Interval Aerobic Exercise and Selenium Nanoparticle Stimulate Autophagy in Mice with Cancer Cachexia
Kia Ranjbar1*, Hamid Agha Alinejad1*, Shirin Shahbazi2, Mahdieh Molanouri Shamsi1, Soodabeh Chekachak1, Jamal Chenari3, Mohammad Hossein Yazdi4, Mehdi Mahdavi5, Peyman Ghasemi1
Affiliation
1Physical Education & Sport Sciences Department, Faculty of Humanities, Tarbiat Modares University, Jala Ale Ahmad Exp, Tehran, Iran
2Department of Medical Genetics, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
3Brain and Spinal Cord Injury Research Center, Neurosciences Institute, Isfahan University of Medical Sciences, Isfahan, Iran
4Department of Pharmaceutical Biotechnology and Biotechnology Research Center, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
5Immunology Department, Pasteur Institute of Iran, 69 Pasteur Ave, Tehran, Iran
Corresponding Author
Kia Ranjbar, Physical Education & Sport Sciences Department, Faculty of Humanities, Tarbiat Modares University, Jala Ale Ahmad Exp, Tehran, Iran; E-mail: kia.ranjbar@modares.ac.ir,
Hamid Agha Alinejad, Physical Education & Sport Sciences Department, Faculty of Humanities, Tarbiat Modares University, Jala Ale Ahmad Exp, Tehran, Iran; E-mail: halinejad@modares.ac.ir
Citation
Ranjbar, K., et al. Interval Aerobic Exercise and Selenium Nanoparticle Stimulate Autophagy in Mice with Cancer Cachexia. (2018) Int J Cancer Oncol 5(1): 35- 40.
Copy rights
© 2018 Ranjbar, K. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Abstract
Autophagy can be activated by several stimuli, such as pathological and physiological stress like exercise training. Nevertheless, it is possible that exercise training modifies basal autophagy during cancer and whether autophagy is required for skeletal muscle adaptation to training in pathological condition. The purpose of the present work was to study Selenium nanoparticles and aerobic interval training on muscle wasting and autophagy in cancer. Selenium nanoparticle supplementation accelerated cachexia symptoms in tumor-bearing mice, while exercise training prevented muscle wasting in tumor-bearing mice. Also, aerobic interval training and cancer enhanced the autophagy markers expression in EDL skeletal muscle in tumor-bearing mice, but the underlying mechanisms in the two conditions are likely different. Nonetheless, combining exercise training and Selenium nanoparticles encountered cachexia and muscle wasting and likewise decreased tumor volume in 4T1 breast cancer mice. These results demonstrated that increased basal autophagy is required for exercise training-induced skeletal muscle adaptation concurrent with selenium nanoparticles and improvement of physical performance in cancer.
Introduction
It is intimately known that well-ordered exercise training boosts muscle mass, physical performance and cause to health advantages including to control muscle mass in many pathological conditions, such as cancer, HIV, metabolic and neurodegenerative diseases[1-4]. As noted, Skeletal muscle wasting is critical problem, which occurs as part of cancer cachexia and a major cause of fatigue in patients[5,6]. Skeletal muscle wasting is induced by cancer even with normal energy intake and is not prevented by nutritional supplementation[6-8]. Muscle-wasting induced cancer is also associated with severe fatigue, which affects 70 - 100% of cancer patients[9,10]. Fatigue related to muscle wasting is a distressing symptom that negatively impacts on physical function and can lead to affected cancer patients changing their employment status and reducing their overall quality of life[11]. Although exercise training has been suggested as a promising countermeasure to prevent muscle wasting in cancer, but different studies have emphasized that a single therapy may not be completely successful in the treatment of muscle wasting[12-14].
Along with exercise training, nutritional interventions have been suggested as a beneficial patron to prevent muscle wasting and improve physical performance[14,15]. Selenium (Se) is indispensable trace element in the body due to anti-oxidative properties as well as pro-oxidative effect, and has great importance in nutrition and medicine. Due to well biological activities and low toxicity of SeNPs, they are drawing more attention. One of the efficient strategies to hinder and treat cancer is to use antioxidant according to some studies[15,16]. Nevertheless, at least one study suggested that combination of dietary supplement and antioxidant could not support skeletal muscle and caused cachexia in tumor-bearing mice[17]. However, the impact of anti-oxidative and anti-carcinogenic of dietary selenium has mentioned in some study reports[18,19]. Furthermore, it is observed that a form of selenium with stronger biology, Se NPs, has considerable and meaningful anti-carcinogenic effect in animal models with breast cancer[15,20] and could be an encouraging method to prevent and treat cancer.
Insufficient understanding of basic biological mechanisms of skeletal muscle wasting has turned to the main setbacks to manage it effectively. Anyway, autophagic-lysosomal proteolysis has been included as one of the barriers too different cellular processes including degradation of various substrates, trafficking and recycling of molecules among internal organelles to and from the exterior of the cell, posttranslational maturation of secretary products, and storage of undigested material are behind donated by lysosomal degradative system[21]. The Main process to degrade cellular constituent is autophagy; and, in order to recycle biomolecules for the synthesis of essential constituents, the speed of degradation can be increased under stress condition, resulting in organelle damage, or under marked nutrient restriction (starvation)[21,22]. It is demonstrated that autophagy, under such conditions, conducts large-scale proteolysis in most visceral tissues, such as the liver; nevertheless, the role and importance of autophagy in the skeletal muscle has been appeared just during recent years[23].
As mentioned above, the factors playing significant role in skeletal muscle loss in cancer cachexia are autophagic modification and oxidative stress; to suggest a simple and considerable treatment option[23], these factors integrate Se NPs, as an antioxidant, with the anabolic action of aerobic interval training. In the present study, this hypothesis tested that skeletal muscle inflammation can be modified by Se NP supplementation and aerobic interval training and thereby muscle atrophy and functional disorder in cachexia is reduced. Moreover, evidence indicates that cancer-related fatigue can be improved by aerobic interval training. Meanwhile, no convincing evidence to demonstrate that preserving muscle mass is associated with exercise induced reduction in fatigue.
Materials and Methods
Animals
Three- to 5-week-old female inbred BALB/c mice, weighing mean 17 g, were obtained from the Pasteur Institute of Iran (Tehran, Iran). First they were divided into four groups: control (C), trained (T), selenium (Se), selenium- trained (SeT) for six weeks and then they divided eight groups: control (C), trained (T), selenium (Se), selenium- trained (SeT), control-cancer (CC), trained-cancer (TC), selenium-cancer (SeC), selenium- trained-cancer (SeTC) each containing 8 mice. The mice were housed in a 12-h light: 12-h dark cycle and temperature-controlled animal facility with free access to tap water and food pellets. Animal were maintained in the Central Animal House, School of Medical Science of TMU.
Preparation of Biogenic Selenium Nanoparticles
Se NPs were prepared using a method described previously [24,25]. A solution of 5.2 mM selenium dioxide (Merck, Germany) was prepared and aqueous ascorbic acid solution (5.2 mM) was added into the mixture with continuous stirring (300 rpm) with a magnetic stirrer. The resulting reaction mixture was centrifuged and washed three times with double-distilled water. A stock solution of (1 mg/ml) Se NPs was prepared and used for further oral administration in doses of 100 and 200 ll per mouse. All treatments were administrated by oral gavages. Untreated or control mice were gavaged with vehicle alone[15].
Body weight and food intake
Body weight and food intake were measured three days a week during supplementation and aerobic interval training. Body mass and food intake were monitored daily after inoculation.
Tumor Induction and tumor size
The 4T1 cell line was cultured for induction of tumors in inbred Balb/c mice. For this purpose, 200 μl of RPMI containing 4 T1cells at concentration of 1 × 106 cells/ml was injected into the mammary glands of female mice. All mice were followed until a tumor nodule was observed. Tumor volume was measured by a digital vernier caliper (Mitutoyo, Japan) on a weekly basis, and reported as cm3 using the following formula (Mohsenikia et al., 2013):
V = 1 / 6 (_ LWD), where L = length, W = width and D = depth
Interval exercise training protocol
Prior to the initiation of exercise training, mice in exercise groups were assigned to the treadmill for 5 days. Acclimation entailed running at the end of their dark cycle (0700) at gradually increasing speeds (10, 12, 16, 18, 20, 22 meter/min) and 0% inclination. Following the acclimation, the exercise protocol began at 16 - 18 m/min, 0% grade, 40 - 50 min, and 5 days/week for 5 weeks (Riggs et al., 2010). No electrical stimulation used, and mice were encouraged to run by a gentle tap on the tail or hindquarters. In addition to we used to incremental test peak velocity and VO3 max determination, however, starting from a speed of 6 m.min-1, the exercise intensity was increased by 3 m.min-1, every 3 min, with an incline of 0%. Exercise continued until exhaustion, which was defined as an inability to maintain the running speed despite contact with a gentle tap on the tail or hindquarters more than 5 times. All measurements were made by the same investigator. The last stage completed by the mouse was defined as the peak velocity (vPeak) (Figure 1).
Figure 1: Time window representation of the experimental design including time points of different measurements during exposure time.
Blood and Tissue collection
At the end of treatment (after three months) to avoid acute exercise response, mice were euthanized 48 h after the last exercise session. During anesthesia period (the animals under anesthesia with Ketamine (100 mg/kg) and Xylazine (10 mg/kg), blood (1.5 ml) was intra cardially withdrawn. Animals were finally euthanized using cervical dislocation. Blood samples were then centrifuged at 4,000 g for 10 min. The tibialis anterior (TA), extensor digitorum longus (EDL), plantaris (PLAN), gastrocnemius (GAST) and soleus (SOL) muscles, the liver, heart (HRT) and epididymal fat, were carefully excised, blotted on filter paper and weighed on an analytical balance. Blood sample and all of the tissues were frozen directly in liquid nitrogen and stored at –80°C for subsequent analyzes
Real-time RT-PCR
Beclin, LAMP-2, p62, LC3 and mTOR mRNA expression were determined by real-time RT-PCR as described in detail elsewhere. Total RNA was extracted from 20 - 40 mg of EDL and soleus muscle tissue using Trizol solution (Invitrogen, USA), and cDNA synthesis (Qiagen, Germany) was performed using Qiagen cDNA synthesis kit according to the manufacturer’s instructions. Real-Time PCR was performed using the Bio-Rad iCycler Thermal Cycler. Briefly, 1 _g of RNA was added to the reaction mixture (gDNA Wipeout Buffer, Quantiscript Reverse- Transcriptase, Quantiscript RT Buffer, RT Primer Mix, and RNase-free water) followed by incubation at 42°C for 15 min. Reverse transcription was terminated at 95°C for 3 min, and then samples were stored at –20°C until used for Real-time PCR analysis. CDNA synthesis of RNAs (1 _g total RNA as input) was carried out according to the kit protocol (Stratagene, USA). To determine the relative expressions of genes, qRT-PCR was performed using SYBR Green dye. Thermal cycling program was as follows: 94°C for 3 min followed by 30 cycles of 94°C for 0.5 min, 54°C for 1 min, and 72°C for 0.5 min. GAPDH mRNA was used for normalization of the gene expression analysis.
Statistical analysis
All statistical analyses were performed with SPSS statistical software (version 16) with the significance level set at P < 0.05. The results are presented as means + SEM. Data were tested for normality and homogeneity of variance using a ShapiroeWilk and Levene’s test, respectively. Three-way ANOVA was used to assess main effects of exercise training, nanoparticles and cancer as well as interaction between training, nanoparticles and cancer for all variables in mice bearing model of breast cancer. Tukey’s post hoc test was used to determine significant differences between individual groups. Moreover, SPSS nonparametric statistics tests including Mann-Whitney, Wilcoxon and Kruskal-Wallis were used for weights and inverted screen tests data.
Results
Body mass and food intake
As explained in methods, mice experienced the protocols for controls (C and CT), aerobic interval training (E and ET), Se NP administrators (Se and SeT) and Se NP administration with aerobic interval training (SeE and SeET) in order to consider the impact of Se NP supplementation and exercise training on cancer cachexia in 4T1 breast cancer mice. Within first 3 weeks after tumor injected, body mass decreased by Se NP administration [Table 1]. Getting a direct criterion of body weight, tumor weight of all sacrifice was deducted from total body mass. Se NPs and training showed no considerable impact body mass of tumor-bearing mice. But, body mass of mice without tumor in SeT group had decreased and trained animals were of higher body mass.
Table 1
| Group | Body weight (g) | Food intake (g) | ||||
|---|---|---|---|---|---|---|
| Initial | Middle | Final | Initial | Middle | Final | |
| C | 18.61 | 19.75 | 21.05 | 2.7 | 2.37 | 2.43 |
| E | 20.16 | 20.81 | 21.51 | 3.01 | 2.81 | 2.46 |
| Se | 18.36 | 19.38 | 20.28 | 2.88 | 2.38 | 2.41 |
| SeE | 18.7 | 19.03 | 20.3 | 2.87 | 2.8 | 2.7 |
| CT | 19.38 | 20.6 | 22.38 | 2.84 | 1.96 | 1.49 |
| ET | 19.19 | 20.2 | 22.12 | 2.8 | 2.58 | 2.33 |
| SeT | 19.06 | 20.25 | 21.66 | 2.77 | 2.66 | 2.24 |
| SeET | 18.33 | 19.8 | 20.67 | 3.12 | 2.57 | 2.44 |
Food intake showed a meaningful effect on cancer and Se NPs reduced food intake within fifth and sixth weeks after tumor injected. Additionally, after food intake, considerable training x cancer x Se NPs acted mutually/interacted within fifth and sixth weeks after tumor injected. Food intake in CT group was lower compared with SeET group based on Post-hoc analysis [Table 1]. Moreover, training and cancer had important impact on mice with fat mass so that, tumor-bearing mice faced with fat mass decrease using Se NPs (a meaningful cancer x Se NPs interaction, P < 0.05; Table 1) while aerobic interval training hindered the fat in tumor-bearing mice to be lost (a meaningful cancer x training interaction, P < 0.05; Table 1).
Our findings exhibited the cachexia promotion in 4T1 breast cancer mice within fifth and sixth weeks after tumor injected using aerobic interval training along with Se NPs supplementation caused the appetite arouse and resulted in fat and body mass sustaining 4T1 breast cancer BALB/C mice. Moreover, aerobic exercise training enhanced the Heart weight/body weight ratio and this demonstrated evidence for physiological effects of aerobic exercise training (P < 0.05; Table 1, Figure 2).
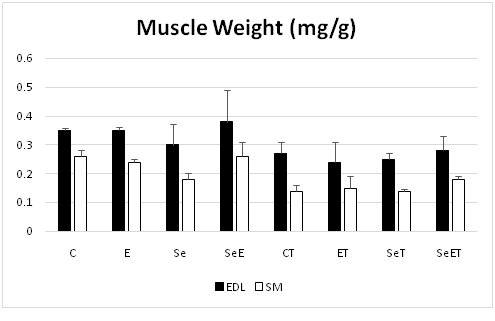
Figure 2
Tumorigenesis and spleen weight
Tumor volumes were measured via external palpation weekly and were reported elsewhere[14]. Shortly, significant effect of Se NP supplementation on tumor volume was clear and recognizable during all other weeks after tumor injection apart from the first week of measurement. In addition, it was observed that training has affected tumor volume considerably within the fifth and sixth weeks after the tumor injected. Surprising to know that, tumor volume in SeET group experienced both aerobic interval training and Se NP administration was lower in comparison with other tumor-bearing groups according to existed data[14].
As Spleen weight was positively related to tumorigenesis, it was recorded as an important factor; and Morphy et al (2011) suggested it as an indirect measure of systemic inflammation[24]; Moreover, our study mentioned a significant effect of cancer in spleen weight (P < 0.05)[14]. Furthermore, in spleen weight, training x cancer x Se NPs start interaction significantly (P < 0.05). Compared with three other tumor-bearing groups, sleep weight in CT group was higher according to Post-hoc analysis (P < 0.05) suggesting that inflammation has reduced after applying exercise training and Se NPs supplementation.
Skeletal muscle size and function
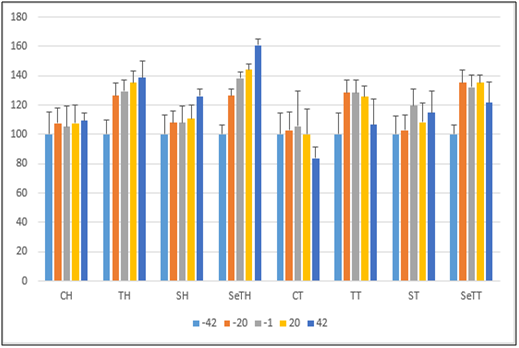
In present study, skeletal muscle mass and functional characteristics were evaluated in order to specify the impact of Se NPs supplementation and exercise training on skeletal muscle loss in mice with 4T1 breast cancer; and it was concluded that mice with 4T1 breast cancer suffered from skeletal muscles atrophy (figure 2)[14]. On the other hand, aerobic exercise training could have supportive role for skeletal muscle mass in some huge muscle such as quadriceps, because cancer influenced the mass of the quadriceps, gastrocnemius, tibialis anterior and plantaris muscles (figure 2)[14] also EDL and soleus largely. Nevertheless, an important effect observed in mass of quadriceps and EDL muscles was because of training (figure 2). Moreover, muscular strength was largely under effect of aerobic interval training according to weights test results obtained before tumor injected[14]. Similarly, muscular strength was influenced meaningfully by cancer after tumor injected[14]. On the other hand, decrease in weight lifting and inverted screen tests scores observed in tumor-bearing mice. but, as weights test results demonstrated, aerobic interval training is responsible for supporting the muscle strength in tumor -bearing mice[14]. Moreover, to specify independent variables during time, speed maximal test must be evaluated; and it can be concluded that just after tumor injected, maximal speed would reduce in CT group (tumor -bearing mice) (Figure 3.)
Figure 3: Incremental test.
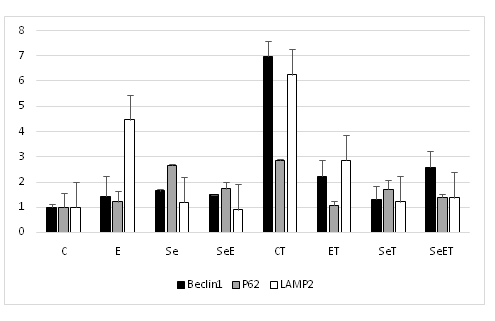
Skeletal muscle expression of Beclin1, P62 and LAMP2s
Markers of autophagy in EDL muscle are presented in (Figure. 4). Inoculation with 4T1 tumors significantly increased muscle expression of Beclin1 in EDL of tumor-bearing-mice additionally selenium affects in Beclin1, p62 and LAMP2 significantly. Also, interaction between exercise*selenium*cancer have been significant effect on Beclin1, p62 and LAMP2in EDL muscle.
Figure 4: Gene expression of autophagy in EDL.
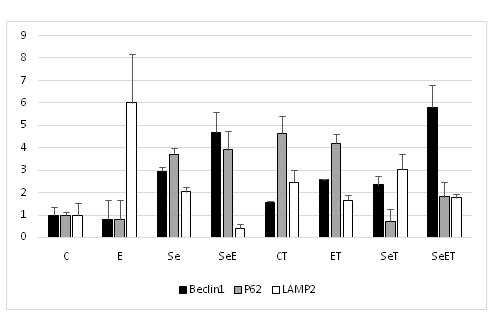
Markers of autophagy in soleus muscle are presented in (Figure. 5). Autophagic markers in soleus muscle did not differ significantly between the tumor bearing and normal groups (Figure. 5). On the other hand, Beclin1 expression in soleus muscle of the selenium tumor bearing groups was significantly lower than those of the untrained group.
Figure 5: Gene expression in soleus.
Discussion
During recent years, autophagy has known as a main catabolic cellular process that made researchers interested in muscle investigation. most of these studies came to this conclusion that there is relation between initiation of autophagy in catabolic muscle types and increased protein volume/change, such as starvation, hypoxia and especially cancer cachexia[25]. Nevertheless, what is still left unknown is regulation and functional role of basal autophagy in skeletal muscle that must be conformed with therapeutic strategies like exercise training and nutritional interventions in cancer[13]. Since Beclin-1 as an indicator of autophagy induction, LAMP-2 is the receptor for chaperone-mediated autophagy, and p62 as a marker of substrate sequestration and eventual degradation were assessed. Our findings demonstrate that muscle wasting, autophagy process, and functionality (measured by incremental speed test) could be modified by aerobic interval training and Se NP supplementation. What makes tumor volume decreased and prohibits form cachexia especially muscle loss in tumor-bearing mice is how to combine aerobic interval training with Se NP supplementation. Additionally, controlling muscle loss and modification of autophagy expression in EDL skeletal muscle are being done through aerobic interval training. Moreover, functionality was evaluated by means of incremental test and this conclusion was drawn that aerobic exercise training or Se NP supplementation could support aerobic fitness during cancer; furthermore, the promotion and preservation of other factors in fitness like muscle endurance and strength was done.
Results of present study indicated that interaction between exercise*selenium*cancer could result in modify of autophagy in EDL muscle separately. Nevertheless, increase of autophagy in tumor group was rather more than the group experienced exercise activities. Furthermore, increase in autophagy has occurred in fast muscle of the group received Selenium nanoparticles. Regarding the results of this study and reviewing the history of previous researches, autophagy increase in fast contraction muscles such as EDL muscle is nearly due to lower antioxidant characteristics compared with fast-contracted muscle[17]. In addition, increase of autophagy level in exercise group can be due to compensation effect and homeostasis control of protein cycle that is necessary during exercise; and finally, this mechanism changes lead to preservation of muscle function. In this direction, results of functional test in muscle practical factors such as muscle power and finding of incremental running test confirmed the autophagy increase as a compensation indicator[14]. On the contrary, autophagy increase in tumor group along with decrease in body function can be considered as a cellular result of stress that could decrease life quality significantly through stimulation of Liz protein paths and by reducing muscle atrophy.
Regarding results of present study, though, selenium has had positive impact on preservation of function during applying incremental running test, especially in cancer period, but selenium consumption could not prevent muscle tissue loss, fat and body weight within cancer period; and sometimes it helps to speed up the lose weight. It was demonstrated in different studies that selenium results in muscle tissue loss by controlling the path of Akt phosphophrektokinaz. Moreover, the results of various studies showed that selenium could not prohibit form muscle dystrophy in patient. In this direction, Kal and colleagues ‘study showed that selenoprotein deficiency in transgenic mice has led to stimulation of muscle growth following exercise activity.
In this study, selenium consumption, especially within last weeks of period, almost could prevent from tumor growth and preserve the function of mice with cancer in increased running test. Considering the results of Molanouri et al as well Yazdi et al, exhibited consumption of selenium nanoparticles nearly could make a supportive effect for body immune system[14,15].
Conclusion
In summary, the present study demonstrates that autophagy is increased by cancer and interaction between exercise*selenium*cancer could result in modify of autophagy, although the underlying mechanisms in the two conditions are likely different. Modification of autophagy in exercise and selenium groups can prevent probably atrophy or generally cachexia as well as improve muscle functions. On the other hand, increase of autophagy in cancer can strengthen cachexia and destroy muscle function during cancer.
Conflict of interest: The authors of this research article have no financial and personal conflict of interest statement.
Acknowledgments: This work was supported by the Research Center of Tarbiat Modarres University (TMU), Tehran, Iran. We wish to thank research center of Tarbiat Modarres University (TMU).
References
- 1. Alves, C.R. da Cunha, R., da Paixão, T. F., et al. Aerobic exercise training as therapy for cardiac and cancer cachexia. (2015) Life Sci 125: 9-14.
- 2. Argilés, J.M., Busquets, S., López-Soriano, F.J., et al. Are there any benefits of exercise training in cancer cachexia? (2012) J Cachexia Sarcopenia Muscle 3(2): 73-76.
- 3. Penna, F., Costamagna, D., Pin, F., et al. Autophagic degradation contributes to muscle wasting in cancer cachexia. (2013) Am J Pathol 182(4): 1367-1378.
- 4. Hayat, M.A. (Ed.) Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging: Volume 3-Role in Specific Diseases (2013) Academic Press.
PubMed||CrossRef||Others
- 5. Pin, F., Minero, V.G., Penna, F., et al. Interference with Ca2+-Dependent Proteolysis Does Not Alter the Course of Muscle Wasting in Experimental Cancer Cachexia. (2017) Front Physiol 8: 213.
- 6. Segatto, M., Fittipaldi, R., Pin, F., et al. Epigenetic Targeting of Bromodomain Protein BRD4 Counteracts Cancer Cachexia and Prolongs Survival. (2017) Nat Commun 8(1): 1707.
- 7. Evans, W.J. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. (2010) Am J Clin Nutr 91(4): 1123S-1127S.
- 8. Brook, M.S., Wilkinson, D.J., Atherton, P.J. Nutrient modulation in the management of disease-induced muscle wasting: evidence from human studies. (2017) Curr Opin Clin Nutr Metab Care 20(6): 433-439.
- 9. Chaillou, T., McPeek, A., Lanner, J.T. Docetaxel does not impair skeletal muscle force production in a murine model of cancer chemotherapy. (2017) Physiol Rep 5(11): e13261.
- 10. Brown, J.M. Evaluation of the Phase Training Model of Cancer Rehabilitation. (2016) Uni Northern Colorado.
PubMed||CrossRef||Others
- 11. Ahlberg, K., Ekman, T., Gaston-Johansson, F., et al. Assessment and management of cancer-related fatigue in adults. (2003) Lancet 362(9384): 640-650.
- 12. Winningham, M.L. Strategies for managing cancer-related fatigue syndrome. (2001) Cancer 92(S4): 988-997.
PubMed||CrossRef||Others
- 13. Aversa, Z., Costelli, P., Muscaritoli, M. Cancer-induced muscle wasting: latest findings in prevention and treatment. (2017) Ther Adv Med Oncol 9(5): 369-382.
- 14. Shamsi, M.M., Chekachak, S., Soudi, S., et al. Combined effect of aerobic interval training and selenium nanoparticles on expression of IL-15 and IL-10/TNF-α ratio in skeletal muscle of 4T1 breast cancer mice with cachexia. (2017) Cytokine 90: 100-108.
- 15. Yazdi, M.H., Mahdavi, M., Varastehmoradi, B., et al. The immunostimulatory effect of biogenic selenium nanoparticles on the 4T1 breast cancer model: an in vivo study. (2012) Biol Trace Elem Res 149(1): 22-28.
- 16. Forootanfar, H., Adeli-Sardou, M., Nikkhoo, M., et al. Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. (2014) J Trace Elem Med Biol 28(1): 75-79.
- 17. Assi, M., Derbré, F., Lefeuvre-Orfila, L., et al. Antioxidant supplementation accelerates cachexia development by promoting tumor growth in C26 tumor-bearing mice. (2016) Free Radic Biol Med 91: 204-214.
- 18. Navarro-Alarcon, M., Cabrera-Vique, C. Selenium in food and the human body: a review. (2008) Sci Total Environ 400(1): 115-141.
- 19. Balsano, C., Alisi, A. Antioxidant effects of natural bioactive compounds. (2009) Curr Pharm Des 15(26): 3063-3073.
- 20. Chang, Y., He, L., Li, Z., et al. Designing Core–Shell Gold and Selenium Nanocomposites for Cancer Radiochemotherapy. (2017) ACS Nano 11(5): 4848-4858.
- 21. Qi, Z., He, Q., Ji, L., et al. Antioxidant supplement inhibits skeletal muscle constitutive autophagy rather than fasting-induced autophagy in mice. (2014) Oxidative Med Cell Longevity 10.
- 22. Rao, V.A., Klein, S.R., Bonar, S.J., et al. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. (2010) J Biol Chem 285(45): 34447-34459.
- 23. Kenific, C. M., Debnath, J. Cellular and metabolic functions for autophagy in cancer cells. (2015) Trends Cell Biol 25(1): 37-45.
- 24. Murphy, E.A., Davis, J.M., Barrilleaux, T.L., et al. Benefits of exercise training on breast cancer progression and inflammation in C3 (1) SV40Tag mice. (2011) Cytokine 55(2): 274-279.
- 25. Amaravadi, R., Kimmelman, A.C., White, E. Recent insights into the function of autophagy in cancer. (2016) Genes Dev 30(17): 1913-1930.