J Nanotechnol Material Sci 5(1): 3543.
Copy rights
© 2018 Hiremath, L. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Cellulose acetate; Heavy metals; Bio composite membranes; Nanofibers; Adsorption
Abstract
In the present era of scarcity of water resources, effective treatment of wastewater is a major prerequisite for growing economy. Developing cost-effective technologies to solve the water pollution problems are critical to sustainable development of human society. Various treatments, recent advanced processes in nonmaterial sciences have been attracting the attention of scientists. The Nano composite membranes play very important role in wastewater treatment for removal of hazardous materials such as toxic heavy metals, dyes and microbes. Bio based Nano fibers are increasingly considered in purification technologies due to their high mechanical properties, high surface area and versatile surface chemistry. Cellulose is the most abundant natural bio polymer. The low cost raw cellulosic material has gained increasing attention due to stiffness, biodegradability and renewability. Cellulose fibers are hydrophilic in nature, so it becomes necessary to increase surface roughness for the development of composites with enhanced properties. Cellulose acetates (CA) are esters of cellulose; CA is useful material in industry with applications in the field of coating, film and membrane separation. Through electro spinning CA can be transformed into nanofiber materials by using solvents. The CA nanofibers were functionalized with thiol groups using thioglycolic acid to form thiol cellulose nanofiber membrane (TCM). The cellulose nanofibers also surface modified with additives like phytic acid, dithizone, SDS, L-dopa, Fe3O4 Nano particles, SiO2 for enhance adsorption properties of membranes. In this review, highlights on surface modification and functionalized of cellulosed- based nanofibers by various methods, characteristics of nanofiber membranes and adsorption behavior for removal of pollutants from wastewater.
Introduction
The global population has increased from 5.3 billion in 1990 to 7.8 billion in 2018. With this increase, there has been a race towards rapid industrialization to provide so called better quality of living. This has resulted in the rise of both air and water pollution. Numerous measures have been taken to curb the extent of toxic waste especially in water resources, as water is one of the fundamental essentials of our everyday life. Although the numbers have increased over the years, 663 million people worldwide still lack improved drinking water resources, while 159 million people still use surface water[1].
In recent years, the development of membrane technology has geared up as a mean to overcome water pollution effectively. Statistics show that membrane technologies contribute up to 53 % of the total world processes for clean water production[2]. The increasing demand in membrane technology is mainly due to their features itself as well as the ability to apply them in sustainable industrial designs. Ideally, membranes should have characteristics such as increased life span, good mechanical, thermal, and chemical stability, low cost, and minimum maintenance. The use of membranes also enables the improvement of industrial design whereby less land space is required for the setting up of various processes, energy consumption is low, and no additional chemical is needed when compared to conventional technologies. This renders membranes as an economical technology with potential in various fields such as separation, filtration, and catalysis for water treatment.
Membranes have been used for water treatment for more than 45 years. A wide range of materials are available for the fabrication of membranes. These materials can generally be grouped into ceramic-based and polymeric-based membranes. Ceramic-based membranes are popular as they are thermally and chemically more stable, have high porosity, and have a longer life span though, they are more expensive and brittle. Polymer-based membranes on the other hand offer flexibility in design, are cheaper, can remove dissolved ions and organics more efficiently[3]. Nevertheless, they also have some disadvantages including high hydrophobicity, exposure to biofouling, low fluxes, and low mechanical strength. We realize that there is abundant literature on both ceramic- and polymeric-based membranes[4-8]. Particularly, recent reviews on polymer Nano composites for membrane applications have emerged[9]. Majority of these reviews are focused on synthetic polymers. Reviews related to natural polymer composite membranes can be hardly found in the literature[10,11]. As such, this chapter has been organized to give the readers an overview of the different types of natural polymer composite membranes and their functions. This chapter highlights the application of novel cellulose-, chitosan-, and natural rubber-based composite membranes in sustainable technologies between 2010 and 2015.
Table 1: Summary of Natural polymer (Cellulose) composite membranes (Types: TFC- Thin film composites, MM- Mixed matrix, PI- Polymer Inclusion).
| Natural polymer matrix | Additives | Type | Function | Application | Refs. |
|---|---|---|---|---|---|
| Chitosan/cellulose | Ag NPs | MM | Antimicrobial | Anti-biofouling | [12] |
| Polyacrylic acid–Ag NPs | MM | Antimicrobial | Anti-biofouling | [13] | |
| Dithizone | TFC | Adsorption | Adsorption of lead | [14] | |
| 5,10,15,20-Tetrakis (1-methyl-4-pyridinio) porphyrin tetra (p-toluene sulfonate) | TFC | Adsorption | Removal of cadmium | [15] | |
| Cellulose | CuO NPs | MM | Antimicrobial | Disinfection of water | [16] |
| TiO2 Nano thorn | TFC | Filtration/catalysis | Removal of MB and humic acid | [17] | |
| Dendrimer–Ag | MM | Catalysis/antimicrobial | Removal of rhodamine B/disinfection of water | [18] | |
| Cellulose/PVC | Fe3O4NPs | MM | Adsorption/filtration | Removal of lead | [19] |
| Cellulose acetate | SDS | TFC | Filtration | Rejection of pesticides | [20] |
| L-dopa | TFC | Filtration | Antifouling | [21] | |
| Alkyl derivative of resorcinarene | PI | Adsorption/filtration | Removal of Pb(II), Cd (II), and Zn(II) | [22] | |
| Iron NPs | MM | Filtration | Rejection of phosphates and organic pollutants | [23] | |
| Cellulose acetate/PEG-600 | Ag | MM | Antimicrobial | Salt rejection/anti-biofouling | [24] |
| Cellulose acetate/PAN | Ag NPs | TFC | Filtration/antimicrobial | Salt rejection/anti-biofouling | [25] |
| Cellulose acetate/PANI | Phytic acid | MM | Adsorbent | Removal of Hg(II) and Cr(VI) | [26] |
| Cellulose acetate/PEG | SiO2 | MM | Filtration | Salt rejection | [27] |
| Cellulose acetate/cellulose triacetate | Boehmite | MM | Filtration | Salt rejection | [28] |
| Cellulose triacetate | Modified ZnO | MM | Filtration | Separation of rhodamine B | [29] |
| Aliquot 336 | PI | Adsorption | Separation of acid violet 90 and acid yellow 127 | [30] | |
| Cyphers | PI | Filtration/adsorption | Separation of Zn(II) | [31] | |
| Activated carbon (AC) | MM | Adsorption/filtration | Removal of uranium | [32] | |
| 2-(10-carboxydecylsulfanyl) benzoic acid methyl monoester | PI | Filtration | Separation of Pb(II) | [33] | |
| Cyanex 923 | PI | Adsorption/filtration | Removal of phenol | [34] | |
| Hydroxyl ethyl cellulose/sodium alginate | Humic acid | MM | Adsorption | Adsorption of Cd(II) | [35] |
| Humic acid | MM | Adsorption | Removal of MB and rhodamine B | [36] |
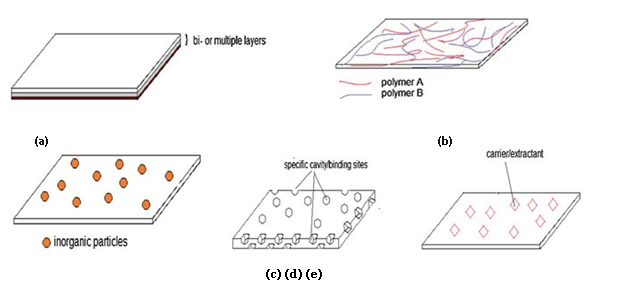
Figure 1.1: Structures of various natural composite membranes: a thin-film composite, b blends,
C mixed matrix, d molecular imprinted, and e polymer inclusion (Noor Hana, 2016)
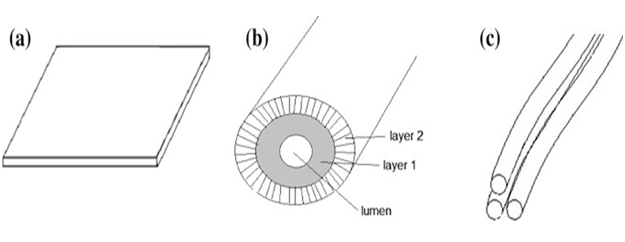
Figure 1.2: Various shapes of natural composite membranes: a flat sheet, b hallow fiber, and c Nano fibrous.
Main Functions and Characteristics of Natural Composite Membranes
As natural polymer composite membranes can be either inert or catalytically active, the role of these membranes can be categorized based on the following areas:
• Filtration: This process can be further classified according to the pore size of the filter. This includes microfiltration, ultrafiltration, Nano filtration, and reverse and forward osmosis. A detailed summary on various classes, their respective pore sizes, and the materials these filters are capable of removing has been given elsewhere[37,38].
• Adsorption: This process involves the capture of substances by physical or chemical means. Physisorption is a reversible reaction that allows substance recovery possible, while the latter is not. Adsorption has been extensively applied for their removal of multivalent heavy metal ions, for example, Pb(II), Cd(II), and Cr(IV)[39]. Organic substances such as phenol, dyes and art emidine can also be removed and recovered by using molecular imprinted membranes with high selectivity.
• Catalysis: In these membranes, metal or semiconductor nanoparticles are normally incorporated to serve as the active component (catalyst) for a certain catalytic reaction[40,41,17]. When semiconductors such as TiO2 or ZnO are employed, a light source (e.g., UV, sunlight) is needed to initiate the reaction. Thus far, the application of catalytic membranes is limited to the degradation of synthetic dyes and nitro aromatic compounds and photocatalytic antimicrobial purposes.
• Antimicrobial/fouling: One of the biggest challenges of membranes in water treatment application is fouling or particularly biofouling. Fouling is the buildup of unwanted particulates onto a wetted surface. This layer of particulate tends to cause several losses in performance and reduces the life span of membranes. Furthermore, it results in high cleaning maintenance.
• Generally, there are three approaches to reduce the biofouling problems in a membrane:
• Suppress the adhesion of biologic particulates onto the membrane;
• Biocide leaching or toxic release;
• Contact killing.
 Figure 2: Commonly used cellulose structures in membrane technology.
Figure 2: Commonly used cellulose structures in membrane technology.
The first strategy involves the alteration of the surface hydrophilicity, roughness, or charges of the membranes. In contrast, the latter two strategies address the biofouling problems by incorporating inorganic species as the antimicrobial agent in the membranes. Silver Nano particles, carbon Nano tubes oxide nanoparticles and ammonium salts are some of the examples. Organic antimicrobial agents can also be employed. However, this is less favorable due to their poorer ability to with stand adverse processing conditions as compared to inorganic antimicrobial agents.
The application of cellulose-based membranes in water filtration has been greatly explored since the 1960s. In fact, cellulose acetate membranes were the first membranes used in commercial reverse osmosis plants in 1965 (History Reverse Osmosis link, 2015). To date, cellulose filter membranes with various pore sizes can be found commercially to remove different sizes of substances. Cellulose membranes have been used in a broad range of filtration spectra that cover from microfiltration to osmosis processes[42,43]. They are capable of removing bacteria, viruses, antibiotics, pesticides, synthetic dyes, oil and grease, heavy metals, and even dissolved salts. The features of cellulose filter membranes can be improved by adding additives. Bentonite[44,45], boehmite[46], modified coal[47], and Sodium Dodecyl Sulfate (SDS)[20] are incorporated into cellulose to increase the water permeability of the filter membranes (higher flux) by modifying the membranes’ surface porosity and hydrophilicity. Other additives such as silica[24], iron Nano particles[24] and Carbon Nano Tubes[48] also improve the cellulose membrane in terms of their durability and thermal and mechanical properties. Nonetheless, a decrement in salt rejection and mechanical strength has been observed when Poly Octahedral Slices Quioxane (POSQ) nanoparticles are applied as the additives[49]. In most cases, adsorption occurs besides filtration. Positively charged heavy metal ions are favorably adsorbed on to the negatively charged cellulose membrane, while water permeates through the interconnected pores of the membrane. However, due to the low surface area of cellulose, the absorptive sites are occupied quickly by metal ions and cause a drastic drop in metal ion rejection. Completing agents (e.g. humic acid, phytic acid), adsorbent particles (e.g. iron oxide, graphite, silica) or carriers (e.g. Calix resorcinarens) are integrated into the cellulose membranes to increase the metal removal efficiency from water. A common feature between these additives is their capability to form interaction with metal ions. For example, activated carbon and iron oxides offer negatively charged surfaces to adsorbed U(VI) and Pb(II), respectively, through complexation[19,32]. Nevertheless, the metal removal efficiency of cellulose composite membranes is also dependent on other parameters such as pH, do pant, concentration of metal ions, and temperature[36]. The fabrication of silver–cellulose membranes and their biofouling resistance properties have been reported in many studies[50,51]. Silver nanoparticles not only increase the surface hydrophilicity of the membrane, brutal so release the silver ions (biocides) that will eventually kill bacteria. Nevertheless, the strength of the bio-fouling resistance of silver–cellulose membranes is dependent on the amount, size and the location of silver nanoparticles in the membrane[52] conducted SEM-EDS mapping studies on a series of Ag polymer membranes and correlated them with their antimicrobial properties. They demonstrated that the silver nano particles located on the player of the membranes are easier to reach out and hence exert higher antibacterial properties. Copper oxide particles in the cellulose membranes also show superior anti-bacterial properties[16]. Similar to silver; its origin of toxicity comes from the leaching of metal ions. Photo catalytic antibacterial properties of semiconductor oxides especially TiO2 and ZnO in cellulose membranes have also been explored. Under the irradiation of light, reactive oxygen species such as OH-, H2O2, and O</span2- are generated. These oxygen species can penetrate through bacteria’s cell wall and inhibit their growth[53,17]. Besides imparting antimicrobial properties, the photo catalytic properties of these membranes can also be used for the degradation of pollutants in water. Furthermore, the presence of semiconductor oxides can lead to simultaneous improvement in membrane stability, flux permeability, and porosity[54,55,15,17] have prepared multifunctional TiO2 and TiO2/ZnO- cellulose membranes for water purification. These membranes were used for concurrent filtration and photo degradation to remove methylene blue and humic acid with high flux and anti-fouling properties. To what extent is it ‘green’? This is one of the questions which frequently pop up when the topic of discussion circulates around renewable materials. When discussing natural composite membranes, the extent to which it is ‘green’ can be reflected from several aspects that is the preparation, materials and function of the membrane.
Electro spinning is a technology for preparation of ultra- long one-dimensional fiber materials with diameters in the scale of nanometer, which exhibits high specific area. Therefore, electro spinning membrane may have a great potential for the adsorption of metal ions. It is well known that electro spinning nanofibers have found applications in bio- medicine[56,57], tissue engineering[58,73] and armored fabric materials[59]. Introduction of functional groups onto the surface of electro spinning nanofibers could be further improved their utilization as adsorbents to remove heavy metal ions. Indeed, sulfinyl group[60], Carboxyl[61,62], amino[63,64], thiol group[65,71,74] and thioether group[66] have been used and resultant materials showed excellent adsorption capacity towards metal ions for example, Cu(II)[40], Cr(VI)[68], Cr(III)[67,75]. In this work, we further explored the composite of CA/PVP by functionalizing with thiol groups. Nanofiber membranes were prepared using a mixture of CA/PVP via an electro spinning technique. Subsequently, the PVP of the membranes was partially removed and the CA was further deacetylated before functionalization with thiol groups. Characterization of the obtained nanofibers was completed using infrared spectroscopy, scanning electron microscopy, and thermo gravimetric analysis. The adsorption behaviors and kinetics of the materials towards selected metal ions, Cu (II), Cd(II) and Pb(II), were investigated. The durability of the material was estimated using repetitive adsorption-desorption. The nanofiber membranes are prepared using an electro spinning technique, and then the membranes are treated with water and alcoholic KOH to partially remove polyvinylpyrrolidone and deacetylate the cellulose acetate in order to increase binding site when further being functionalized with thiol groups using thioglycolic acid. In the earlier worker’s other references, the membranes were modified directly without being treated with water or other solvent. The advantage of the proposed thiol-functionalized cellulose Nano-fibers is greater adsorption capacity and faster adsorption speed.
Experimental
Cellulose Acetate (CA) is a useful material in industry with applications in the fields of coating, film and membrane separation. Through electro spinning, CA can also be transformed into nanofiber materials. In electro spinning, choosing appropriate solvent is vital because the solvent dictates not only whether a material can be electro spun into nanofibers, but also the quality of the nanofibers. It was reported that a mixed solvent of acetone/dimethyl acetyl amide (DMAc) (2/1) was the most versatile solvent for the electro spinning of CA (Liu H et. al, 2002). CA can also be mixed with other polymers to produce nanofibers, for example, CA/PolyVinylPyrrolidone (PVP) fibers were successfully prepared using electro spinning[42].
Preparation of the cellulose nanofiber membranes
Deacetylation
The CA/PVP nanofiber membranes were immersed in deionized water to partially remove the PVP component under super sonification for 3 h. After being dried at 80 °C under vacuum for 24 h, the membranes were deacetylated in alcoholic KOH solution (0.5 mol L-1) for 3 h. The regenerated cellulose membranes (RCM) were then rinsed with distilled water for several times and dried under vacuum at 80°C.
DSacetyl of RCM
RCM (0.1 g) was added to a solution of NaOH (0.2 mol L-1) in a mixed solvent (EtOH/H2O = 5/4, 10 mL) at ambient temperature for 72 h under stirring. The excessive base was titrated with HCl (0.1 mol L-1) using phenolphthalein as an indicator.
Modification of RCM with thioglycolic acid
RCM (6 g) were placed in a mixture of thioglycolic acid (50 mL), THF (50 mL) and suphuric acid (98%, 0.2 mL) in a flask (250 mL). The reaction mixture was gently stirred at 40 °C for 2 days. Then the membranes were successively washed with distilled water till neutrality before being further washed with ethanol for a few times to remove the water of the material as much as possible. The thiol-functionalized cellulose nanofiber membranes (TCM) were dried under vacuum at 30 °C for 2 days before being used in next step.
The optimization of adsorption conditions
Throughout this work, the membrane used for investigations was 50 mg and the volume of solution was controlled at 20 mL. The temperature for all experiments was controlled at 27 °C.
Effect of acidity
TCM (50 mg) was placed in a conical flask containing of solution (20.0 mL, 50 mg L-1) of M(II) (M = Cu, Cd, Pb). The initial pH of the solutions was adjusted to between 2.0 and 7.0 using either HCl (0.1 mol L-1) or NaOH (0.1 mol L-1) aqueous solution. The solution was kept at 27 °C under agitation for 36 h. The content of the metal ion remained in the solution was analyzed using AAS.
Effect of contacting time
TCM (50 mg) was added to M(II) solution (20 mL, 50 mg L-1). To the mixture was added HCl (0.1 mol L-1) or NaOH (0.1 mol L-1) aqueous solution to adjust the pH value. Agitation of the solution was performed at 27 °C for 12 h. The concentrations of the metal ions remained in the solution were measured every 2 h using AAS.
Adsorption isotherms
To a conical flask (50 mL) was added TCM (50 mg) and the metal ions solution (20 mL), whose pH value was adjusted to 6. The concentration of the metal ions varied from 10 to 80 mg L-1. The solution was kept at 27 °C under agitation for 12 h. The concentration of the metal ion remained in the solution was analyzed using AAS.
Desorption of the metal ions and repetitive adsorption
TCM (50 mg) adsorbed with the metal ions as described above (initial concentration 50 mg L-1) was used in the de- sorption investigation. The desorption of metal ions was carried out in HCl aqueous solutions (25 mL) of varying concentrations, ranging from 1 to 8 mol L-1. The conical flasks were kept at 27 °C under agitation for 36 h. The concentrations of the metal ions in the solutions were analyzed using AAS. After the desorption at optimal acidity, the membranes were rinsed with distilled water to remove any residual solution and then dried in vacuum at 30 °C for 2 days before being used in next round of adsorption-desorption investigation.
Electro spinning and grafting of thiol groups
A mixed solvent of acetone and DMAc was used for electro spinning. Various ratios of CA/PVP were used for the preparation of the nanofiber membranes. The morphology of the five CA/PVP composite fibers is shown in Figure 1. As shown by their SEM images, all of the electro spun nanofibers showed smooth surfaces and were cylindrical in shape. But it is noticeable that the smoothness of the fiber decreased with the increase of the ratio of CA/PVP. Com- pared to the fibers at other ratio, the material obtained at the ratio of 25/9 exhibited relatively even distributions in diameters. Therefore, the ratio of 25/9 was used throughout this work. To increase the surface area of the fibers, the water solubility of PVP was exploited. As shown in Figure 2, the electro spun CA/PVP composite fibers remained in a cylindrical form with slight decrease in diameter after being treated with water and grooves were found on the surface of the washed fibers which would lead to larger surface area. The water-treated fibers were further functionalized with thiol groups. To expose as many hydroxyl groups on the fiber surfaces as possible, the materials were deacetylated in alcoholic KOH solution[68].
The deacetylation efficiency was obtained using back-titration[69]. It was found that DS acetyl value decreased from 2.45 to 0.47. Figure 2 shows that the deacetylation altered significantly the morphologies of the nanofibers and fusions between the nanofibers were also observed. Furthermore, the CA nanofibers were slightly swollen after the deacetylation. The swelling could be attributed to the exposed hydroxyl groups which show strong affinity towards water. The deacetylated membrane was further functionalized with thiol groups to generate TCM using the procedure described in the literature[70]. In the reaction, thioglycolic acid reacted with the hydroxyl group to form ester bond to achieve the functionalization. The morphologies of the nanofibers before and after the functionalization (Figures 3 and 4) were hardly distinguishable.
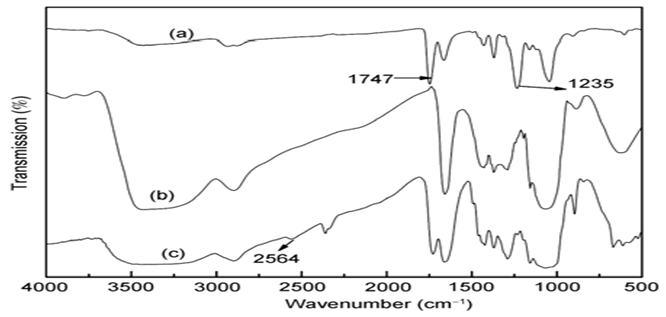
Infrared spectra of the washed electro spun CA/PVP membranes, RCM and TCM were shown in Figure 5. The broad band between 3400 and 3600 cm-1 in the infrared spectra of CA/PVP membranes is attributed to the hydroxyl group. After the deacetylation, additional hydroxyl groups were exposed on the surface of the materials, which is in agreement with the enhancement of the broad band. Figure 5(a) shows the characteristic absorption bands for the ester carbonyl (C=O, 1747 cm-1) and C–O–C skeleton (1235 cm-1) (Ma Z, Ramakrishna S.2008)[71]. But these bands disappeared entirely after the deacetylation in alcohol as shown in Figure 5(b), which suggests nearly complete deacetylation on the surface of the material (RCM). In Figure 5(c) is shown the infrared spectrum of the membrane functionalized with thioglycolic acid. The restoration of a strong and sharp band near 1747 cm-1 indicates the presence of the ester carbonyl group resulted from the functionalization. The weak absorption band of –SH group at 2573 cm-1[72] further supports the success of functionalization.
Nanofiber membranes were prepared using a mixture of CA/PVP via electro spinning technique. The nanofiber membranes were further converted into membranes possessing thiol functional group. The prepared material showed improved surface area after the bleaching of PVP before the functionalization (Figure 6). TGA characterization showed that the thermal stability of the nanofibers (RCM, TCM) was slightly compromised compared to the precursors (CA and PVP). The functionalization of the materials to achieve the desired material (TCM) was confirmed using infrared[73-75].
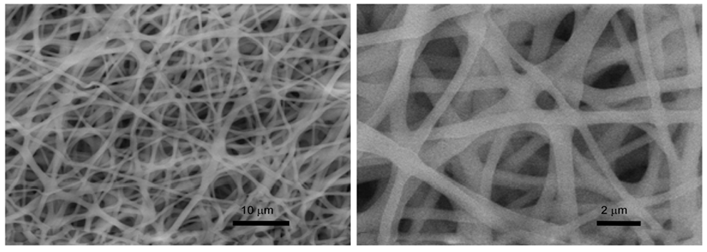
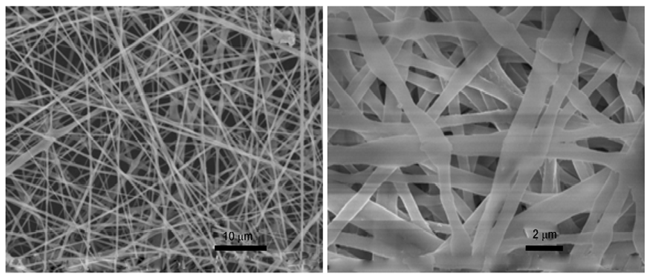
Figure 3: SEM images of RCM.
Figure 4: SEM images of TCM.
Figure 5: The infrared spectra of (a) the water-bleached CA/PVP mem- branes, (b) RCM and (c) TCM.
Figure 6: TGA curves of CA/PVP, RCM and TCM.
Conclusion and future prospect
Developing cost-effective technologies to solve the water pollution problems are critical for sustainable development of human society. The low cost raw cellulosic material has gained increasing attention due to stiffness, biodegradability and renewability. Cellulose fibers are hydrophilic in nature, so it becomes necessary to increase surface roughness for the development of composites with enhanced properties. Surface modification and functionalization of cellulosed- based nanofibers has got quite interesting and economical route for removal of pollutants from wastewater. In recent years, the development of membrane technology has geared up as a means to overcome water pollution effectively.
References
- 1. Progress on Drinking Water and Sanitation. (2015) WHO.
Pubmed||Crossref||Others
- 2. Mezher, T., Fath, H., Abbas, Z., et al. Techno-economic assessment and environmental impacts of desalination technologies. (2011) Desalination 266(1-3): 263–273.
- 3. Kabsch-Korbutowicz, M., Urbanowska, A. Comparison of polymeric and ceramic ultrafiltration membranes for separation of natural organic matter from water. (2010) Environ Protect Eng 3(1): 125–135.
Pubmed||Crossref||Others
- 4. Leong, S., Razmjou, A., Wang, K., et al. TiO2 based photocatalytic membranes: a review. (2014) J Membr Sci 472: 167–184.
- 5. Kim, J., Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: review of manufacturing procedures and performance improvement for water treatment. (2010) Environ Pollut 158(7): 2335–2349.
- 6. Mohammad, A.W., Teow, Y.H., Ang, W.L., et al. Nanofiltration membranes review: recent advances and future prospects. (2015) Desalination 356: 226–254.
- 7. Ismail, A.F., Padaki, M., Hilal, N., et al. Thin film composite membrane recent development and future potential. (2015) Desalination 356: 140–148.
- 8. Fane, A.G., Wang, R., Hu, M.X. Synthetic membranes for water purification: status and future. (2015) Angew Chem Int Ed Eng l549(11): 3368–3386.
- 9. Yin, J., Deng, B. Polymer-matrix nanocomposite membranes for water treatment.(2015) J Membr Sci 479: 256–275.
- 10. Lau, W.J., Gray, S., Matsuura, T., et al. A review on polyamide thin film nanocomposite (TFN) membranes: history, applications, challenges and approaches. (2015) Water Res 80: 306–324.
- 11. Carpenter, A.W., de Lannoy, C.F., Wiesner, M.R. Cellulose nanomaterials in water treatment technologies. (2015) Environ Sci Technol 49(9): 5277–5287.
- 12. Liu, C.X., Zhang, D.R., He, Y., et al. Modification of membrane surface for anti-biofouling performance: effect of anti-adhesion and anti-bacteria approaches. (2010) J Membr Sci 346: 121–130.
- 13. Lin, S.,Chen, L.,Huang, L., et al. Novel antimicrobial chitosan–cellulose composite films bioconjugated with silver nanoparticles. (2015) Ind Crops Prod 70: 395–403.
- 14. Zhang, L.,Bai, R. Novelmulti-functional membrane technology for visual detection and enhanced adsorptive removal of lead ions in water and wastewater. (2011) Water Sci Technol 11:113–120.
- 14. Zhang, L., Zhao, Y.H., Bai, R. Development of a multifunctional membrane for chromatic warning and enhanced adsorptive removal of heavy metal ions: application to cadmium. (2011) J Membr Sci 379: 69–79.
- 16. Booshehri, A.Y., Wang, R., Xu, R. SimplemethodofdepositionofCuOnanoparticleson a cellulose paper and its antibacterial activity. (2015) Chem Eng J 262: 999–1008.
- 17. Bai, H., Liu, Z., Sun, D.D. A hierarchically structured and multifunctional membrane for water treatment. (2012) Appl Catal B111–112: 571-577.
- 18. Bendi, R., Imae, T. Renewable catalyst with Cu nanoparticles embedded into cellulose nano-fiber film. (2013) RSC Adv 3: 16279–16282
- 19. Gholami, A., Moghadassi, A.R., Hosseini, S.M., et al. Preparation and characterization of polyvinyl chloride-based nanocomposite nanofiltration-membrane modified by iron oxide nanoparticles for lead removal from water. (2014) J Ind Eng Chem 20(4): 1517–1522
- 20. Ghaemi, N., Madaeni, S.S., Alizadeh, A., et al. Fabrication of cellulose acetate/sodium dodecyl sulfate nanofiltration membrane: characterization and performance in rejection of pesticides. (2012) Desalination 290: 99–106.
- 21. Azari, S., Zou, L., Cornelissen, E., et al. Facile fouling resistant surface modification of microfiltration cellulose acetate membranes by using amino acid l-DOPA. (2013) Water Sci Technol 68(4): 901–908.
- 22. Zawierucha, I., Kozlowski, C., Malina, G. Removal of toxic metal ions from landfill leachate by complementary sorption and transport across polymer inclusion membranes. (2013) Waste Manag 33(10): 2129–2136.
- 23. Velu, S., Rambabu, K., Muruganandam, I. Preparation, characterization and application of cellulose acetate-iron nanoparticles blend ultrafiltration membranes. (2013) J Chem Pharm Res 5: 1418–1428.
Pubmed||Crossref||Others
- 24. Gul, S., Waheed, S., Ahmad, A., et al. Synthesis, characterization and permeation performance of cellulose acetate/polyethylene glycol-600 membranes loaded with silver particles for ultralow pressure reverse osmosis. (2015) J Taiwan Institute Chem Eng 57: 129-138.
- 25. Perera, D.H.N., Nataraj, S.K., Thomson, N.M., et al. Room-temperature development of thin film composite reverse osmosis membranes from cellulose acetate with antibacterial properties. (2014) J Membr Sci 453: 212–220.
- 26. Li, R., Liu, L., Wang, F. Removal of aqueous Hg(II) and Cr(VI) using phytic acid doped polyaniline/cellulose acetate composite membrane. (2014) J Hazardous Mater 280: 20–30.
- 27. Ahmad, A., Waheed, S., Khan, S.M., et al. Effect of silica on the properties of cellulose acetate/polyethylene glycol membranes for reverse osmosis. (2015) Desalination 355: 1–10.
- 28. Zirehpour, A., Rahimpour, A., Seyedpour, F., et al. Developing new CTA/CA-based membrane containing hydrophilic nanoparticles to enhance the forward osmosis desalination. (2015) Desalination 371: 46–57.
- 29. Akin, I., Ersoz, M. Preparation and characterization of CTA/m-ZnO composite membrane for transport of Rhodamine B. (2014) Desalination Water Treat 57(7): 3037-3047.
Pubmed||Crossref||Others
- 30. Salma, A., Ounissa, K.S., Fadila, H., et al. Equilibrium and kinetic modeling of acid dye removal from aqueous solution by polymer inclusion membrane (PIMs). (2014) Desalination Water Treat 57(8): 3707-3719.
- 31. Baczynska, M., Regel-Rosocka, M., Nowicki, M., et al. Effect of the structure of polymerin clusion membranes on Zn(II) transport from chloride aqueous solutions. (2015) J Appl Polym 132(30): 42319.
- 32. Rodriguez, R.V., Montero-Caberera, M.E., Esparza-Ponce, H.E., et al. Uranium removal from water using cellulose triacetate membranes added with activated carbon. (2012) Appl Rad Isotopes 70: 872–881.
- 33. Oberta, A., Wasilewski, J., Wodzki, R. Structure and transport properties of polymer inclusion membranes for Pb(II) separation. (2011) Desalination 271:132–138.
- 34. Perez-Silva, I., Galan-Vidal, C.A., Ramirez-Silva, M.T., et al. Phenol removal process development from synthetic wastewater solutions using a polymer inclusion membrane.(2013) Ind Eng Chem Res 52: 4919–4923.
- 35. Chen, J.H., Ni, J.C., Liu, Q.L., et al. Adsorption behavior of Cd(II) ions on humic acid-immobilized sodium alginate and hydroxyl ethyl cellulose blending porous composite membrane adsorbent. (2012) Desalination 285: 54–61.
- 36. Shenvi, S.S., Isloor, A.M., Ismail, A.F., et al. Humic acid based biopolymeric membrane for effective removal of methylene blue and rhodamine B. (2015) Ind Eng Chem Res 54: 4965–4975.
- 37. Noor Hana., Hanif, Baker, A. Natural Composite Membranes for Water Remediation: Toward a Sustainable Tomorrow. (2012) Renewable Energy and Sustainable Technologies for Building and Environmental Applications 25-49.
Pubmed||Crossref||Others
- 38. Bet-Moushoul, E., Mansourpanah, Y., Farhadi, K., et al. TiO2 nano composite based polymeric membranes: a reviews on performance improvement for various applications in chemical engineering processes. (2016) Chem Eng J283: 29–46.
- 39. Zhang, L., Bai, R. Novel multi-functional membrane etechnology for visual detection and enhanced adsorptive removal of lead ions in water and wastewater. (2011) Water Sci Technol 11(1): 113–120.
- 40. Taha, A.A., Wu, Y.N., Wang, H, et al. Preparation and application of functionalized cellulose acetate/silica composite nanofibrous membrane via electrospinning for Cr(VI) ion removal from aqueous solution. (2012) J Environ Manag 112: 10–16.
- 41. Meng, J.,Zhang, X.,Ni, L., et al. Antibacterialcellulose membrane via one-step covalent immobilization of ammonium/amine groups. (2015) Desalination 359(2): 156–166.
- 42. Zhang, L., Hsieh, Y.L. Ultrafine Cellulose Acetate Fibres with Nanoscale Structural Features.(2008) J Nanosci Nanotechno 8(9): 4461–4469.
- 43. History of reverse osmosis filtration.
Pubmed||Crossref||Others
- 44. Pagidi, A., Thuyavan, Y.L., Arthanareeswaran, G., et al. Polymeric membrane modification using SPEEK and bentonite for ultrafiltration of dairy wastewater. (2015) J Appl Polym Sci 132(21): 41651.
- 45. Kiran, S.A., Arthanareeswaran, G., Thuyavan, Y.L., et al. Influence of bentonite in polymer membranes for effective treatment of car wash effluent to protect the ecosystem. (2015) Ecotoxicol Environ Safety 121: 186–192.
- 46. Zirehpour, A., Rahimpour, A., Seyedpour, F., et al. Developing new CTA/CA-based membrane containing hydrophilic nanoparticles to enhance the forward osmosis desalination. (2015) Desalination 371: 46–57.
- 47. Thaci, B., Gashi, S., Daci, N., et al. Effect of modified coal through chemical activation process on performance of heterogenous reverse osmosis membranes. (2015) Environ Protect Eng 41: 53–65.
Pubmed||Crossref||Others
- 48. El-Din, L.A.N., El-Gendi, A., Ismail, N., et al. Evaluation of cellulose acetate membrane with carbon nanotubes additives. (2015) J Ind Eng Chem 26: 259–264.
- 49. Worthley, C.H., Constantopoulos, K.T., Ginic-Markovic, M., et al. A study into the effect of POSS nanoparticles on cellulose acetate membranes. (2013) J Membr Sci 431: 62–71.
- 50. Rodriguez, R.V., Montero-Caberera, M.E., Esparza-Ponce, H.E, et al. Uranium removal from water using cellulose triacetate membranes added with activated carbon. (2012) Appl Rad Isotopes 70: 872–881.
- 51. Shenvi, S.S., Isloor, A.M., Ismail, A.F., et al. Humic acid based biopolymeric membrane for effective removal of methylene blue and rhodamine B.(2015) Ind Eng Chem Res 54(18): 4965–4975.
- 52. Ting, X., ZuLei, Z., HaiQing, L., et al. Characterization in adsorptive behaviors using Cu(II), Cd (II), Pb (II) as models, (2013) Science China Chemistry 56.
Pubmed||Crossref||Others
- 53. Khan, S.B., Alamry, K.A., Bifari, E.N., Asiri, A.M., et al. Assessment of antibacterial cellulose nanocomposites for water permeability and salt rejection. (2015) J Ind Eng Chem 24: 266–275.
- 54. Abedini, R., Mousavi, S.M., Aminzadeh, R. A novel cellulose acetate (CA) membrane using TiO2 nanoparticles: preparation, characterization and permeation study. (2011) Desalination 277(1-3): 40–45.
- 55. Zeng J, Liu S, Zhang L. TiO2 immobilized in cellulose matrix for photocatalytic degradation of phenol under weak UV light irradiation. (2010) J Phys Chem C 114(17): 7806–7811
- 56. Wang, R.M., Wang, H., Wang, Y., et al. Preparation and photocatalytic activity of chitosan-supported cobalt phthalocyanine membrane. (2014) Coloration Technol 130: 32–36.
- 57. Kulkarni, A., Bambole, V.A., Mahanwar, P.A. Electrospinning ofPolymers, Their Modeling and Applications. (2010) Polym Plast Technol Eng 49: 427–441.
- 58. Shalumon, K.T., Binulal, N.S., Selvamurugan, N., et al. Electrospinning of carboxymethyl chitin/poly(vinyl alcohol) nanofibrous scaffolds for tissue engineering applications. (2009) Carbohydr Polym 77(4): 863–869.
- 59. Huang, C., Chen, S., Reneker, D.H., et al. High-strength mats from electrospun poly(p-phenylene biphenyl tetracarboximide) nanofibers. (2006) Adv Mater 18(5): 668–671.
- 60. Ki, C., Gang, E., Um, I., et al. Nanofibrous membrane of wool keratose/silk fibroin blend for heavy metal ion adsorption. (2007) J Membr Sci 302(1-2): 20–26.
- 61. Tian, Y., Wu, M., Liu, R., et al. Electrospun membrane of cellulose acetate for heavy metal ion adsorption in water treatment. (2011) Carbohydr polym 83(2): 743–748.
- 62. Stephen, M., Catherine, N., Brenda, M., et al. Oxolane-2,5-dione modified Electrospun cellulose nanofibers for heavy metals adsorption. (2011) J Hazard Mater 192: 922–927.
- 63. Neghlani, P.K., Rafizadeh, M., Taromi, F.A. Preparation of aminated-polyacrylonitrile nanofiber membranes for the adsorption of metal ions: comparison with microfibres. (2011) J Hazard Mater 186(1): 182–189.
- 64. Kampalanonwat, P., Supaphol, P. Preparation and adsorption behavior of aminatedelectrospunpolyacrylonitrile nanofiber mats for heavy metal ion removal. (2010) Appl Mater Interface 2(12): 3619–3627.
- 65. Wu, Y., Jia, W., An, Q., et al. Multiaction antibacterial nanofibrous membranes fabricated by electrospinning: an excellent system for antibacterial applications. (2009) Nanotechnol 20(24): 245101.
- 66. Teng, M., Wang, H., Li, F., et al. Thioether-functionalized mesoporous fibre membranes: sol-gel combined electrospun fabrication and their applications for Hg2+ removal. (2011) J Colloid Inter- face Sci 355(1): 23–28.
- 67. Sun, M., Ding, B., Yu, J. Sensitive metal ion sensors based on fibrous polystyrene membranes modified by polyethyleneimine. (2012) RSC Adv 2(4):1373–1378
Pubmed||Crossref||Others
- 68. Liu, Y., Wu, Z., Chen, X., et al. A hierarchical adsorption material by incorporating mesoporous carbon into macroporous chitosan membranes. (2012) J Mater Chem 22: 11908–11911.
Pubmed||Crossref||Others
- 69. Liu, H., Hsieh, Y.L. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. (2002) J Polym Sci, Part B:Polym Phys 40: 2119–2129.
- 70. Wang, Q.C., Zhang, L., Zhao, Z.G. A method of preparing mercapto cotton. (2003) CN Patent 200310115874.3
Pubmed||Crossref||Others
- 71. Ma, Z., Ramakrishna, S. Electrospun regenerated cellulose nanofiber affinity membrane functionalized with protein A/G for IgG purification. (2008) J Membr Sci 319(1): 23–28.
- 72. Sharma, R., Ahuja, M. Thiolated pectin Synthesis, characterization and evaluation as a mucoadhesive polymer.(2012) Carbohydr Polym 85(3): 658-663.
- 73. Agarwal, S., Wendorff, J.H., Greiner, A. Progress in the field of electrospinning for tissue engineering applications. (2009) Adv Mater 21(32-33): 3343–3351.
- 74. Wu, S., Li, F., Wang, H., et al. Effects of poly (vinyl alcohol) (PVA) content on preparation of novel thiol-functionalized mesoporous PVA/SiO2 composite nanofiber membranes and their application for adsorption of heavy metal ions from aqueous solution. (2010) Polymer 51(26): 6203–6211.
- 75. Wang, M., Meng, G., Huang, Q., et al. Electrospun 1,4-DHAQ-Doped cellulose nanofiber films for reusable fluorescence detection of trace Cu2+ and further for Cr3+. (2012) Environ Sci Technol 46(1): 367–373.