In Vitro Cytotoxicity Evaluation of Polymerized Resin Cements on the Viability of L-929 Fibroblast Cells
Affiliation
- 1Dental Materials Research Center and Department of Operative Dentistry, Faculty of Dentistry, Mashhad University of Medical Sciences, Mashhad, Iran
- 2Department Immunologist, Buali Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
Corresponding Author
Horieh Moosavi. Dental Materials Research Center and Department of Operative Dentistry, Faculty of Dentistry, Mashhad University of Medical Sciences, Mashhad, Iran. E-mail: dentist_57@yahoo.com , Moosavih@mums.ac.ir
Citation
Moosavi, H., et al. In Vitro Cytotoxicity Evaluation of Polymerized Resin Cements on the Viability of L-929 Fibroblast Cells. (2015) J Dent & Oral Care 1(4): 1-5.
Copy rights
©2015 Moosavi, H. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Resin Cement; Cytotoxicity; L-929; MTT
Abstract
Objective: This study was designed to evaluate the cytotoxicity of two resin-based cements; Bistite II DC, and Multi Bond II, after polymerization on cultured L-929 fibroblast cells.
Methods: Disc-shaped samples were prepared in polyethylene molds with cylindrical cavities (5 mm diameter, 2 mm high) of BistiteII DC and Multi Bond II. After setting of resin cement disc, they were aged for 1, 3, and 5 days in Dulbecco’s Modified Eagle’s Medium (DMEM). Cell viability of L-929 fibroblast cells was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and the difference between the groups was tested by analysis of variance and Tukey tests (a = 0.05).
Result: After 1 day of storage, the Bistite II DC, and Multi Bond II were essentially non-cytotoxic. On days 5 of the experiment, the cell viability of two resin cements did not differ significantly (P > 0.05), but cell viability was slightly reduced on day 5. There was no significant difference in the cytotoxicity between Bistite II DC, and Multi Bond II.
Conclusion: After polymerization, two resin-based cements (Bistite II, and Multi Bond) induced slight cytotoxicity. The sensitivity of cytotoxicity to L-929 cells in depended on the type of resin-based cements.
Introduction
The aesthetic aspect of dental treatment has become increasingly popular in the recent years, especially with the development of improved materials and adhesive techniques using composite resins. The indirect composite resin restoration technique involves extra-oral fabrication of an inlay and its placement with resin cement. It has been reported that for large cavities, indirect restorations bear advantages over direct techniques such as improvements in anatomic form, con-tour, fracture resistance and wear resistance[1]. The major goals of using resin-based adhesive materials are to enhance the bonding strength between restoration and the tooth structure, reduce the micro-leakage in the dentin–restoration interface and scatter the occlusal stress. Resin-based cement is necessary to be used for cementing non-metal prosthesis for the advantages of esthetic and strength. As resin-based adhesive materials come into close and prolonged contact withgingivo–dentin complex, their safety influence on soft tissue is of great interest, especially when the finishing line is locatedin gingival sulcus during tooth preparation[2]. When the remaining thickness of dentin is thin dental adhesive/cement compounds can be eluted from dental materials and can be swallowed by saliva and then they can enter the organism. Dental compounds can be metabolized to very toxic agents in the organism. Furthermore dental compounds can enter the organism by uptake from the blood in the pulp and can then enter the organism by this route. The observed toxicity in cells/organisms is therefore may not be caused by the main molecule but by the toxic intermediates formed in the metabolism of eluted dental compounds. It has been shown that resin-based adhesive materials exert potential harmful effects to the soft tissue. The biological safety of dentin-bonding agents has been extensively studied[3,4], but reports on the biological safety of new resin-based cements to cultured L-929 fibroblast cells are still rare.“In vitro cytotoxicity test” has the advantage of easy control of experimental factors that are often a problem when performing experiments in vivo. In vitro methods are reproducible, cost-effective, relevant, and suitable for the evaluation of basic biological properties of dental materials. According to the possible dangerous effects of these components’ cytotoxicity it is very important to know how they affect the cells. To evaluate the cytotoxicity of resin-based cements on cultured L-929 fibroblast cells completely, this study evaluates the cytotoxicity of two resin-based cements (Bistite II DC, and Multi Bond II) after polymerization on cultured L-929 fibroblast cells. There is a few information about the cytotoxicity and biocompatibility of resin components. Therefore we examined the impact of two dentin bonding agents on L-929 mouse fibroblast cells.
Materials and Methods
Resin-based Cements and Sample Preparation Two resin-based cements were evaluated: Bistite II DC, (Tokuyama Dental Corporation, Japan), and Multi Bond II (Tokoyama, Dental Corporation, Tokyo, Japan). The cements were prepared according to the application instructions (Table 1) under aseptic conditions and were applied into polyethylene rings with diameter of 5 mm and height of 2 mm. Samples were polymerized in accordance with the application instructions (Table 1). Sodium dodecyl sulfate (SDS) was used as positive control and DMEM without serum as negative control.
Table 1: Resin-based cements tested and their instructions of application.
| Material (Abbreviation) Manufacturer Lot no. Composition Procedure | Composition | Procedure |
|---|---|---|
| Bistite II DC (BT) Tokuyama Dental 028012 | Primer 1 (A and B): phosphoric acid monomer, acetone, alcohol, water, and initiator. Primer 2: HEMA, acetone, initiator. Resin cement pastes: Paste-A: NPGDMA, Bis-MPEPP, silica-zirconia filler. Paste-B: MAC-10, silica-zirconia filler, benzoylperoxide, photo-initiator. |
Apply primer 1A + 1B, leave for 30 s, air dry, apply primer 2, leave for 20 s, air-dry, place mixed paste A + B, light cure for 20 s. |
| Multi Bond II (MB) Tokuyama Dental 0780Z1 | Primer: phosphoric acid monomer, water, acetone, UDMA, co-activator. Liquid: MMA, UDMA, HEMA, MTU-6, borate catalyst. Powder: PMMA, co-activator. |
Apply primer for 20 s and gently air dry for 10 s. Powder: liquid: 1:3 Mix for 5 s, apply to disc surface. |
Abbreviations: HEMA: 2-hydroxyethyl methacrylate, MAC-10: methacryloyl un decanedicarboxylic acid, MMA: methyl methacry- late, PMMA: poly methyl methacrylate, UDMA: urethane dimethacrylate, MTU-6: 6-methacryloxyhexyl 2-thiouracil-5-carboxylate.
Cell Culture
The mouse fibroblast cell line (L929) was obtained from National Cell Bank of Iran (NCBI, Tehran, Iran) and used for the experiment. Cells were cultivated as a monolayer culture in DMEM medium (Gibco, Detroit, USA), supplemented with 10% FBS (Gibco, Detroit, USA), 100 IU/ml penicillin and 100 μg/ml streptomycin (Gibco, Detroit, USA) at 37°C in a fully humidified air atmosphere containing 5% CO2. For the experiments, cells were removed from the flasks using a 0.25% trypsin-EDTA solution (Gibco, Detroit, USA). Briefly, cells (2×104 cells/well) were seeded onto 96-well microplate and allowed to adhere overnight. Then, each cell line was exposed to increasing concentrations of aqueous extract (5-2000 μg/ml) for 24 h. The first column of each microplate was assumed as negative control (containing no extract).
Exposure of Cells to Tested Materials
The sample disks were removed immediately after curing. For the prevention from bacterial contamination, all sample disks were sterilized with UV-radiation for 45 minutes on each side. Then, L929 fibroblasts were treated with resins in two following ways.
Incubation with Medium Containing the Compounds
Prepared disks were placed into 1 ml of freshly prepared DMEM medium for 1,3 and 5 days. Then, L929 fibroblasts were treated with or without pre-incubated medium at a density of 1×104 cells in polystyrene 96-well tissue culture plates (Costar) for 24h at 37°C in a fully humidified air atmosphere containing 5% CO2.
Direct Contact Testing L929 fibroblasts were seeded at a density of 4×104 cells in polystyrene 24-well tissue culture plates (Costar) and the cell layer was exposed to a flat surface of the disk for 1,3 and 5 days. Paper disks with the resembling diameters were used as negative controls. The interface area of 12.5% and the volume ratio was 2.74 cm²/ ml, which is within the recommended range of 0.5– 6.0 cm²/ ml suggested by ISO 10993- 5 and International Organization for Standardization.
Cytotoxicity Assessment Using MTT Assay
Cytotoxicity of cells was measured using MTT (Sigma, St Louis, USA) colorimetric assay. The assay is based on the metabolic reduction of soluble MTT by mitochondrial enzyme activity of viable cells into an insoluble colored Formosan product, which can be measured spectrophotometrically after dissolving in dimethyl sulfide (DMSO). To assay the cytotoxicity, MTT solution (5 mg/ml in phosphate buffered solution) was added to each well and the plate was incubated for 3 h at 37°C. Then, DMSO was replaced and dissolved any Formosan crystals. The optical density (OD) was read on an Elisa reader (Microplate reader MR 600, Dynatech, USA) at a wavelength of 545 nm. The inhibitory rate of cytotoxicity was calculated by the following formula:
Cytotoxicity percent = [1- (ODcontrol - ODtreated)/ODcontrol]×100
Cytotoxicity was rated based on cell viability relative to controls as[5]:
Non-cytotoxic => 90% cell viability;
Slightly cytotoxic = 60– 90% cell viability;
Moderately cytotoxic = 30– 59% cell viability;
Severely cytotoxic = ≤ 30% cell viability.
Morphological alteration of the L-929 cells was observed directly by phase contrast microscope and photographed by a Nikon camera.
Statistical Analysis
Five replicates of each concentration were performed in each test. All assays were repeated four times to ensure reproducibility. The significance of differences were evaluated using SPSS 11.0 software by one way analysis of variance (ANOVA) and Bonferroni’s post doc test. All results were expressed as mean ± SEM. A probability level of P < 0.05 was considered statistically significant.
Results
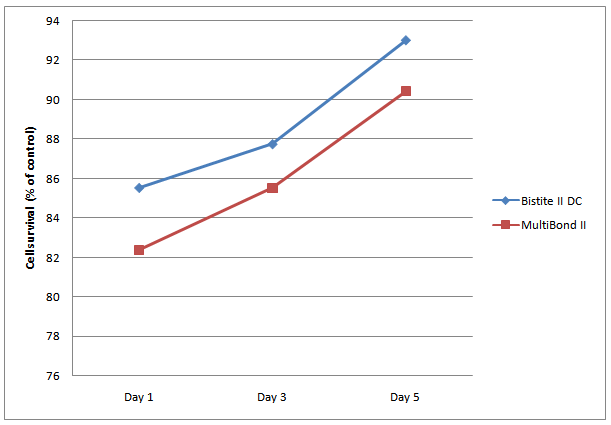
The Relative growth rates (RGRs) of cultured L-929 cells are shown in Table 2. The RGR of cells exposed to Bistite II DC and MultiBond II were 85.54%, and 82.39%, respectively, while 100% in negative control group and 0.00% in positive control group.
Table 2: Relative growth rate (RGR, %) and cytotoxic rates.
| Experimental groups | Day 1 | Day 3 | Day 5 | |||
|---|---|---|---|---|---|---|
| RGR | SD | RGR | SD | RGR | SD | |
| Bistite II DC | 85.54 | 2. 71 | 87.75 | 2. 49 | 93.00 | 3. 38 |
| Slight | Slight | Non | ||||
| Multi Bond II | 82.39 | 1. 45 | 85.52 | 1. 04 | 90.42 | 1. 34 |
| Slight | Slight | Non | ||||
| Positive control | 4.06 (SD 0.02) severely cytotoxic | |||||
| Negative control | 100** non-cytotoxic | |||||
*Denote significant differences between the experiment groups and negative control with p < 0.01.
There was no significant difference in the cytotoxicity between the Bistite II DC and MultiBond II group. There was significant difference between the experiment group and negative control groups. At each concentration level, there was no significant difference in the cytotoxicity between Bistite II DC and MultiBond II. The RGR increased along with the increasing the time of cements curing (Figure1).
Figure 1: Cytotoxicity trends of the Bistite II DC and MultiBond II resin cements during the total period of the experiment.
Discussion
Cytotoxicity of dental resins and their component materials has been shown in several studies. In addition, a recent study has shown that degradation derivatives of dental resins could cause comparable toxic effects as the raw monomers[6,7]. In vitro cytotoxicity tests should be performed with cells homologous to the human tissue of ultimate concern[8-10]. Cells in the resting stage seem to reflect the in vivo condition more closely than do cells in the growing phase[11]. Therefore, the cytotoxicity of resin-based cements was examined on confluent cells in this study. In the present study, the mean relative growth rate of mouse L-929 cells exposed to BistiteII DC, and MultiBond II groups, while 100% in negative control group and 0.00% in positive control group. That means that the two resin-based cements have slight cytotoxicity after polymerization. At the same time, the rank orders with respect to cytotoxicity were found to be as follow: BistiteII DC < MultiBond II. There was no significant difference in the cytotoxicity between BistiteII DC, and Multi Bond II. Significant differences were observed between the groups of this study after 1 day of incubation, with the MultiBond II being moderately cytotoxic. However, ageing reduced the cytotoxicity of the MultiBond II cement resin. Previous studies have also demonstrated decreased cytotoxicity of dental composites with increasing pre-incubation periods that is almost immeasurable after 7 days[12]. On day 5 of the experiment, cell viability was almost 100 per cent in all groups; however, a mild cytotoxic effect was observed on day 5 in two groups. This may be due to water absorption that provokes monomer release[13]. Variable cytotoxic trends over the total period of such experiments have been reported[14]. It was demonstrated that different light-cured bonding systems resulted in cell toxicity levels significantly lower than that of a chemically cured system (Concise). In their study, the cytotoxicity of all substances diminished after 7 days of pre-incubation, with Concise still being the material with the highest cytotoxicity level[15]. Moderate cytotoxicity of the MultiBond II on day 1 of the present experiment was probably related to insufficient polymerization or severe toxicity of its liquid activator[16]. In clinical application of MultiBondII, a layer of activator is applied both to the tooth surface and to the indirect restoration base with the adhesive sandwiched between them. If the adhesive is very thin, the activator/adhesive ratio increases which may cause more residual un-polymerized monomers within the system. On the other hand, an increase in adhesive thickness produces an inhomogeneous polymerization pattern due to insufficient activator penetration, which may result in cytotoxicity. The latter phenomenon may have little significance in the current study because the dimensions of the specimens were controlled. Since cement resin contain higher ratios of resin diluents, higher cytotoxicity of this type of materials was expected in the present study, but the results showed excellent biocompatibility. This finding is in agreement with the results of study who compared hybrid, condensable, and flowable composites and found no toxic effects of flowable composite during the 2 day interval, while the other groups were moderately cytotoxic[17]. It was concluded that since cell culture toxicity data are highly model dependent, the test protocols to screen the toxicity of dental materials should be standardized to obtain comparable results[18]. Several studies have indicated that cytotoxic effects in cell culture are mainly caused by released monomers. Curing of resin-based cement is usually not complete, unconverted monomers can be released from resin into an adjacent aqueous phase and can diffuse through dentin to the pulp space[19,20]. The sensitivity of cytotoxicity to cultured L-929 cells depended on the resin-based cements tested. This may be due to differences in the content and component of monomers or additives of two resin-based cements. Bistite II DC is dual-cure resin cement based on Bis- MPEPP. It contains MAC-10, a special adhesive monomer. It has been shown that some methacrylate monomer (Bis-MPEPP) is strong cytotoxic to fibroblast[21,22]. MultiBond II is self-cure dental adhesive resin cement based on MMA (methyl methacrylate). It contains 4-methacryloxyethyl trimellitate anhydride (4-META), a high performance bonding monomer, and tri-n-butylborane (TBB), a catalyst. BistiteII DC is dual-cure resin cement, also contains 4-META. Compared with other polyfunctional methacrylate monomers, MMA has a low potential for irritation and 4-META may not affect the cytotoxicity induction[23]. This may be the reason why there was no significant difference in the cytotoxicity between Bistite II DC and MultiBond II at the same concentration and both showed slight cytotoxicity. However the sensitivity of cytotoxicity to human pulp cells depended on the resin-based cements and the concentration of the elution. Bistite II DC is lower cytotoxic agent than MultiBond II resin-based cement. The present findings suggest that additional care should be taken when manipulating cement resin, especially MultiBond II resin-based cement. This is emphasized by the fact that disposable gloves are permeable to methyl methacrylate and its derivatives[24,25]. It has been recommended that complete evacuation of remnant activators should be accomplished after cement resin setting with water spray and suction. Care should be taken to remove excess cement resin around the indirect restoration after polymerization, especially in areas where the cement resin may be in close contact with oral tissues, such as the subgingival and interproximal areas[26,27]. It should be borne in mind that in vitro cytotoxicity tests do not completely represent the cytotoxic properties of materials in the oral environment. It is known that the oral mucosa is generally more resistant to toxic substances than cell cultures because of the musin and keratin layers[28]. In addition, the sublethal effects of adhesive materials during prolonged exposure, which may cause estrogenic effects, should not be neglected. Further research is required to focus on the longterm effects of these materials.
Conclusion
Within the limitation of the present study, the MultiBond II showed moderate cytotoxicity, this subsided considerably during longer incubation, while the Bistite II DC showed suitable biocompatibility. So care should be undertaken during the manipulation of cement resin base materials.
Acknowledgment
The authors would like to acknowledge the research chancellor of Mashhad University of Medical Sciences for providing this research with financial support (grant number # 900744). There is no conflict of interest.
References
- 1. Wassell, R.W., Walls, A.W., McCabe, J.F. Direct composite inlays versus conventional composite restorations: three-year clinical results. (1995) Br Dent J 179(9): 343- 349.
- 2. Schedle, A., Franz, A., Rausch-Fan, X., et al. Cytotoxic effects of dental composites, adhesive substances, compomers and cements. (1998) Dent Mater 14(6): 429- 440.
- 3. Chen, R.S, Liu, C.C., Tseng, W.Y., et al. Cytotoxicity of three dentin bonding agents on human dental pulp cells. (2003) J Dent 31(3): 223- 229.
- 4. Huang, F.M., Chang, Y.C. Cytotoxicity of dentine-bonding agents on human pulp cells in vitro. (2002) IntEndod J 35(11): 905- 909.
- 5. Sjögren, G., Sletten, G., Dahl,J.E. Cytotoxicity of dental alloys, metals, and ceramics assessed by millipore filter, agar overlay, and MTT tests. (2000) J Prosthet Dent 84(2): 229- 236.
- 6. VandeVannet, B.M., Hanssens, J.L. Cytotoxicity of two bonding adhesives assessed by three-dimensional cell culture. (2007) Angle Orthod 77(4): 716- 722.
- 7. Emmler, J., Seiss, M., Kreppel, H., et al. Cytotoxicity of the dental composite component TEGDMA and selected metabolic by-products in human pulmonary cells. (2008) Dent Mater 24(12): 1670- 1675.
- 8. Yesilsoy, C., Feigal, R.J. Effects of endodontic materials on cell viability across standard pore size filters. (1985) J Endod 11(9): 401- 407.
- 9. Chang, Y.C., Huang, F.M., Cheng, M.H., et al. In vitro evaluation of the cytotoxicity and genotoxicity of root canal medicines on human pulp fibroblasts. (1998) J Endod 24(9): 604- 606.
- 10. Chang, Y.C., Tai, K.W., Huang, F.M., et al. Cytotoxic and nongenotoxic effects of phenolic compounds in human pulp cell cultures. (2000) J Endod 26(8): 440- 443.
- 11. Huang, F.M., Chang, Y.C. Cytotoxicity of resin-based restorative materials on human pulp cell cultures. (2002) Oral Surg Oral Med Oral Pathol Oral RadiolEndod 94(3): 361- 365.
- 12. Nalçaci, A., Oztan, M.D., Yilmaz, S. Cytotoxicity of composite resins polymerized with different curing methods. (2004) IntEndod J 37(2): 151- 156.
- 13. Lassila, L.V., Vallittu, P.K. Denture base polymer AlldentSinomer: mechanical properties, water sorption and release of residual compounds. (2001) J Oral Rehabil 28(7): 607- 613.
- 14. Sigusch, B.W., Völpel, A., Braun, I., et al. Influence of different light curing units on the cytotoxicity of various dental composites. (2007) Dent Mater 23(11): 1342- 1348.
- 15. Jonke, E., Franz, A., Freudenthaler, J., et al. Cytotoxicity and shear bond strength of four orthodontic adhesive systems. (2008) Eur J Orthod 30(5): 495- 502.
- 16. Terhune, W.F., Sydiskis, R.J., Davidson, W.M. In vitro cytotoxicity of orthodontic bonding materials. (1983) Am J Orthod 83(6): 501- 506.
- 17. Nalçaci, A., Oztan, M.D., Yilmaz, S. Cytotoxicity of composite resins polymerized with different curing methods. (2004) Int Endod J 37(2): 151- 156.
- 18. Franz, A., König, F., Lucas, T., et al. Cytotoxic effects of dental bonding substances as a function of degree of conversion. (2009) Dent Mater 25(2): 232- 239.
- 19. Gerzina, T.M., Hume, W.R. Effect of dentine on release of TEGDMA from resin composite in vitro. (1994) J Oral Rehabil 21(4): 463- 468.
- 20. Gerzina, T.M., Hume, W.R. Diffusion of monomers from bonding resin-resin composite combinations through dentine in vitro. (1996) J Dent 24(1-2): 125- 128.
- 21. Ratanasathien, S., Wataha, J.C., Hanks, C.T., et al. Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts. (1995) J Dent Res 74(9): 1602- 1606.
- 22. Geurtsen, W., Lehmann, F., Spahl, W., et al. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. (1998) J Biomed Mater Res 41(3): 474- 480.
- 23. Fujisawa, S., Atsumi, T. Cytotoxicities of a 4-META/MMA-TBBO resin against human pulp fibroblasts. (2004) Dent Mater J 23(2): 106- 108.
- 24. Afsahi, S.P., Sydiskis, R.J., Davidson, W.M. Protection by latex or vinyl gloves against cytotoxicity of direct bonding adhesives. (1988) Am J Orthod Dentofacial Orthop 93(1): 47- 50.
- 25. Lönnroth, E.C., Wellendorf, H., Ruyter, E. Permeability of different types of medical protective gloves to acrylic monomers. (2003) Eur J Oral Sci 111(5): 440- 446.
- 26. Terhune, W.F., Sydiskis, R.J., Davidson, W.M. In vitro cytotoxicity of orthodontic bonding materials. (1983) Am J Orthod 83(6): 501- 506.
- 27. Tell, R.T., Sydiskis, R.J., Isaacs, R.D., et al. Long-term cytotoxicity of orthodontic direct-bonding adhesives. (1988) Am J Orthod Dentofacial Orthop 93(5): 419- 422.
- 28. Sjögren G, Sletten G, Dahl, J.E. Cytotoxicity of dental alloys, metals, and ceramics assessed by millipore filter, agar overlay, and MTT tests. (2000) J Prosthet Dent 84(2): 229- 236.