Intra-Bone Marrow-Hematopoietic Stem Cell Transplantation; A Powerful Tool For Hematopoietic Stem Cell Transplantation
Yasushi Adachi1,2, Ming Li2, Susumu Ikehara2*
Affiliation
- 1Department of Surgical Pathology, Toyooka Hospital, Toyooka, Hyogo, Japan
- 2Department of Stem Cell Disorders, Kansai Medical University, Hirakata, Osaka, Japan
Corresponding Author
Susumu, Ikehara. M.D., Ph.D. Professor of Stem Cell Disorders, Kansai Medical University, 2-5-1 Shinmachi, Hirakata City, Osaka 573-1010, Japan; Tel:81-072-804-2450; E-mail: ikehara@hirakata.kmu.ac.jp
Citation
Ikehara, S., et al. Intra-Bone Marrow- Hematopoietic Stem Cell Transplantation; A Powerful Tool for Hematopoietic Stem Cell Transplantation. (2016) J Stem Cell Regen Bio 2(1): 29- 30.
Copy rights
© 2016 Ikehara, S. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Hematopoietic stem cell transplantation; Intra-bone marrow; Hematopoietic diseases
Introduction
Hematopoietic stem cell transplantation (HSCT) was originally developed to treat hematopoietic diseases, congenital immunodeficiency, leukemia, and so on. Subsequently, the technique has been used to treat solid tumors, autoimmune diseases and metabolic disorders. Although improvements have been made to the protocol, the associated risks are still high. To minimize these risks, we have established a new HSCT protocol, intra-bone marrow-hematopoietic stem cell transplantation (IBM-HSCT). IBMHSCT reduces graft versus host disease (GVHD), and accelerates hematopoiesis of donor bone marrow cells, resulting in the rapid recovery of hematopoiesis after the BMT.
In 1968, R.A. Good et al. performed the first hematopoietic stem cell transplantation (HSCT), in which bone marrow cells (BMCs) were utilized as HSCs, in humans[1]. This procedure was successfully carried out on an immunodeficient male baby. HSCT had originally been developed to treat immunological and hematopoietic diseases, but was subsequently applied to solid tumors, autoimmune diseases, metabolic disorders, and so on. HSCT protocols have been gradually improved, and the risks of HSCT have been reduced, but are still unacceptably high[2]. However, with a better safety profile and ease of application, HSCT could be applied to a far wider range of diseases.
HSCT has usually been performed by injecting donor hematopoietic cells containing hematopoietic stem cells into the recipient’s peripheral vein. However, a considerable number of injected cells become trapped in the lung and are prevented from migrating into the recipient’s bone marrow[3,4].
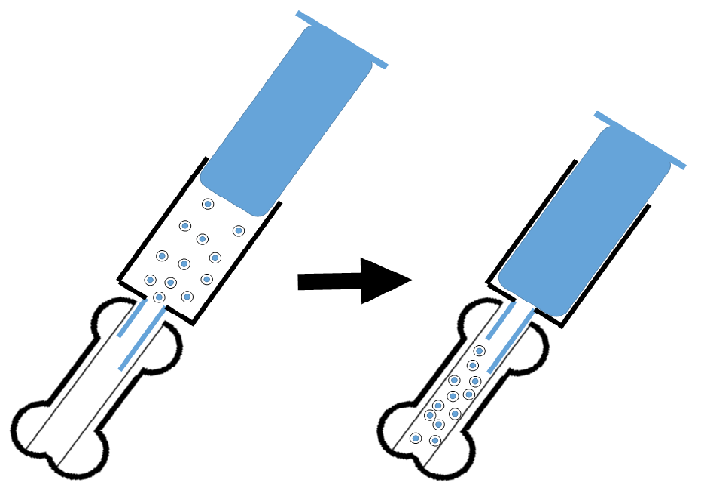
In order for the HSCT to be more effective, we developed a new protocol, in which donor HSCs are directly injected into the recipient’s bone marrow cavity, resulting in the injection of many more donor HSCs into the recipient’s bone marrow cavity than the conventional intravenous method[5] (Figure). IBM-HSCT accelerates donor hematopoiesis, resulting in improved survival rates of the recipients after BMT[5,6]. This method also obviates the risk of the hematopoietic cells becoming trapped. Since 2001, we have published around 50 papers describing the method, which we have successfully applied to several animal models of autoimmune diseases, diabetes, senescence accelerated mouse, and so on. For example, the MRL/lpr mouse, which is a lupus model, is known to be chimeric-resistant[7]. This mouse is also radiosensitive (< 8.5 Gy), whereas its abnormal hemopoietic stem cells are radioresistant (> 8.5 Gy); conventional BMT (8.5 Gy irradiation plus allo BMT) has a transient effect on the autoimmune diseases, which tend to recur 3 months after the BMT. However, when we transplanted BMCs from normal mice into the bone marrow cavity of MRL/lpr mice, the hemopoietic cells were replaced with the donor cells and the mice subsequently did not show any autoimmune status, resulting in prolonged survival[5].
Figure 1: Procedure of IBM-HSCT: Hematopoietic cells containing hematopoietic stem cells are directly injected into the bone marrow cavity.
Intra-bone marrow injection is a useful and effective method for HSCT. It has also been applied to humans using cord blood and has shown better results than the conventional intravenous injection method[8,9]. Our hope is that IBM-HSCT is further developed and is used in a wide variety of fields.
References
- 1. Good, R.A., Meuwissen, H.J., Hong, R., et al. Bone marrow transplantation: correction of immune deficit in lymphopenic immunologic deficiency and correction of an immunologically induced pancytopenia. (1969) Trans Assoc Am Physicians 82: 278-85.
- 2. Gooley, T.A., Chien, J.W., Pergam, S.A., et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. (2010) N Engl J Med 363(22): 2091-101.
- 3. Cui, J., Wahl, R.L., Shen, T., et al. Bone marrow cell trafficking following intravenous administration. (1999) Br J Haematol 107(4): 895-902.
- 4. Kushida, T., Inaba, M., Hisha, H., et al. Crucial role of donor-derived stromal cells in successful treatment for intractable autoimmune diseases in mrl/lpr mice by bmt via portal vein. (2001) Stem Cells 19(3):226-35.
- 5. Kushida, T., Inaba, M., Hisha, H., et al. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. (2001) Blood 97(10): 3292-9.
- 6. Li, Q., Hisha, H., Yasumizu, R., et al. Analyses of very early hemopoietic regeneration after bone marrow transplantation: comparison of intravenous and intrabone marrow routes. (2007) Stem Cells 25(5): 1186-94.
- 7. Ikehara, S., Yasumizu, R., Inaba, M., et al. Long-term observations of autoimmune-prone mice treated for autoimmune disease by allogeneic bone marrow transplantation. (1989) Proc Natl Acad Sci USA 86(9):3306-10.
- 8. Frassoni, F., Varaldo, R., Gualandi, F., et al.The intra-bone marrow injection of cord blood cells extends the possibility of transplantation to the majority of patients with malignant hematopoietic diseases. (2010) Best Pract Res Clin Haematol 23(2): 237-44.
- 9. Frassoni, F. Intra-bone route of administration offers new perspectives for safer transplantation of hematopoietic stem cells. (2010) Cytotherapy 12(1): 5-6.