Localization of Electrochemical Reactions in Electrocatalytic Processes on Nanocomposite Electrodes
Affiliation
Institute of General & Inorganic Chemistry of National Academy of Science, Kyiv, Ukraine
Corresponding Author
Danilov, M.O. Institute of General & Inorganic Chemistry of Nat. Acad. Sci., Palladina avenue 32/34, Kyiv-142, Ukraine, Tel: (38044) 424-22-80; E-mail: danilovmickle@rambler.ru
Citation
Danilov, M.O. Localization of Electrochemical Reactions in Electrocatalytic Processes on Nanocomposite Electrodes. (2015) J Nanotech Mater Sci 2(2): 55-62.
Copy rights
© 2015 Danilov, M.O. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Reaction localization principle; Nanocomposites; Catalysts; Electrocatalysis; Electrochemical power sources.
Abstract
A principle of reaction localization is proposed, which explains the catalytic properties of nanocomposites materials in the reaction under study. By changing the route of electrochemical reactions from the catalyst to the support or vice versa one can influence electrocatalytic properties. The catalytic activity can be judged from the difference in the electrochemical overpotential of separation of the reactant under investigation in a particular medium between the catalyst and the support, The action of this principle was demonstrated on the basis of oxygen reduction and hydrogen oxidation reactions for fuel cells and for the reaction of electrochemical hydrogen accumulation at a hydride electrode. For an oxygen electrode with carbon support in an alkaline medium, materials with high oxygen evolution overpotential are good catalysts. For a hydrogen electrode with carbon support in an alkaline medium, materials with low hydrogen evolution overpotential are good catalysts. In the case of electrochemical hydrogen accumulation at intermetallic compounds with low hydrogen evolution overpotential in an alkaline medium, catalysts with high hydrogen evolution overpotential must be used. Making use of this principle one can carry out goal-directed synthesis or select new organic or inorganic catalytic materials.
Introduction
Electrochemical power sources and fuel cells, in which electrocatalytic processes take place, belong to alternative or minor energetics. At the present time, the power generated by all current sources in the world equals the power generated by all nuclear, thermal and water power stations. In view of the sharp deterioration of the environmental conditions and depletion of oil and gas resources, which give rise to energy crisis, the development of electrochemical generators becomes very topical. Electrocatalytic reactions and processes are the basis of any electrochemical energy generation system. Research in the field of developing highly efficient catalysts has been done for a long time and fairly successfully. But it is of semi empirical character.
The review on electrocatalysis[1] shows that the main mechanism with the aid of which the laws governing it were explained was based on the activated-chemisorption model. At the present time, a number of papers[2-4] show an alternative mechanism, which is known as incipient hydrous oxide/adatom mediator (IHOAM) model[5]. The need for new approaches to catalysis became necessary after Haruta et al[6] had revealed an astonishing catalytic activity of gold nanoparticles deposited onto substrates. Describing IHOAM in their paper, Burke et al[5] laid special emphasis on the role of nonequilibrium surface states of metal, in which active atoms on the surface may undergo fast redox electron transitions at low potentials and serve as mediators in electrocatalytic processes. Surface atoms or oxide groups (active centers) are regarded not merely as “anchors” for adsorption; these particles with low surface coverage undergo chemical or electrochemical transformations as a part of electro-catalytic processes. References[7,8] propose two mechanisms for platinum: activated chemisorption and IHOAM, which act for different reactions at this catalyst. Reference[9] shows the role of intensive parameters, such as Tafel slope and zero-charge point for oxide electrodes, with the aid of which electron and geometric factors in electrocatalysis can be separated. As follows from these papers, however, there is no unified theory which makes it possible to describe electrocatalytic processes.
It is known that all electrocatalytic processes are based on the compromise potential of the electrochemical reactions proceeding at the electrode. The value of this compromise potential depends on the potentials of the electrochemical reactions that proceed simultaneously at this electrode. Therefore, by localizing electrochemical reactions or varying their route one can influence the electrocatalytic properties of the material.
The aim of our work was to study and substantiate the effect of change or localization of the route of electrochemical reactions on the electrocatalytic properties of nanocomposites materials.
Experimental
Multiwalled carbon nanotubes (MWCNT) were used as the catalyst support. They were prepared by catalytic ethylene pyrolysis on a catalyst[10]. The product was obtained as black powder with a bulk density of 25-30 g dm-3. The outer diameter of nanotubes was about 10-30 nm, the specific surface was 230 m² g-1, and the mineral impurity content of unpurified product was 15-20 wt%. The catalyst impurities were removed from MWCNTs by treatment with a hydrofluoric acid solution. The oxygen electrodes were prepared by pressing. The hydrophobic layer contained 0.07 g cm-2 acetylene black with 25% polytetrafluoroethylene, and the active layer contained 0.02 g cm-2 MWCNTs, modified by manganese dioxide, with 5% polytetrafluoroethylene. The hydrogen electrodes were prepared by pressing from various compositions based on MWCNTs and catalysts with addition of 25% polytetrafluoroehylene as binder. The investigations were carried out in a fuel cell mockup. The mockup for testing gas-diffusion electrodes is described in Ref[11]. The electrolyte was a solution of analytical grade 6M KOH. For the oxygen electrode, zinc was used as the auxiliary electrode. For the hydrogen electrode, the auxiliary electrode was a nickel oxide electrode. In all investigations, the reference electrode was a silver-chloride electrode connected through a salt bridge. All potentials are given with respect to silver-chloride reference electrode. The electrochemical curves were taken under galvanostatic conditions. The hydrogen and oxygen source was a U-shaped electrolyzer with alkaline electrolyte. Hydrogen and oxygen were supplied to the gas electrodes under an excess pressure of 0.01 mPa. Before operation, the gas electrodes were blown through with appropriate gas for an hour. In electrochemical tests, the electrolyzer current exceeded by a factor of three the current generated at the gas electrode. The study of the polarization curves were performed on potentiostat P-8S Elins (Russia) on a standard three-electrode scheme. As auxiliary electrode used cylindrical platinum electrode with area of 7 cm². The linear polarization and chronopotentiometry technique were employed to determine the electrode process kinetics. The linear polarization was performed between the steady potential in 6M KOH solution to +0.5 V with scan rate of 1 mV/s. In all investigations, the reference electrode was a silver-chloride electrode connected through a salt bridge. The intermetallic compound La0.84Co0.16Ni4.83Fe0.04 with a gravimetric composition of under 40 nm was investigated as a hydride electrode material for nickel-metal hydride batteries. Coatings were deposited on intermetallic compound particles by chemical reduction from aqueous solutions of metal salts with reductants. The electrodes made of these materials were tested in a nickel-metal hydride battery mockup under galvanostatic conditions. The charge and discharge current magnitude was the same (100 mA cm-2). A cermet nickel oxide electrode was prepared by pressing powders onto a nickel net. A PTFE emulsion was used as the binder. Before the beginning of investigations, five training charge-discharge cycles were performed. The tests were carried out in a 5M solution of potassium hydroxide with addition of 1M lithium hydroxide[12].
Results and Discussion
During the electrocatalytic reaction at the electrode, the reagent supply stage, the electrochemical act of electron transfer, and reagent withdrawal take place. By using a heterogeneous catalyst-support composites with different energy barriers for different electrochemical reaction stages one can change the catalytic activity of this composites. The principle of reaction localization manifests itself to the greatest extent in nanostructured catalytic compositions, which is associated with rapid diffusion of reactant across the interface between catalyst and support particles. Carbon-base nanomaterials with deposited catalysts are best suited for such composites.
Consider the following examples of the application of the principle of electrochemical reaction localization:
- 1. For oxygen reduction reaction, which proceeds in fuel cell oxygen electrodes;
- 2. For hydrogen oxidation reaction, which proceeds in fuel cells at hydrogen electrodes;
- 3. For hydride formation reaction, which proceeds at the hydride electrode of nickel-metal hydride battery.
Consider the principle of reaction localization for the oxygen electrode of a fuel cell. The use of a heterogeneous composites of carbon-base material and catalyst with high oxygen evolution overpotential makes it possible to localize electrochemical reactions. In this case, adsorption of molecular oxygen on the carbon support will take place, and at the catalyst with high oxygen evolution overpotential, electron attachment with bond rupture takes place, which accelerates the oxygen ionization process.
Therefore, the most active catalysts for oxygen reduction in an alkaline medium for oxygen electrode are materials with high molecular oxygen evolution overpotential in alkaline medium, i.e. materials with high value of the coefficient a in the Tafel equation:
η = a + b lg I [1]
If a series of metals is made up in order of increasing oxygen evolution overpotential in alkaline medium, it will be as follows[13]:
Co < Fe < Ni < Cd < Pb < Au < Pt [2]
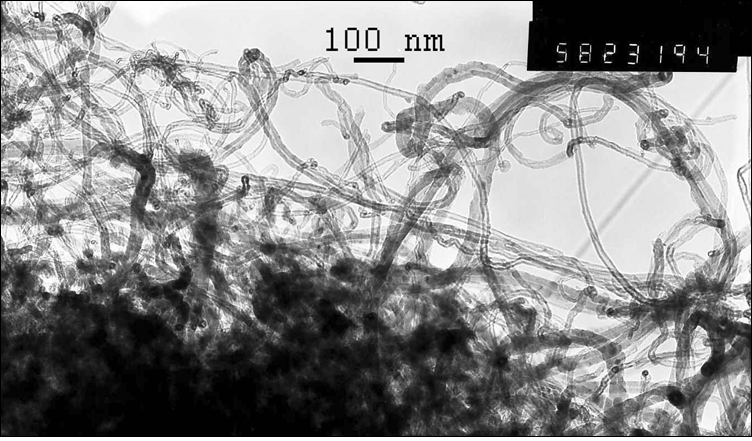
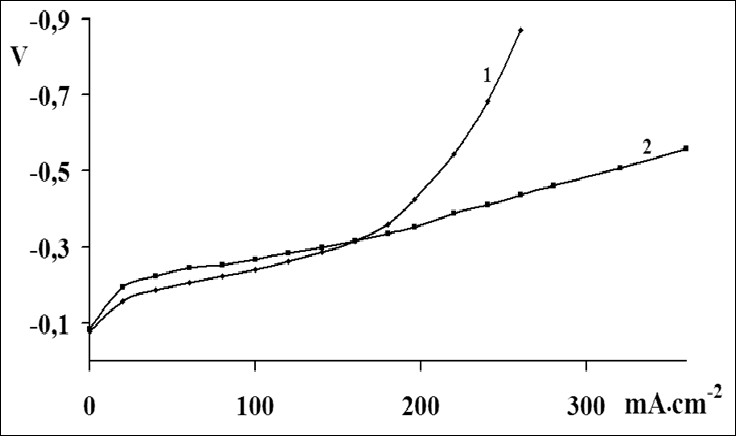
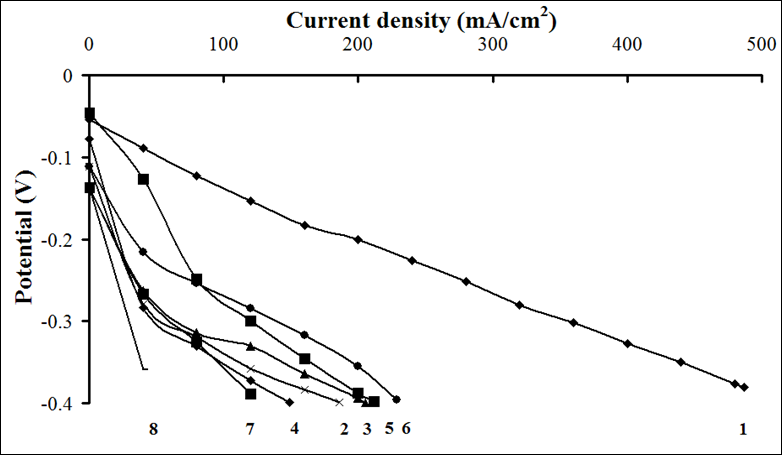
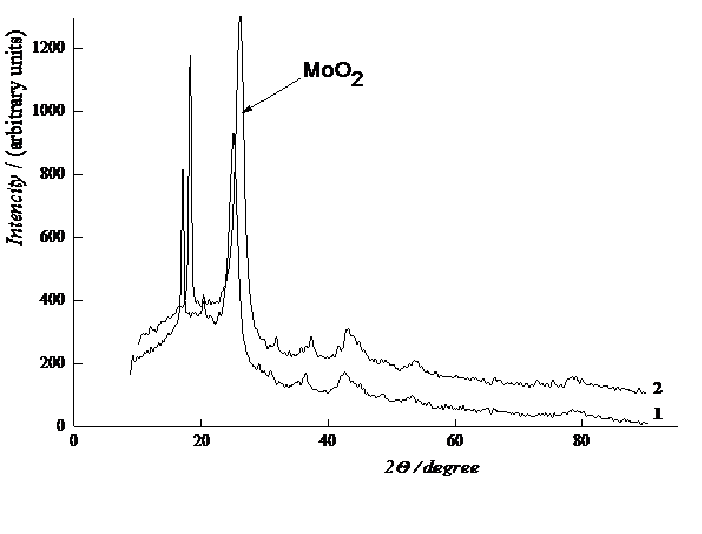
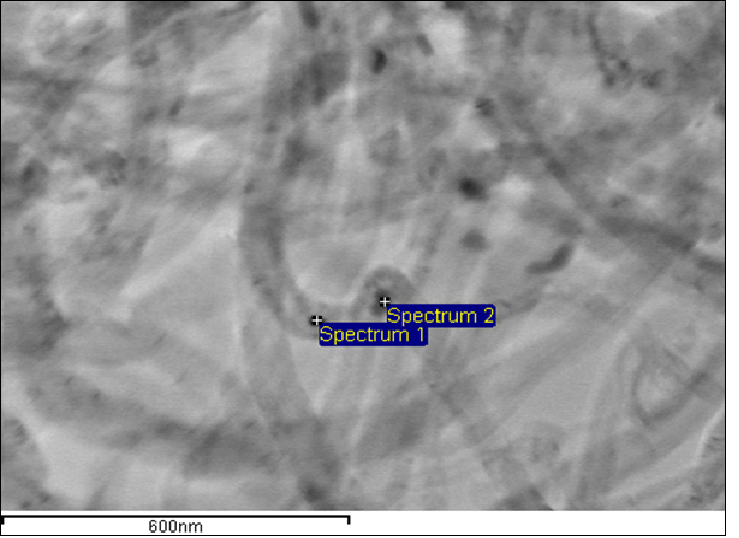
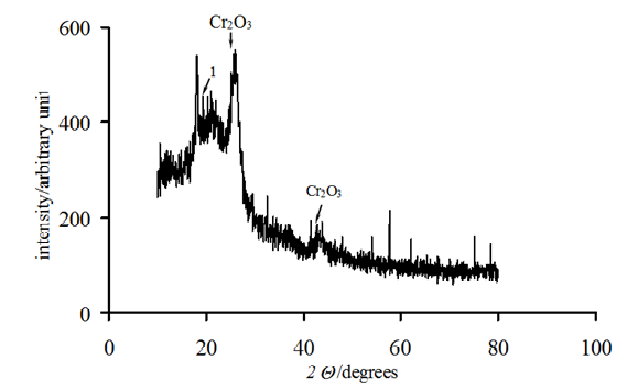
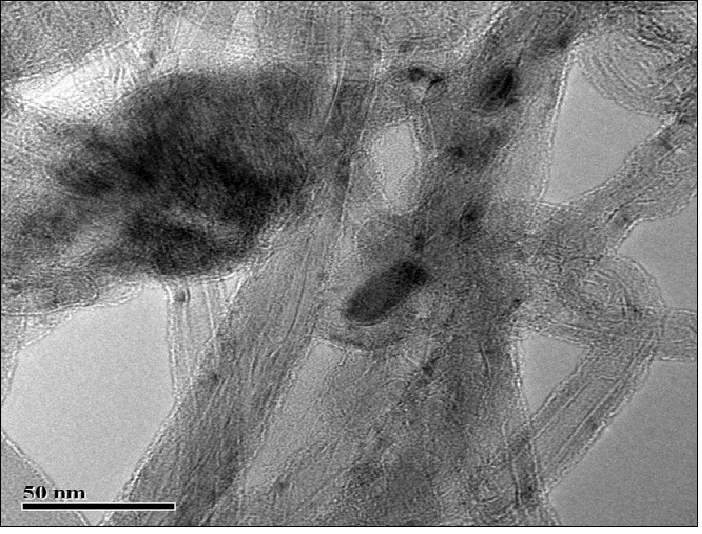
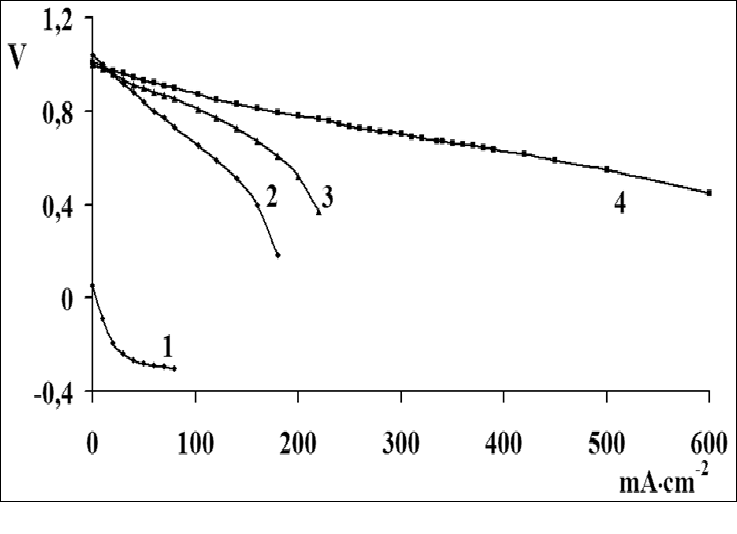
The catalytic activity of these metals will also increase for oxygen electrode in alkaline medium. The value of the coefficient a depends on the nature of material, specific surface, orientation of the single-crystal faces, solution composition, the degree of surface oxidation[13]. Metals and oxides were used as catalyst for investigations. Platinum, nickel and lead were chosen from metals[14,15]. Manganese[16], molybdenum[17], chromium[18], cobalt and niobium oxides were used as metal oxides. The catalysts were deposited by electrochemical and chemical methods developed by us[14-18]. As an example, we chose MWCNTs with deposited manganese dioxide (Figure 1)[16], which has a high oxygen evolution overpotential and hence a high catalytic activity. From the above series of catalysts[2] we chose for comparison the cheapest material (lead). A nanocomposites of carbon nanotubes with 10% lead content was prepared by the method of reduction of metal compounds[16]. Figure 2 shows current-potential curves for oxygen electrodes based on a composites of WCNT s with 10% manganese dioxide (Figure 2, curve 1) and MWCNTs with 10% lead content (Figure 2, curve 2). As is seen from Figure 2, the electrodes containing a 10% nanocomposites of lead and MWCNTs operate at current densities of over 150 mA cm-2 better than the composites based on manganese dioxide with MWCNTs. Thus, the fact that lead, which has a high oxygen evolution overpotential, has a better catalytic activity in the oxygen reduction reaction supports the principle of reaction localization. Figure 3 shows plots of potential against current density for oxygen electrodes with active mass based on composites of carbon nanotubes with different catalysts deposited on them: metallic platinum, lead, nickel (Figure 4) and molybdenum (Figure 5), chromium (Figure 6), cobalt (Figure 7), manganese and niobium oxides. The coefficients a in the Tafel equation for the reaction of molecular oxygen evolution at these electrodes have been calculated at small current density values and low polarization. Table 1 lists values of the coefficient a, obtained by us, for oxygen evolution at a Ni electrode with different materials deposited on the surface and electrical characteristics of oxygen electrodes fabricated from nanocomposites based on carbon nanotubes with the same catalysts deposited on them. As is seen from Fig. 3 and Table 1, there is a correlation between the value of the coefficient α for molecular oxygen evolution at the materials under investigation and the electrical characteristics of oxygen electrodes based on nanocomposites of the same materials deposited on carbon nanotubes. The larger value of the coefficient α for the reaction of molecular oxygen evolution at the catalyst under investigation, give the higher the electrochemical characteristics of oxygen electrodes based on nanocomposites consisting of these catalysts and carbon nanotubes.
Table 1: Electrochemical characteristics of various catalytic materials
| Catalyst | Current density (mA/cm²) at 350 mV for oxygen electrodes | Coefficient α for oxygen evolution reaction |
|---|---|---|
| Platinum | 487 | 0.76 |
| Lead | 228 | 0.72 |
| Manganese dioxide | 211 | 0.7 |
| Niobium oxide | 205 | 0.7 |
| Molybdenum oxide | 186 | 0.62 |
| Cobalt oxide | 148 | 0.61 |
| Chromium oxide | 120 | 0.62 |
| Nickel | 40 | 0.52 |
Figure 1: Micrographs of nanocomposites based on multiwalled carbon nanotubes with deposited manganese dioxide (10 mass. %).
Figure 2: Current-potential curves for oxygen electrodes for fuel cells with different nanocompositions:
1 - MWCNTs containing manganese dioxide (10 mass. %);
2 - MWCNTs containing lead (10 mass. %).
Figure 3: Dependence of potential on current density for oxygen electrodes with active layer (0.02 g/cm²) based on composites of multiwalled carbon nanotubes with different deposited catalysts (10 wt%): (1) platinum, (2) molybdenum oxide, (3) niobium oxide, (4) cobalt oxide, (5) manganese dioxide, (6) lead, (7) chromium oxide, (8) nickel.
Figure 4: Micrographs of nanocomposites based on multiwalled carbon nanotubes with deposited metallic nickel (10 mass. %).
Figure 5A: Micrographs of nanocomposites based on multiwalled carbon nanotubes with deposited molybdenum oxide (10 mass. %);
Figure 5B: X-ray phase analysis samples of composites MWCNT with deposited MoO2.
Figure 6A: Micrographs of nanocomposites based on multiwalled carbon nanotubes with deposited 10 mass. % of chromium oxide;
Figure 6B: X-ray phase analysis samples of composites MWCNT with deposited 10 mass. % of chromium oxide. 1- Cr(OH)3;
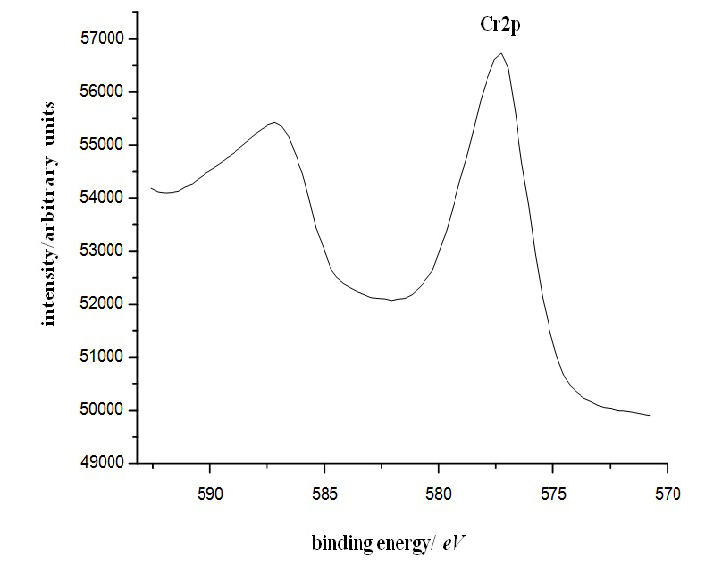
Figure 6C: Results of X-ray photoelectron spectroscopy of a sample of composite based on chromium oxide and carbon nanotubes.
Figure 7: Micrographs of nanocomposites based on multiwalled carbon nanotubes with deposited 10 mass. % of cobalt oxide.
Another support for this principle is the fact that the oxide compounds of Ni with variable valence, which also have a high oxygen evolution overpotential in alkaline solutions, possess catalytic properties. Iron, which is a catalytic poison, has, as we see, for manganese dioxide a lower oxygen evolution overpotential in the oxygen reduction reaction. This fact is an indirect support for the principle of reaction localization.
Consider the requirements to catalysts for the hydrogen electrode of a fuel cell with alkaline electrolyte. Here, in accordance with the principle of reaction localization, catalysts with low hydrogen evolution overpotential in alkaline medium must be used. In this case, adsorbed hydrogen atoms with bond rupture in the molecule are formed on the catalyst, and on carbon-base nanomaterials, electron detachment with desorption of hydrogen ions takes place. The series of metals in order of decreasing coefficient a for hydrogen evolution in an alkaline medium is as follows[13]:
Metal Pb < Sn < Zn < Cd < Cu < Ti < Fe < Ag < Ni < Al < Co < Pd < Pt [3]
a 1.36 1.28 1.2 1.05 0.96 0.83 0.76 0.73 0.65 0.64 0.6 0.53 0.31
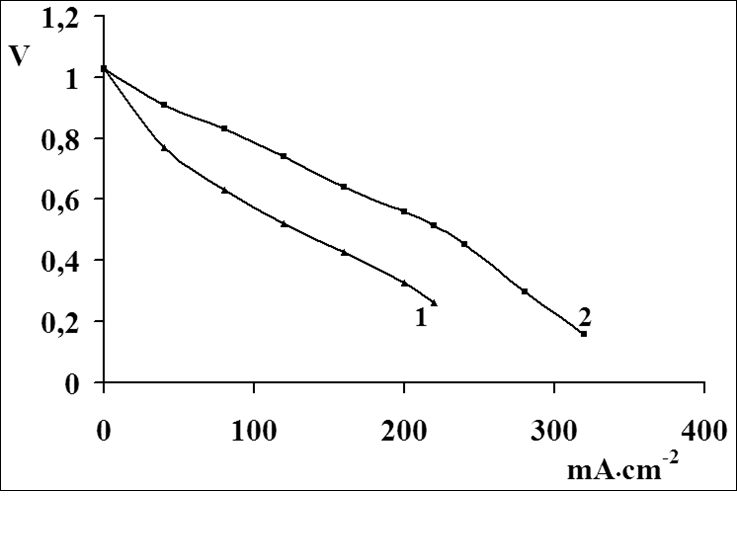
Materials consisting of nanocompositions of MWCNTs with 10% palladium content and mixtures of powders of the Ni2Al3 and Mg2Ni alloys with 50% MWCNT content were chosen for investigations. From these materials, hydrogen electrodes for fuel cell were made by pressing. Figure 8 shows current- potential curves for these hydrogen electrodes, which were obtained in a KOH-based 6M electrolyte. As follows from the electrochemical characteristics of the electrodes, these materials are arranged according to activity for hydrogen electrode in the following series: Pd (curve 4, Figure 8), Ni2Al3 (curve 3, Figure 8), Mg2Ni (curve 2, Figure 8), and pure carbon nanotubes (curve 1, Figure 8). This series fully coincides with the arrangement of metals in a series with decreasing hydrogen evolution overpotential in an alkaline medium. Let us now see how heterogeneous composition is affected by the introduction of a deliberately bad catalyst for hydrogen electrode. Following the principle of electrochemical reaction localization, lead is such a catalyst. Using the above method for the deposition of metals on carbon nanotubes, we have obtained composites containing: MWCNTs with 5% Pd (Figure 9, curve 2) and MWCNTs with 5% Pd and 10% Pb deposited on them (Figure 9, curve 1). As is seen from Figure 9 (curve 1), introduction of lead in MWCNT-Pd composites deteriorates the characteristics of the fuel cell hydrogen electrode. This confirms once move the possibility of predicting catalytic properties of materials with the aid of the principle of electrochemical reaction localization. Thus, the principle of reaction localization is also substantiated for catalysts of fuel cell hydrogen electrode.
Figure 8: Current-potential curves for hydrogen electrodes for fuel cells with different active-layer composites:
1 - 0.029 g cm-2 MWCNT; 2 - 0.029 g cm-2 Mg2Ni with 0.029 g cm-2 MWCNT; 3 - 0.029 g cm-2 Ni2Al3 with 0.029 g cm-2 MWCNT; 4 - 0.029 g cm-2 MWCNT with 10 mass. % of palladium.
Figure 9: Current-potential curves for hydrogen electrodes for fuel cells with different active-layer composites:
1- 0.029 g cm-2 MWCNT with 5 mass. % of Pd and 10 mass. % of Pb;
2 - 0.029 g cm-2 MWCNT with 5 mass. % of Pd.
Now consider the reaction of electrochemical hydrogen accumulation which proceeds at the metal-hydride (MH) electrode of nickel-metal hydride battery. The principle of reaction localization was studied for a LaNi5-type hydride-forming intermetallic compound and various metals-catalysts deposited on the surface of metallide particles.
Consider the processes occurring in a nickel-metal hydride battery. They are characterized by effective migration of hydroxyl ions from the positive to the negative electrode during charge and from the negative to the positive electrode during discharge without change in electrolyte volume. The discharge reaction equation is:
NiOOH + H2O + e ↔ Ni(OH)2 + OH- Eo = +0.490 V (nickel oxide electrode)
МН + ОН- ↔ М + Н2О + е Еo = - 0.828 V (metal-hydride electrode)
The overall discharge reaction is:
MH + NiOOH ↔ M + Ni(OH)2 Eo = +1.318 V
The same reactions proceed reversibly during charge. Besides, during charge an oxygen evolution reaction proceeds at nickel oxide electrode in addition to the conversion Ni(OH)2 to the NiOOH. It starts at 80% conversion into NiOOH proceeds according to the scheme: 2ОН - → 1/2О2 + 2е + Н2О
In the case of overcharge of MH electrode, a reaction of evolution of gaseous hydrogen occurs, which is chemically absorbed by this electrode in the case of proper battery design. To avoid strong overcharge of nickel oxide electrode, an excess amount of it must be taken when designing the battery. The main problem of nickel-metal hydride batteries is MH corrosion reaction in the presence of dissolved oxygen. The rate of the corrosion process becomes especially high at a temperature above 40°C:
2 МН + О2 → 2М(ОН)
To prevent this reaction, the authors propose microcapsulation[19,20]. Another way of reducing corrosion is the use of polymeric electrolyte or good separators. In addition to corrosion prevention, microcapsulation plays a big role in the metal hydride formation process. To coat intermetallic compounds, metals with high molecular hydrogen evolution overpotential in alkaline medium must be used. At such metals, the water adsorption reaction with electron attachment is localized, as a result of which adsorbed hydrogen is formed, which diffuses subsequently into intermetallic compound to form a metal hydride. The role of the catalyst with high hydrogen overpotential reduces to the formation of adsorbed hydrogen and prevention of its recombination on the charge of the electrode. Therefore, the series of catalysts for use in hydride electrode will be reverse to the series of catalysts [2] for fuel cell hydrogen electrode.
Here the characteristics of the catalysts improve from platinum to lead with increasing coefficient a from the Tafel equation.
Powders of the intermetallic compound La0.84Co0.16Ni4.83Fe0.04 with < 40 nm grain size with coatings of the following composition: nickel-phosphorus and cobalt-phosphorus alloys, copper, silver and palladium were obtained for investigation[12].
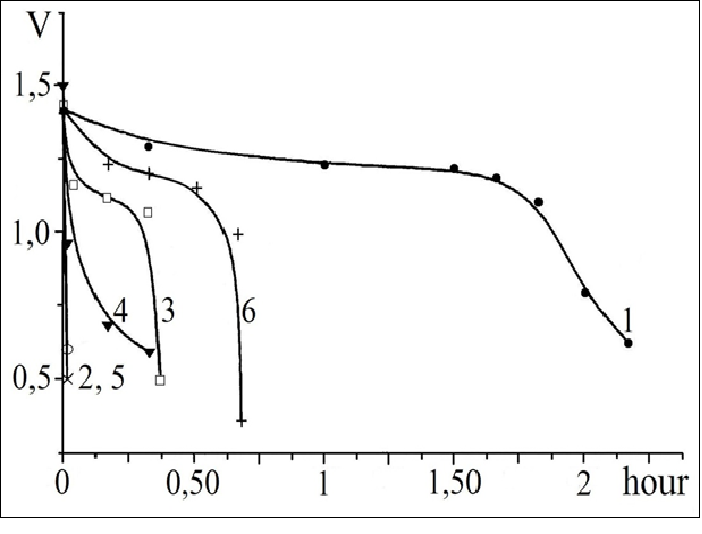
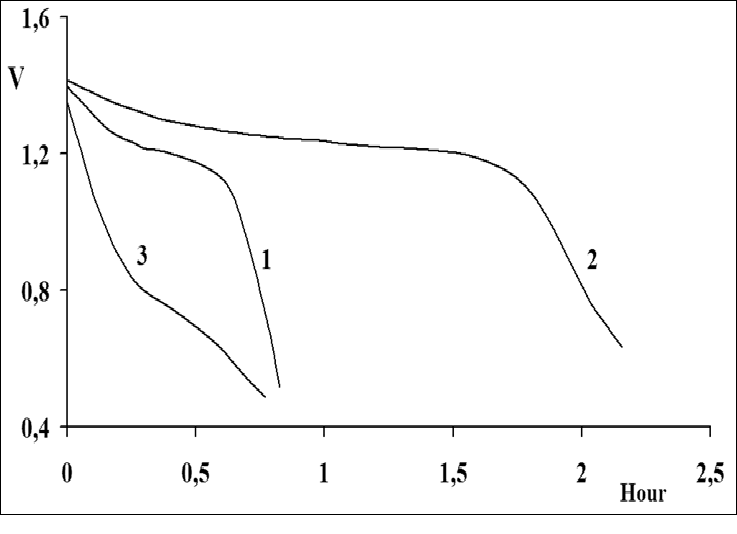
Figure 10 shows discharge curves for a Ni-MH battery with anode composed of different metallide powders coated with 9% Cu (Figure 10, curve 1), 3% Ag (Figure 10, curve 6), 0.1% Pd (Figure10, curve 3), 9% Co (Fig. 10, curve 4), 5% Ni (Figure 10, curve 5) and the starting metallide La0.84Co0.16Ni4.83Fe0.04 (Figure 10, curve 2). According to the value of discharge capacity, they may be arranged, depending on the type of coating, in the following order: Cu, Ag, Pd, Co-P, Ni-P. This series differs slightly from the series in which the coefficient a decreases: Cu (0.96), Ag (0.73), Ni (0.65), Co (0.60), Pd (0.53). This is most likely accounted for by the nonoptimal number and size of the clusters of the deposited metals. This assumption is substantiated by the nonlinear dependence of electrode discharge capacity on the percentage of copper (Figure 11). It has been found that the discharge capacity of electrodes with 2.5% and 9% copper (Figure 11, curves 1,2) is higher than that of electrodes with 5% copper (Figure 11, curve 3).
Figure 10: Discharge curves for nickel-metal hydride current sources with hydride electrode based on a coated intermetallic compound:
1 - Cu; 2 - Ni; 3 - Pd; 4 - Co; 5 - Starting intermetallic compound La0.84Co0.16 Ni4.83 Fe0.04. Discharge current magnitude: 100 mA cm-2.
Figure 11: Discharge curves for nickel-metal hydride current sources with hydride electrodes based on the intermetallic compound La0.84Co0.16 Ni4.83 Fe0.04 with different amount of deposited copper:
1- 2.5 %; 2- 9 %; 3- 5 %. Discharge current magnitude: 100 mA cm-2.
These experiments and literature data[19] showed that the use of materials with high hydrogen evolution overpotential and hence the coefficient a in the Tafel equation makes it possible to improve the characteristics of hydride electrode. Thus, the principle of reaction localization is applicable to hydride electrodes too.
When considering the above variants of using the principle of electrochemical reaction localization, it may be formulated as follows. For any electrocatalytic reaction proceeding in a particular electrolyte at an electrode consisting of an inhomogeneous catalyst – support system, materials for the catalyst and support with diametrically opposite properties in this electrolyte with respect to the catalyzed reaction must be selected. For example, for oxygen reduction reaction in an alkaline electrolyte, if a carbonaceous material having relatively low oxygen evolution overpotential has been chosen as the catalyst support, then a material with high oxygen evolution overpotential in alkaline medium must be taken as the catalyst for this medium. The catalytic activity of different inhomogeneous composites can be estimated from the value of the difference in the evolution overpotential of the reagent under investigation at the catalyst and support in a particular medium. A limitation in selecting materials on the basis of the principle of electrochemical reaction localization is the corrosion stability of the chosen materials in a particular electrolyte.
Thus, the principle of reaction localization allows one to explain why some materials or others are catalysts for the chosen reaction; and the catalytic activity of the chosen inhomogeneous composition can be judged from the difference in the electrochemical evolution overpotential of the reagent under investigation between the catalyst and support. The larger this difference, the higher the catalytic activity of the composition obtained.
Making use of this principle, one can perform goal-directed synthesis or selection of catalytic materials for electrochemical power sources and for other electrochemical systems and reactions.
Conclusion
1. A principle of electrochemical reaction localization has been proposed, which makes it possible to estimate the activity of electrodes, consisting of catalyst and support, for electrochemical power sources from the difference in the evolution overpotential of the reagent under investigation between the catalyst and support.
2. In accordance with the principle of electrochemical reaction localization, good catalysts for the fuel cell oxygen electrode in an alkaline medium with carbon support are materials with high molecular oxygen evolution overpotential in alkaline medium. The higher the a values from the Tafel equation, the more active the catalyst.
3. In accordance with the principle of electrochemical reaction localization, good catalysts for the fuel cell hydrogen electrode in alkaline medium with carbon support are materials with low hydrogen evolution overpotential in alkaline medium. The lower the a values from the Tafel equation, the more active the catalyst.
4. In accordance with the principle of electrochemical reaction localization, additions of catalysts with high hydrogen evolution overpotential in alkaline medium must be used for the hydride electrode of nickel-metal hydride battery for intermetallic compounds with low hydrogen evolution overpotential in alkaline medium. The higher the a value from the Tafel equation, the better the characteristics of hydride electrode.
5. The principle of electrochemical reaction localization allows one to synthesize or select novel organic or inorganic materials for electrochemical power sources and to assess them by the value of electrochemical overpotential, i.e. by the coefficient a from the Tafel equation. The larger the difference in the value between the catalyst and support, the higher activity of the electrode.
References
- 1. Pletcher, D. Electrocatalysis: present and future. (1984) J Appl Electrochem 14(4): 403-415.
- 2. Burke, L.D. Premonolayer oxidation and its role in electrocatalysis. (1994) Electrochimica Acta 39(11-12): 1841-1848.
- 3. Burke, L.D., Nugent, P.F. The electrochemistry of gold: II the electrocatalytic behaviour of the metal in aqueous media. (1998) Gold Bulletin 31(2): 39-50.
- 4. Burke, L.D., Collins, J.A., Murphy, M.A. Redox and electrocatalytic activity of copper in base at unusually low, premonolayer potentials. (1999) J Solid State Electrochem 4(1): 34-41.
- 5. Burke, L.D., Kinsella, L.M., O’Connell, A.M. Importance of Metastable States in Electrocatalytic Processes at Metal Surfaces in Aqueous Media. (2004) Russian Journal of Electrochemistry 40(11): 1105-1114.
- 6. Haruta, M., Yamada, N., Kobayashi, T., et al. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. (1989) J Catal 115(2): 301-309.
- 7. Parsons, R., VanderNoot, T. The oxidation of small organic molecules: A survey of recent fuel cell related research. (1988) J Electroanalyt Chem 257(1-2): 9-45.
- 8. Burke, L.D., Nugent, P.F. Dichromate reduction on gold and platinum electrodes in aqueous acid solutions. (1997) Electrochimica Acta 42(3): 399-411.
- 9. Guerrini, E., Trasatti, S. Recent developments in understanding factors of electrocatalysis. (2006) Russian Journal of Electrochemistry 42(10): 1017-1025.
- 10. Melezhik, A.V., Sementsov, Y.I., Yanchenko, V.V. Synthesis of Fine Carbon Nanotubes on Coprecipitated Metal Oxide Catalysts. (2005) Russian Journal of Applied Chem 78(6): 917-923.
- 11. Danilov, M.O, Melezhik, A.V. Carbon nanostructures as hydrogen sorbent for anode of a chemical power cell. (2004) Russian Journal of Applied Chem 77(12): 1958-1961.
- 12. Veziroglu, T.N., Zaginaichenko, S.Y., Schur, D.V., et al. ICHMS2001, VII International conference “Hydrogen materials science and chemistry of metal hydrides”. (2001) Alushta, ADEF-Ukraine 338-341.
- 13. Antropov, L.I., Beknazarov, A. Theoretical Electrochemistry. Honolulu, University Press Pacific, 2001, Chap. 28.
- 14. Danilov, M.O., Kolbasov, G.Y., Melezhyk, A.V. Electrodes For Fuel Cells Based On Carbon Nanotubes And Catalysts. Carbon Nanomaterials in Clean Energy Hydrogen Systems. Editors: Baranowski, B. Zaginaichenko, S.Yu., Schur, D.V., et al. (2009) Springer 279-281.
- 15. Danilov, M.O., Kolbasov, G.Y., Melezhyk, A.V. Electrocatalytic properties of nanostructured compositions for alkaline fuel cells in Proceedings of the 8th ABA Brno. (2007) Advanced Batteries and Accumulators, Brno 131-135.
- 16. Danilov, M.O., Melezhyk, A.V. Carbon nanotubes modified with catalyst-promising materials for fuel cells. (2006) J. Power Sources 163(1): 376-381.
- 17. Danilov, M.O., Ivanova, N.D., Melezhyk, A.V., et al. Molybdenum oxide-carbon nanotubes nanocomposites for fuel cell oxygen electrode of the 9th ABA, Ju. Vondrak, Ed. Brno. (2008) Advanced Batteries and Accumulators, Czech Republic 83-85.
- 18. Danilov, M.O., Kolbasov, G.Ya. Electrochemical method for the preparation nanocomposites based on carbon nanotubes and chromium oxides for oxygen electrodes. (2010) J Solid State Electrochem 14(12): 2169-2172.
- 19. Yu, J.S., Lee, H., Lee, P.S., et al. Effect of Cu powder as an additive material on the properties of Zr-Based pasted alloy electrodes for Ni/MH batteries. (2000) J Electrochem Soc 147(7): 2494-2497.
- 20. Sakai, T., Ishikawa, H., Oguro, K. Effects of Microencapsulation of Hydrogen Storage Alloy on the Performances of Sealed Nickel/Metal Hydride Batteries. (1987) J Electrochem Soc 134(3): 558-562.