Infection Features of Acute Leukemia Patients During Induction Chemotherapy: A Retrospective Analysis of 203 Cases
Ruijuan Zhang, Nannan Zhang
Affiliation
Department of Hematology, the Second Hospital of Shanxi Medical University, Taiyuan, China
# Ruijuan Zhang and Nanan Zhang contributed equally
Corresponding Author
Linhua Yang, Department of Hematology, The Second Hospital of Shanxi Medical University, Taiyuan, China, E-mail: yanglh5282@163.com
Citation
Yang, L., et al. Infection Features of Acute Leukemia Patients during Induction Chemotherapy: A Retrospective Analysis of 203 Cases. (2017) Int J Hematol Ther 3(1): 1- 6.
Copy rights
© 2017 Yang, L. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Acute leukemia; Induction chemotherapy; Infection; Risk factor; Complete remission
Abstract
Infection is a major complication of chemotherapy for Acute Leukemia (AL). Two hundred and three patients with newly diagnosed AL were recruited in this study. By analyzing infection incidence (82.8%, 168/203), infection-related mortality (7.1%, 12/168), clinical features, and risk factors, as well as the correlation with efficacy for the patients during induction chemotherapy, we aimed to understand the significance of infection in patients with them, thereby guiding clinical treatment. All of the patients, 168 cases suffered from infection; among of them, there were clear sites of infection accounted for 94.6% (159/168), and the most infection sites were lungs, gastrointestinal tract, and oral cavity. 213 of pathogens were detected, including gram-negative bacilli (39.0%, 83/213), Gram-positive cocci (34.3%, 73/213), fungi (23.5%, 50/213), and virus (3.3%, 7/213). Diagnosis/clinical diagnosis of the patients with invasive fungal disease accounted for 24.6%, and the main pathogen was Candida. Multivariate analysis of the risk factor of infection showed that was neutropenia (P < 0.001, OR = 14.37, 95% CI 2.576 - 116.518); however, infection did not affect the rate of CR/CRi ( χ² = 2.564, P = 0.109).
Conclusion: During the first induction therapy for newly diagnosed AL patients, the rate of infection and fungal infection was high, Lung is the most common site of infection, Gram-negative bacteria is more common, agranulocytosis increase the chance of infection, it does not affect the complete remission rate.
Introduction
Infection is one of the most common complications in AL, especially during induction chemotherapy[1,2]. The infection- related mortality is quite high, infection could delay chemotherapy, and increase the risk of recurrence[3], and the mortality of febrile neutropenia reaches 10 - 20%[4-6]. Therefore, we retrospectively analyzed the infection status of 203 cases of newly diagnosed AL patients in our hospital during induction chemotherapy, and tried to understand clinical features and risk factors of the infection, and the relationship with the efficacy during initial induction treatment for acute leukemia patients, thereby guiding clinical treatment.
Materials and Methods
Patients
From January 2015 to February 2016, 203 cases of hospitalized patients with newly diagnosed AL during induction chemotherapy at the Department of Hematology of the Second Hospital of Shanxi Medical University, among the patients, 106 cases were male and 97 were female, median age of 47 (16 - 79) years. There were 155 cases of acute myeloid leukemia (AML), in which 32 cases were Acute Promyelocytic Leukemia (APL), 46 cases were Acute Lymphoblastic Leukemia (ALL), including 15 cases of philadelphia chromosome-positive acute lymphoblastic leukemia (ph + ALL) and 2 cases of acute mixed leukemia ( MAL).
Regimen for induction therapy
The “7 + 3” regimen (cytarabine × 7d and anthracycline or anthraquinone × 3d) was performed for induction therapy in patients with AML (non-APL); part of the elderly patients or hypoproliferative acute leukemia(HAL) patients received CIG regimen (cytarabine, idarubicin and recombinant human granulocyte colony - stimulating factor); APL patients were treated with retinoic acid, arsenic trioxide and/or anthracyclines; ph - ALL patients were treated with VDLP ± C regimen (vincristine, daunorubicin, L-asparaginase, and dexamethasone ± cyclophosphamide), whereas ph + ALL patients were imatinib replace L-asparaginase.
Diagnostic criteria of infection and related definition
Diagnostic criteria of infection referred to “The diagnostic criteria of nosocomial infection of Chinese Ministry of Health (trial implementation)”[7], and diagnostic criteria of neutropenia with fever[6,8,9] and Diagnostic criteria and treatment principles of invasive fungai disease in patient with hematonosis or malignancy[10-12]. The axillary temperature of patients during induction chemotherapy was more than 38.0°C or over 37.7°C for one hour, detailed physical examination and microbiology, imaging and laboratory test, exclude drugs and transfusion-related fever.
Statistical analysis
SPSS 17.0 software was used for statistical analysis. Mann-Whitney test was used for comparison in groups of continuous variables. Chi-square test was used for the comparison of categorical variables between groups with risk factors for infection screened by univariate analysis, then Logistic regression was performed. All comparisons were two-tailed test, and P < 0.05 was considered statistically significant difference.
Results
Incidence and mortality of infection
The infection rate was 82.8% (168/203) during induction chemotherapy; 88 cases were male, 80 were female; 14 cases were dead, and 12 cases was due to infection (7.1%, 12/168), 5 males and 7 females, 5 elderly and 7 younger patients; 9 cases were AML (non-APL), 3 were ALL; one case was low-risk, eight were intermediate risk, and three were high risk.
Infection features
The site of infection
Among 168 patients with infection, patients with clear site of infection accounted for 94.6% (159/168), totally 328 cases times. The infected site was as follows: lung (26.1%, 93/328), gastrointestinal tract (17.1%, 61/328), oral cavity (12.4%, 44/328), upper respiratory tract (12.1%, 43/328), blood stream (10.4%, 37/328), cutaneous soft tissue (5.1%, 18/328), sinuses, perianal (3.7% each, 13/328), and urinary tract (1.4%, 6/328). The site of infection of 9 patients was not clear. single-site infection was accounted for 51/168, two-site was 85/168, and more than 3-site was 32/168.
Etiology information
213 pathogens were detected. Among these, 83 pathogens were gram-negative bacilli, including Escherichia coli, Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, onion primary Haw de coli, Enterobacter cloacae, Proteus mirabilis, Proteus vulgaris, Malt bacillus subtilis, Aeromonas bacteria, Corynebacterium diphtheria class, Citrobacter, Serratia marcescens, Pseudomonas yellow perch, and Citrobacter freundii; 11 strains of Acinetobacter baumannii were detected, which was accounted for 13.3% in Gram-negative bacilli, including seven strains of multi-drug resistance, two strains of extensively drug resistance, two strains of sensitivity; the drug-resistance rate of cefoperazone sulbactam, minocycline, and amikacin was 20%, following by 27.3% of imipenem, 30% of levofloxacin, and 36.4% of meropenem; mixed infection was accounted for 90.1% (10/11). 73 pathogens were gram-positive cocci, including Enterococcus (containing vancomycin-resistant enterococci), coagulase-negative staphylococci (containing Staphylococcus haemolyticus), Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus angina, Staphylococcus epidermidis, and Streptococcus songs. 50 pathogens were fungi, including Candida albicans, Candida krusei, C. parapsilosis, Aspergillus fumigatus, lung mold, Aspergillus flavus, and Candida tropicalis. 7 pathogens were virus, accounting for 3.3 %, including EB virus, respiratory syncytial virus, and adenovirus (Table1).
Table 1: Distribution and composition ratio of 10 pathogens.
| Pathogens | Number(n) | Composition ratio(%) |
|---|---|---|
| Gram-negative bacilli | 83 | 39.0 |
| Escherichia coli | 23 | 10.8 |
| Acinetobacter baumannii | 11 | 5.2 |
| Klebsiella pneumoniae | 9 | 4.2 |
| Pseudomonas aeruginosa | 8 | 3.8 |
| Stenotrophomonas maltophilia | 8 | 3.8 |
| Others | 24 | 11.3 |
| Gram-positive cocci | 73 | 34.3 |
| Enterococcus | 43 | 20.2 |
| Coagulase-negative staphylococci | 13 | 6.1 |
| Staphylococcus aureus | 7 | 3.3 |
| Streptococcus | 4 | 1.9 |
| Others | 6 | 2.8 |
| Fungus | 50 | 23.5 |
| Candida albicans | 32 | 15.0 |
| Others | 18 | 8.5 |
| Virus | 7 | 3.3 |
| Epstein-Barr virus | 5 | 2.3 |
| Others | 2 | 1.0 |
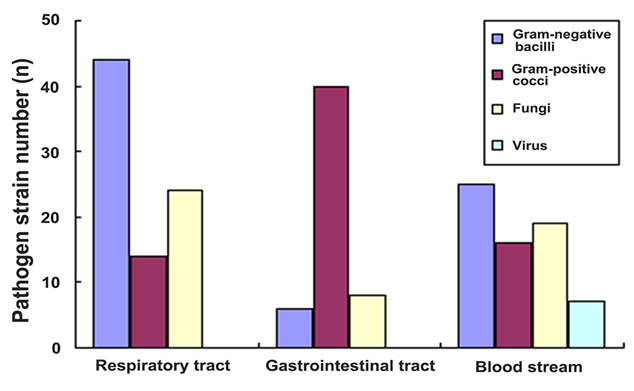
In addition, the spectrum of pathogens was different for different site; the pathogens with the three largest number of strains were analyzed (Figure 1). 82 pathogens were detected in respiratory: 44 strains were bacillus, including 10 strains of Acinetobacter baumannii, 9 strains of maltophilia Aeromonas, 6 strains of Escherichia 6 strains of coli, Burkholderia each; 14 strains were bacteria, including 9 strains of enterococci; 24 strains were fungal, including 15 strains of albicans. 54 pathogens were detected in gastrointestinal: 40 strains were cocci, all being Enterococcus; 6 strains of bacillus, 4 strains of Klebsiella pneumonia, and 2 strains of Proteus; 8 pathogens were fungal, all being Candida, including 6 strains of Candida albicans. 67 pathogens were found from blood culture: 16 strains were gram-positive cocci, including 11 strains of coagulase-negative staphylococci (all were methicillin-resistance) 4 strains of Staphylococcus aureus; 25 strains of bacillus, including 10 strains of E. coli and 4 strains of Pseudomonas aeruginosa. 19 pathogens were fungi, including 10 strains of Candida albicans. 7 pathogens were virus.
Figure 1: The spectrum of pathogens of the three largest number of strains.
By analyzing of infection with different age, it was found that the common infection site of elderly patients was lung (65.2%, 30/46), gastrointestinal tract (30.4%, 14/46), blood flow (26.1%, 12/46), whereas the common infection sites of non-elderly patients was slightly different, including lung (40.1%, 63/157), gastrointestinal tract (29.9%, 47/157), and oral cavity (22.9%, 36/157); both groups there was no significant difference in terms of detection rate of bacillus and fungal and mortality, and detection rate of cocci in non-elderly group was significantly higher than that in older group (Table 2).
Table 2: Analysis of infection for 46 cases of elderly patients and 157 cases of non-elderly patients.
| Group | Elderly(%) | Non-Elderly(%) | χ² | P value |
|---|---|---|---|---|
| Pathogens | ||||
| Gram-negative bacilli | 47.1%(33/70) | 35.0%(50/143) | 2.930 | 0.087 |
| Gram-positive bacilli | 21.4%(15/70) | 40.6%(58/143) | 7.635 | 0.006 |
| Fungus | 27.1%(19/70) | 21.7%(33/143) | 0.421 | 0.516 |
| Mortality | 10.9%(5/46) | 4.5%(7/157) | 1.647 | 0.199 |
Analysis of risk factor
By univariate analysis of infection factors in AL patients during induction chemotherapy, it was showed that neutropenia, neutropenia time > 7 days, AML, and co-infection before chemotherapy were risk factors of infection for AL patients (Table 3). Multivariate analysis found that neutropenia was independent risk factor of infection.
Table 3: Infection risk factors for the AL patients during induction chemotherapy.
| Factor | Uni-variate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Cases(n) | infection(n)(%) | χ² | P value | OR | 95% CI | P value | |
| Sex | 0.100 | 0.752 | |||||
| Male | 106 | 88(83.0) | |||||
| Female | 97 | 80(82.5) | |||||
| Age 1(years old) | 8.601 | 0.197 | |||||
| 16 - 20 | 20 | 14(70.0) | |||||
| 21 - 30 | 37 | 29(78.4) | |||||
| 31 - 40 | 31 | 26(86.7) | |||||
| 41 - 50 | 31 | 26(86.7) | |||||
| 51 - 60 | 39 | 34(87.2) | |||||
| 61 - 70 | 38 | 33(86.8) | |||||
| 71 - 79 | 7 | 6(85.7) | |||||
| Age 2(years old) | 0.307 | 0.579 | |||||
| < 60 | 157 | 129(82.2) | |||||
| ≥ 60 | 46 | 39(85.7) | |||||
| Type of leukemia | 26.528 | 0.000 | 1.972 | 0.252 - 15.444 | 0.518 | ||
| AML(non-APL) | 123 | 110(89.4) | |||||
| ALL | 46 | ||||||
| Ph - | 31 | 16(51.6) | |||||
| Ph + ALL | 15 | 12(80.0) | |||||
| MAL | 2 | 2(100.0) | |||||
| APL | 32 | 26(81.3) | |||||
| Stratification of risk | 0.641 | 0.866 | |||||
| Low risk | 21 | 16(76.2) | |||||
| Intermediate risk | 115 | 97(84.3) | |||||
| High risk | 67 | 55(82.1) | |||||
| ANC | 117.00 | 0.000 | 14.37 | 2.576 - 116.518 | 0.000 | ||
| 0- 0.09 | 138 | 138(100.0) | |||||
| 0.10 - 0.19 | 17 | 11(64.7) | |||||
| 0.20 - 0.29 | 18 | 7(38.9) | |||||
| 0.30 - 0.39 | 5 | 2(40.0) | |||||
| 0.40 - 0.49 | 2 | 1(50.0) | |||||
| ≥ 0.5 | 23 | 10(43.5) | |||||
| Time of neutropenia | 14.240 | 0.000 | 0.926 | 0.143 - 6.019 | 0.936 | ||
| > 7 days | 152 | 140(92.1) | |||||
| ≤ 7 days | 51 | 28(54.9) | |||||
| Chemotherapy prior to infection | 82.817 | 0.000 | 1.026 | 0.162 - 6.518 | 0.978 | ||
| Yes | 134 | 116(86.6) | |||||
| No | 69 | 16(23.2) | |||||
| Glucocorticois | 23.781 | 0.000 | 0.029 | 0.001 - 0.841 | 0.039 | ||
| Yes | 46 | 28(60.9) | |||||
| No | 157 | 140(89.2) | |||||
| Diabetes | 0.001 | 0.979 | |||||
| Yes | 20 | 16(80.0) | |||||
| No | 183 | 152(83.1) | |||||
Note: b: regression coefficient, OR: odds ratio, 95% CI: 95% confidence interval.
50 cases of patients with diagnosis/clinical diagnosis invasive fungal disease, composed of 26 males and 24 females. Univariate analysis showed that age ≥ 60 years old, neutropenia, and neutropenia time > 14 days were risk factors. Multivariate analysis showed that age ≥ 60 years old and neutropenia were risk factors of fungal infection (Table 4).
Table 4: Risk factors of fungal infection for AL patients during induction therapy.
| Factor | Uni-variate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Cases(n) | infection(n)(%) | χ² | P value | OR | 95% CI | P value | |
| Age | 7.287 | 0.007 | 2.604 | 1.160 - 5.842 | 0.020 | ||
| ≥ 60 years old | 46 | 17(37.0) | |||||
| < 60 years old | 157 | 33(21.0) | |||||
| Sex | 2.951 | 0.086 | 0.611 | 0.301 - 1.239 | 0.072 | ||
| Male | 106 | 26(24.5) | |||||
| Female | 97 | 24(24.7) | |||||
| Type of leukemia | 3.234 | 0.357 | |||||
| AML | 125 | 35(27.3) | |||||
| Non-AML | 78 | 15(19.2) | |||||
| Stratification of risk | 5.302 | 0.151 | |||||
| Low and intermediate risk | 136 | 32(23.5) | |||||
| High risk | 67 | 18(26.9) | |||||
| ANC | 23.373 | 0.000 | 15.961 | 2.036 - 125.091 | 0.006 | ||
| < 0.5 | 153 | 50(32.7) | |||||
| ≥ 0.5 | 50 | 0 | |||||
| Time of neutropenia | 10.627 | 0.001 | 1.960 | 0.888 - 4.327 | 0.096 | ||
| > 14 days | 66 | 30(45.5) | |||||
| ≤ 14 days | 137 | 20(14.6) | |||||
| Chemotherapy prior to infection | 1.070 | 0.301 | |||||
| Yes | 134 | 36(26.9) | |||||
| No | 69 | 14(20.3) | |||||
| Diabetes | 5.647 | 0.172 | |||||
| Yes | 20 | 6(30.0) | |||||
| No | 183 | 44(24.0) | |||||
| Glucocorticoids | 2.143 | 0.367 | |||||
| Yes | 46 | 18(39.1) | |||||
| No | 157 | 32(20.4) | |||||
Correlation of infection with efficacy Comparison of 168 cases of infection with 35 patients with non-infection in sex, age, risk stratification of leukemia, and chemotherapy, resulted in comparable balance. CR /CRi rate of the two groups of patients was not significant difference (Table 5).
Table 5: Correlation analysis of infection and efficacy for AL patients.
| Group | Cases(n) | CR/CRi(n)(%) | χ² | P value |
|---|---|---|---|---|
| Infected group | 168 | 125(75.6%) | 2.564 | 0.109 |
| Non-infected group | 35 | 28(88.6%) |
Discussion
In this study, our data showed incidence of infection was 82.8% in 203 patients with newly diagnosed AL during induction chemotherapy, and the infection-related mortality was 7.1%, which was the main cause of death, in line with the study by Masmoudi., et al[13,14]. Moreover, sex, age, type of leukemia, risk stratification, and multi-site infection factor were not the factors for death. Clear site of infection were accounted for 94.6% (159/168), the most common site of infection was lung, which was consistent with the study by Cannas., et al[1,2,15,16]. 213 pathogens were isolated, gram-negative bacilli still predominantly[1,2,6,13,16], where E. coli was the most common pathogen, followed by Acinetobacter baumannii, which was in line with the studies of Chinese CHINET monitoring network of drug-resistance bacteria in 2012[17,18]. Similarly, the high rate of detection, high rate of multi-drug resistance and extensively drug-resistance[19], often accompanying by a mixed infection, and the common site of infection in lung should be paid attention. Gram-positive cocci is followed by pathogen, unlike other epidemiological investigation[1,2,6,13], predominantly Enterococcus, illustrating that empirical antibiotic therapy should consider local epidemiological investigation. Although Staphylococcus aureus and coagulase-negative staphylococci were reduced, coagulase-negative staphylococci was still the most common pathogen for infection in blood stream[15,17], but the methicillin-resistant rate was increased (100%), which was significantly higher than that in other studies[20], probably relating to less strains of coagulase-negative staphylococcus in our study. The study also found that the infection spectrum of pathogen in different infected site was significantly different: respiratory was mainly gram-negative bacilli, predominantly drug-resistant strains; Enterococcus was mainly in the gastrointestinal tract, followed by bacillus of blood culture, coccus, fungi, and virus, suggesting that the initial empirical anti-infective therapy should consider different infection pathogens[1].
In this study, incidence of invasive fungal disease (24.6%) was significantly higher than that in CAESAR research[21] (4.94%), and the infection was mainly caused by Candida[1,12,21-23], predominantly in lung[1,21,22]. Univariate and multivariate analysis of factor affecting fungal infection, found that age ≥ 60 years and neutropenia were the risk factors for fungal infection[24]. The virus infection rate was low, mainly with EB virus.
Occurrence and severity of infection are related to the degree of neutropenia and duration[5,25]. In this study, infection of 70% patients started with neutropenia, and the vast majority of patients were with severe neutropenia or the neutropenia time was more than 7 days. Univariate analysis of factors of infection found that neutropenia, especially severe neutropenia, neutropenia > 7 days, AML, co-infection before chemotherapy were the risk factors for infection, which was in line with the study by Chindaprasirt., et al[26,27]. Multivariate analysis showed that neutropenia was the independent risk factor for infection. Unlike Li., et al[22] study, the present study found that diabetes and the use of hormones did not increase infection. In addition, although age was not the risk factor, the feature of infection was different, the fungal infection rate of blood stream in elderly patients was higher than that in younger patients (but with a higher detection rate of coccus).
Unlike other studies[27] that infection increases hospital stay, this study found no increase in hospital stay with infection. 168 cases of infection with a median length of hospital stay was 29 days (18 - 56 days); for non-infected patients, median hospital stay was 28 days (21 - 42 days). The difference was not significant (P = 0.59). However, it has been demonstrated that[28-30], no matter AL patient achieves CR after chemotherapy, or the patient was without clinical contraindications, and even with severe neutropenia, it could not affect the discharge. But we need more clinical validation to confirm this conclusion.
It has been found that fungal infection is a prognostic factor[31]. The analysis of correlation between infection and efficacy for AL patients during induction chemotherapy showed that the CR/CRi rate of 203 cases of AL patients was 76.8% (155/203), and infection was not the factor affecting efficacy, which was not consistent with the above conclusion.
Conflict of interest:None.
References
- 1. Cannas, G., Pautas, C., Raffoux, E., et al. Infectious complications in adult acute myeloid leukemia: analysis of the Acute Leukemia French Association-9802 prospective multicenter clinical trial. (2012) Leuk Lymphoma 53(6): 1068-1076.
- 2. Luo, Y.M., Liu, T.B., Xie, S.T., et al. Clinical features and risk factors for infections in adult acute leukemia after chemotherapy. (2015) Chinese Journal of Hematology 36(12): 1020-1024.
- 3. Malagola, M., Peli, A., Damiani, D., et al. Incidence of bacterial and fungal infections in newly diagnosed acute myeloid leukaemia patients younger than 65 yr treated with induction regimens including ?udarabine: retrospective analysis of 224 cases. (2008) Eur J Haematol 81(5): 354-63.
- 4. Ramzi, J., Mohamed, Z., Yosr, B., et al. Predictive factors of septic shock and mortality in neutropenic patients. (2007) Hematology 12(6): 543-548.
- 5. Sacar, S., Hacioglu, S.K., Keskin, A., et al. Evaluation of febrile neutropenic attacks in a tertiary care medical center in Turkey. (2008) J Infect Dev Ctries 2(5): 359-363.
- 6. Chinese guidelines for the clinical application of antibacterial drugs for agranulocytosis with fever (2016). (2016) Chinese Society of Hematology Chinese Medical Association, Chinese Medical Doctor Association Hematology Branch Chin J Hematol 37(5): 353-359.
- 7. The diagnostic criteria of nosocomial infection (trial implementation). (2001) Natl Med J China 81(5): 314-320.
- 8. Freifeld, A.G., Bow, E.J., Sepkowitz, K.A., et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. (2011) Clin Infect Dis 52(4): e56-93.
- 9. Averbuch, D., Orasch, C., Cordonnier, C., et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia. (2013) Haematologica 98(12): 1826-1835.
- 10. De Pauw, B., Walsh, T.J., Donnelly, J.P., et a1. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. (2008) Clin Infect Dis 46(12): 1813-1821.
- 11. Castagna, L., Bramanti, S., Sarina, B., et a1. ECIL 3-2009 update guidelines for antifungal management?(2012) Bone Marrow Transplant 47(6): 866.
- 12. China Working Group of Invasive Fungal Infections. Diagnostic criteria and treatment principles of invasive fungaI disease in patient with hematonosis or malignancy (fourth revised edition). (2013) Clin J Intern Med 52(8): 704-709.
- 13. Masmoudi, S., Khanfir, A., Maalej-Mezghan, S., et al. Chemotherapy-induced febrile neutropenia: About 186 episodes. Clinical, microbiological and therapeutic characteristics. (2015) Tunis Med 93(4): 217-222.
- 14. Rabagliati, R., Bertin, P., Ceron, I., et al. Epidemiology of febrile neutropenia in adult patients with acute leukemia and lymphoma: Cohort study of public and private hospital of Santiago, Chile. (2014) Rev Chilena Infectol 31(6): 721-728.
- 15. Garcia, J.B., Lei, X., Wierda, W., et al. Pneumonia during remission induction chemotherapy in patients with acute leukemia. (2013) Ann Am Thorac Soc 10(5): 432-440.
- 16. Yan, C.H., Xu, T., Zhen, X.Y., et al. Epidemiology of febrile neutropenia in patients with hematological disease: a prospective multicentre survey in China. (2016) Chinese Journal of Hematology 37(3): 177-182.
- 17. Kaur, M., Gupta, V., Gombar, S., et al. Incidence, risk factors, microbiology of venous catheter associated bloodstream infections--a prospective study from a tertiary care hospital. (2015) Indian J Med Microbiol 33(2): 248-254.
- 18. Zhang, H., Zhang, X., Xu, Y., et al. CHINET 2012 surveillance of antibiotic resistance in Acinetobacter baumannii isolates in China. (2014) Chinese Journal of Infection and Chemotherapy 114(5): 392-397.
- 19. Guardado, A.R., Blanco, A., Asensi, V., et al. Multidrug-resistant Acinetobacter meningitis in neurosurgical patients with intraventricular catheters: assessment of diffierent treatments.(2008) J Antimicrob Chemother 61(4): 908-913.
- 20. Hu, F., Zhu, D., Wang, F., et al. CHINET 2014 surveillance of bacterial resistance in China. (2015) Chinese Journal of Infection and Chemotherapy 15(5): 401-410
- 21. Sun, Y., Huang, H., Chen, J., et al. Invasive fungal infection in patients receiving chemotherapy for hematological malignancy: a multicenter, prospective, observational study in China. (2015) Tumour Biol 36(2): 757-767.
- 22. Li, Y., Xu, W., Jiang, Z., et al. Neutropenia and invasive fungal infection in patients with hematological malignancies treated with chemotherapy: a multicenter, prospective, non-interventional study in China. (2014) Tumour Biol 35(6): 5869-5876.
- 23. Neofytos, D., Lu, K., Hatfield-Seung A, et al. Epidemiology, outcomes, and risk factors of invasive fungal infections in adult patients with acute myelogenous leukemia after induction chemotherapy. (2013) Diagn Microbiol Infect Dis 75(2): 144-149.
- 24. Sun, Y., Meng, F., Han, M., et al. Epidemiology, management, and outcome of invasive fungal disease in patients undergoing hematopoietic stem cell transplantation in China: A Multicenter prospective observational study. (2015) Biol Blood Marrow Transplant 21(6): 1117-1126.
- 25. Freres, P., Gonne, E., Collignon, J., et al. Management of febrile neutropenia in cancer patients. (2015) Rev Med Liege 70(4): 195-200.
- 26. Liu, H., Zhao, J., Xing, Y., et al?Nosocomial infection in adult admissions with hematological malignancies originating from different lineages: a prospective observational study.(2014) PLoS One 9(11): e113506.
- 27. Chindaprasirt, J., Wanitpongpun, C., Limpawattana, P., et al. Mortality, length of stay, and cost associated with hospitalized adult cancer patients with febrile neutropenia. (2013) Asian Pac J Cancer Prev 14(2): 1115-1119.
- 28. Inoue S, Khan I, Mushtaq R, et al. Postinduction Supportive Care of Pediatric Acute Myelocytic Leukemia: Should Patients be Kept in the Hospital?. (2014) Leuk Res Treatment 2014: 592379.
- 29. Sopko, L., Sabty, F.A., Rimajova, V., et al. The feasibility of an early hospital discharge following chemotherapy for the acute myeloid leukemia. (2012) Bratisl Lek Listy 113(5): 298-300.
- 30. Walter, R.B., Taylor, L.R., Gardner, K.M., et al. Outpatient management following intensive induction or salvage chemotherapy for acute myeloid leukemia. (2013) Clin Adv Hematol Oncol 11(9): 571-577.
- 31. Pechlivanoglou, P., Le, H.H,, Daenen, S., et al. Mixed treatment comparison of prophylaxis against invasive fungal infections in neutropenic patients receiving therapy for haematological malignancies: a systematic review. (2014) J Antimicrob Chemother 69(1): 1-11.