Localized Oral Mucous Membrane Pemphigoid: Successful Treatment with Intralesional Triamcinolone Acetonide Injection
Elif PEKER1, Güzin Neda HASANOGLU ERBASAR2*, Burcu SENGÜVEN3
Affiliation
- 1Gazi University, Faculty of Dentistry, Department of Oral and Maxillofacial Surgery, Ankara, Turkey
- 2Yıldırım Beyazıt University, Faculty of Dentistry, Department of Oral and Maxillofacial Surgery, Ankara, Turkey
- Gazi University, Faculty of Dentistry, Department of Oral Pathology, Ankara, Turkey
Corresponding Author
Guzin Neda Hasanoslu Erbasar, Dögol Caddesi. MEB Kampüsü H Blok 06560 Besevler-Ankara-Türkiye, Tel: 0090 555 526 67 51; Fax: 0090 312 2151930; E-mail: neda986@gmail.com
Citation
Hasanoglu Erbasar, N.G., et al. Localized Oral Mucous Membrane Pemphigoid: Successful Treatment with Intralesional Triamcinolone Acetonide Injection. (2016) J Dent Oral Care 2(5): 1- 5.
Copy rights
© 2016 Hasanoslu Erbasar, N.G. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Mucous membrane pemphigoid; Topical steroids; Triamcinolone acetonide; Intralesional injection
Abstract
Mucous membrane pemphigoid (MMP) is a rare, chronic and vesiculobullous disorder that classified as autoimmune disease.MMP can affect various mucous membranes but predominantly occurs in the oral cavity. In most of cases oral lesions may be initial manifestation or the only sign of the disease. It is predominantly seen in the fifth decade of life. It affects women more commonly than men. Children are rarely affected from this disease. MMP may cause extremely variable severity due to different clinical presentations as vesicles, erosions, pain, and occasional scarring. Oral lesions as gingival inflammation and scarring are not life theatening but lead to functional limitations. The primary goals of the treatment are to cease the progression of the disease, reduce the symptoms, and prevent the sequelae of tissue ulseration and consecutive scarring. The mild MMP lesions may be successfully managed with topical corticosteroids. However, the lesions that do not respond to local treatments or become widespread and also the diseases which show rapid progress can be treated can be treated with systemic glucocorticoids and immunosuppressive agents such as azathioprine, mycophenolatemofetyl, cyclosporine, and dapsone, rituximab and intravenous immunoglobulin. In this report two cases of oral MMP treated successfully with intralesional triamcinolone acetonide are presented along with a review of literature.
Introduction
Mucous membrane pemphigoid (MMP) is a rare, chronic and vesiculobullous disorder that classified as autoimmune disease. MMP affects frequently the mucosal surfaces of the mouth and also cutaneous involvement may be seen, approximately, one-quarter of patients. When it affects the gingiva, it is referred to as “desquamative gingivitis”. MMP is the most common group of autoimmune mucocutaneous diseases that affects the oral cavity[1-3].
It is predominantly seen in the fifth decade of life. It affects women more commonly than men. Children are less frequently affected. MMP is usually associated with numerous target antibodies in the basement membrane and and weaken the attachment to the connective tissue. The MMP is a heterogeneous clinical and immunologic entity that is reflected in pathogenetic mechanisms, clinical presentations and response to therapy[4-7].
MMP may cause extremely variable severity due to different clinical presentations as vesicles, erosions, pain, and occasional scarring. Oral lesions as gingival inflammation and scarring are not life theatening but lead to functional limitations. On the other hand, involvement of the conjunctiva as erosions with scarring and cicatrization may lead to blindness and the involvement of respiratory tract lesions in larynx, esophagus and lower airway may result in airway loss[1].
The diagnosis of MMP is mainly based on the patients history, clinical examination and biopsy with histologic or direct immunoflorescent examination that showing deposition of IgG, IgA, and C3 in the basal membrane zone (BMZ). When performing a biopsy, it should involve the vesicle and surrounding tissue not the erosion itself. Cytology smears are not indicated. Separation of the intact epithelium from the underlying connective tissue should be seen on biopsy specimen with an often chronic inflammation in the connective tissue[8].
In this study two cases of oral MMP with special emphasis on their clinical features and treatments are presented along with a review of literature.
Case Report
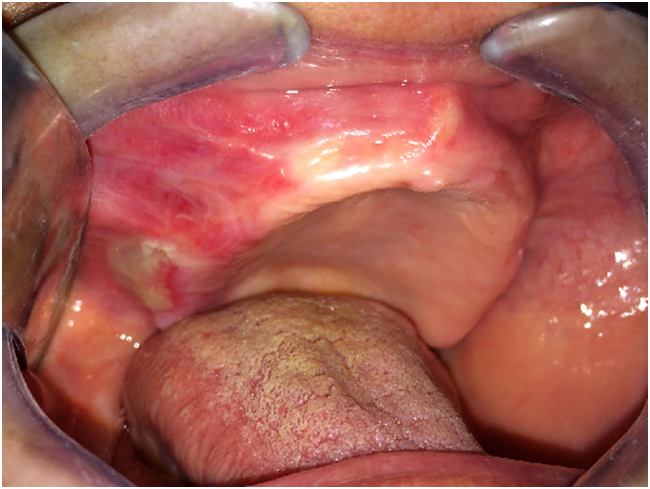
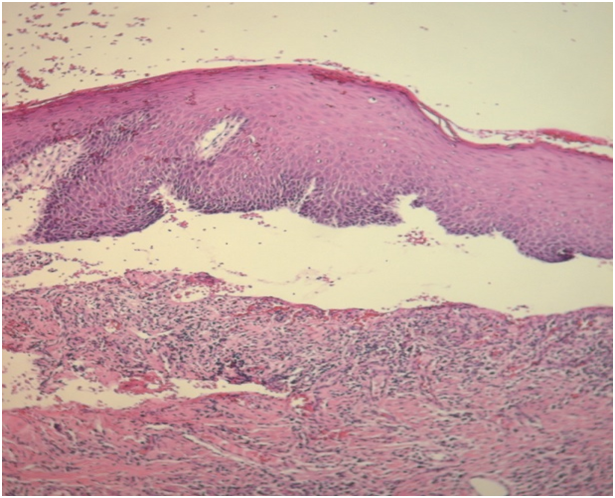
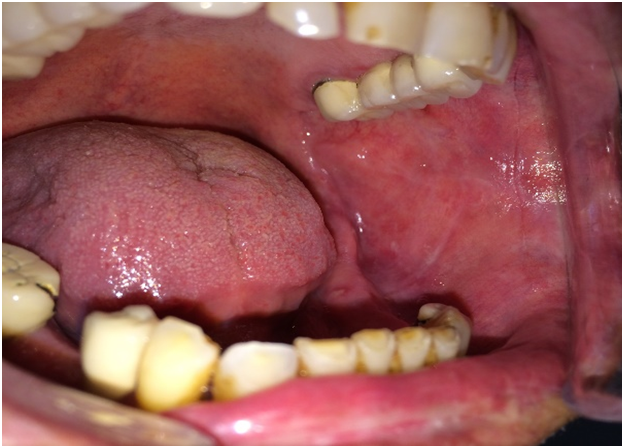
Case 1 was 64-year-old female patient was referred to the department of Oral and Maxillofacial Surgery with a complaint of burning sensation and pain of the right upper gums and palate for the past 6 months. She had no systemic disease or ongoing medical therapy at the referral time to our clinic. We revealed mild desquamation on the right posterior maxillar area (Figure 1). Gentle manipulation on the normal mucosa induced a positive Nikolsky’s sign. An incisional biopsy was performed on this area and intact gingiva was submitted for histopathological examination. Biopsy specimens from the oral mucosa revealed detachment of epithelial basement membrane and subepithelial lamina propria with slight chronic inflammation, suggestive of MMP (Figure 2).
Figure 1: Intraoral examination of the first patient.
Figure 2: Histopathology revealed subepithelial cleft and basal cell degeneration, along with slight chronic inflammation. (H&E,x100)
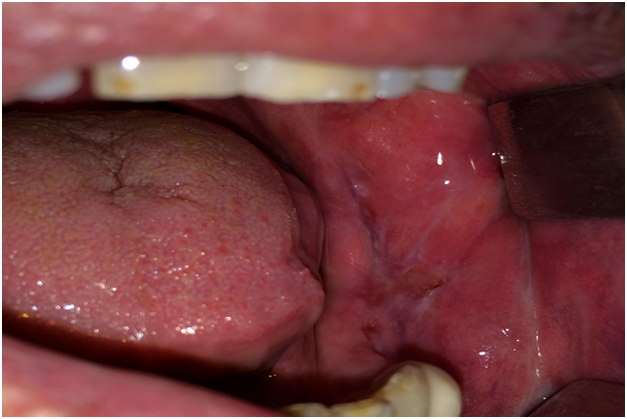
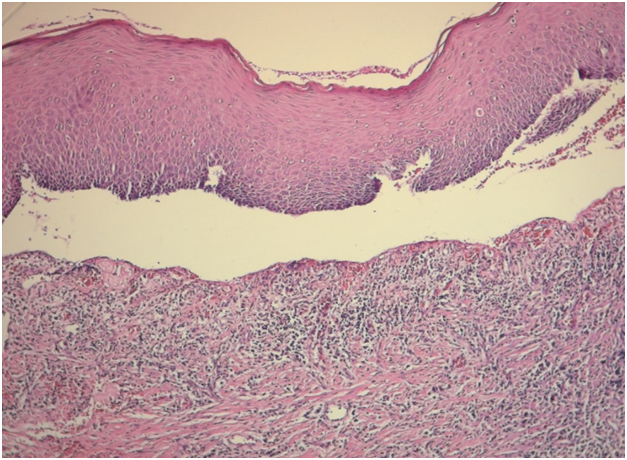
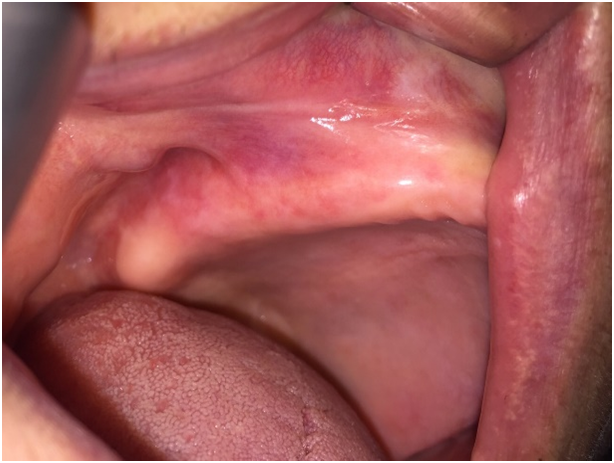
Case 2 was a 61-year-old man who was referred to our department with a chief complaint of pain and sensitivity of the mouth for 4 months which increases with intake of spicy food. The medical history was not significant and he was otherwise in good health. Oral examination revealed mild desquamation of the buccal mucosa (Figure 3). Also gentle pressure of the normal mucosa induced a positive Nikolsky’s sign. An incisional biopsy was performed on this area and submitted for histopathological examination. Biopsy specimens from the oral mucosa revealed partial junctional separation at the level of the basement membrane, suggestive of MMP (Figure 4).
Figure 3: Intraoral examination of the second patient.
Figure 4: Histopathology showed the presence of stratified squamous epithelium supported by connective tissue stroma and partial junctional separation at the level of the basement membrane. (H&E,x200)
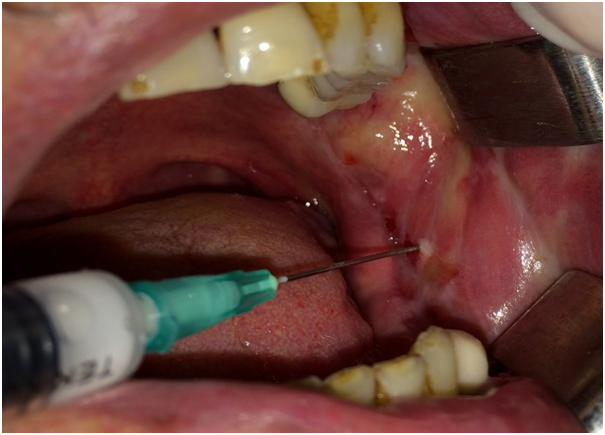
Both of the patients had an ophthalmologic and dermatological examination and no associated ocular, cutaneous or genital lesions were reported. Patients were initially treated with topical 0.1% triamcinolone acetonoide per 3 - 4 times a day over a period of 4 weeks. The first patient showed no response and the second patient showed poor response to topical steroids; for this reason they were were treated with intralesional triamcinolone acetonoide injections (Figure 5). In the first case, complete healing without scar was obtained after 6 sessions. Whereas complete resolution without scar formation was observed after 3 sessions in the second case. At the end of the 2-year follow-up period both patients remained disease free (Figure 6 and 7).
Figure 5: Intralesional injection of triamcinolone acetonide in the second case.
Figure 6: Intraoral examination of the firstpatient at the end of the 2-year follow-up.
Figure 7: Intraoral examination of the second patient at the end of the 2-year follow-up.
Discussion
MMP is a rare chronic autoimmune blistering disease that identified by deposition of IgA and/or IgG directed against proteins of the basement membrane complex. The activation of complement system by immunoglobulins attracts the leukocytes. Mainly neutrophils cause the release of proteolytic enzymes and subsequently cleave fibrils in the basement membrane that eventuate in blister formation[2,6]. In the literature MMP has been termed by several names including cicatricial pemphigoid, benign mucous membrane pemphigoid, oral pemphigoid, and ocular cicatricial pemphigoid. In 2002,Chan[1] suggested the term of mucous membrane pemphigoid; because some serious complications, for instance ocular involvement may cause blindness or laryngeal scarring may result death[9], make the term “bening” inappropriate and the disease does not always induce scarring, as in example of gingival involvement, the term of cicatricial does not involve all cases. Lastly site-spesific terms exclude the patients with multiple mucous membranes involvement[1,2].
MMP can affect various mucous membranes but predominantly occurs in the oral cavity. In most of cases oral lesions may be initial manifestation or the only sign of the disease[2,3,7].
In the oral cavity MMP can be also manifested as desquamative gingivitis which is characterized by broad desquamation and/or erosion of the buccal side of attached gingival of anterior teeth. Additionally gentle pressure on the normal mucosa may induce a positive Nikolsky’s sign[2] which was also present in our both cases.
Patients with MMP usually complain about pain, dysphagia, or peeling of the mucosa which adversly impact their quality of life[10]. Likewise both of our patients had complained of pain and discomfort that were especially increased during intake of spicy foods.
But most critical complication of MMP is scarring which is spesifically poses important problem when the conjunctiva, esophagus, or larynx get involved[7].
The primary goals of the treatment are to cease the progression of the disease, reduce the symptoms, and prevent the sequelae of tissue ulseration and consecutive scarring. The management of oral MMP can be challenging and differs based on the severity of disease[6].
In general there is no approved management protocol for the oral MMP. Several treatments methods have been used but probably due to the rarity of the disease large-scale clinical drug trials could not been conducted[7]. The mild lesions may be successfully managed with topical corticosteroids.Right along with fluocinolone acetonide or clobetasol proprionate, triamcinolone acetonide is the third indicated topical steroid for MMP but the form of ointment or aqueous solution is not sufficient to control the disorder. This synthetic glucocorticoid is more effective when interlesionally injection is desired hereby high concentration of the drug locally delivered with no systemic side effects[3]. MMP generally occurs in elderly people more than 50 years of age[7] which is similar to the presented patients. This age group can be highly associated with multiple medical problems that pose an obstacle for the systemic therapy. For that reason perilesional/intralesional triamcinolone injections may be an efficient choice between local and systemic therapy[3]. While our patients’ lesions not respond to the topical corticosteroids; intra lesional triamcinolone injections was preferred as the second line of therapy. Kalinska et al.[3] reported four patients with oral MMP who was successfully treated with multiple sessions (varied between 3 to 18) of perilesional and/intralesional triamcinolone injections.
However, lesions that not respond to local treatments or become widespread and also the diseases which show rapid progress can be treated with systemic glucocorticoids and immunosuppressive agents such as azathioprine, mycophenolate mofetyl, cyclosporine[6,11].
Dapsone is a well-known drug that has been used in the treatment of the infectious diseases including leprosy and malaria for many years. But more recently it has become a worthwhile therapeutic agent in inflammatory diseases and found effecient in the adjunct therapy in MMP[7]. In the eigthies Rogers et al.[12,13] and Matthews et al.[14] reported successful treatments of oral MMP with dapsone. In 1999 Ciarrocca and Greenberg[7] used dapsone in combination with topical corticosteroids which caused great improvement in patients with oral MMP.Al-Shehhi et al.[15] used dapsone (100 mg/day) combined with topical 0.05% clobetasol propionate cream for localized oral MMP. But due to insufficient improvent of the lesion after 6 months; they swithced the therapeutic regimen to topical tacrolimus (%1 solution) with oral prednisolone and after one month the lesion healed completely.
Recently there is an emerging trend to use rituximab (RTX) in patients with MMP in whom conventional therapy was failed. Although in the literature the majority of patients showed improvement after the use of RTX neither long-term follow-up was present nor optimal protcols were defined for specific subgroups of MMP. Besides, in most cases patients received concomitant immunosuppressive and anti-inflammatory therapy[16-18] which can cause serious adverse events including infections and death[19].
Moreover intravenous immunoglobulin(IVIg) has been demonstrated as an effective adjuvant treatment for MMP. Yeh et al.[20] reported a significant decrease in the autoantibody titers which followed by clinical improvement in 13 patients with extensive MMP that showed no previous response to immunosuppressive therapy or had substantial adverse effects.
Also Letko et al.[21] stated IVIg therapy induced faster control of the acute inflammation by comparison with conventional immunosuppressive therapy in patients with MMP and no recurrences were recorded in IVIg therapy during the follow-up period. However, Iaccheri et al reported IVIg with systemic steroids and immunosuppressive agents failed to control ocular MMP[22]. A tetracycline type antibiotic, minocycline, alone or in combined with nicotinamide has also been reported in the treatment of MMP[23,24]. Although it has effective anti-inflammatory and immunesuppressive properties and shown as a safe alternative for MMP patients; several side effects including vertigo and gastralgia may cause withdrawal of the drug[6,25].
Recently Carrozzo et al.[25] evaluated the therapeutic benefit of minocycline in patients with oral MMP. They reported a major response in 3 patients, a minor response in 4 and no response in 2 patients but also one patient showed permanent remission. More than half of the patients (55%) had to stop the drug because of side effects. Authors concluded that minocycline revealed temporary clinical benefits in oral MMP and patients without circulating autoantibodies against BP180 showed a better clinical response.
When clinical remission is achieved, medications should be slowly tapered to reduce the risk of disease flares. In case that discontinuance of the treatment is not achievable due to relapses, the least effective topical or systemic maintenance regimens should be implemented[6,26].
In addition to pharmacological interventions, proper management of MMP requiring the multidisciplinary approach. Initially patient should be evaluated meticulously by an ophthalmologist whether the conjunctival involvement is present. Also based upon the suspected or known involvement sites clinical co-management between dentist and different physicians such as dermatologists, otolaryngologists, urologists, internists, gastroenterologists may be needed[6,27].
Conclusion
Perilesional injection with triamcinolone can be an efficient and safe choice in patients with oral MMP that showed no improvement with topical corticosteroids. It should be borne in mind that perilesional injection with steroids indicated especially in mild erosive lesions. The management of MMP should be individualized dependent on the severity, extent and progression of the disease. Also clinicians should be in contact with experienced physician in MMP for possible other mucosal involvements. Further clinical trials including different therapeutic regimens with longer follow up is needed.
Conflict of interest:None.
References
- 1. Chan, L.S., Ahmed, A.R., Anhalt, G.J., et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. (2002) Archives of dermatology 138(3): 370-379.
- 2. Hasan, S., Kapoor, B., Siddiqui, A., et al. Mucous membrane pemphigoid with exclusive gingival involvement: Report of a case and review of literature. (2012) J Orofacial Sciences 4(1): 64-69.
- 3. Kalinska-Bienias, A., Kalowska, M., Kwiek, B., et al. Efficacy and safety of perilesional/intralesional triamcinolone injections in oral mucous membrane pemphigoid. (2016) Br J Dermatol 174(2): 436-438.
- 4. Kirtschig, G., Murrell, D., Wojnarowska, F., et al. Interventions for mucous membrane pemphigoid and epidermolysisbullosaacquisita. (2003) Cochrane Database Syst Rev 1: CD004056.
- 5. Lazarova, Z., Yancey, K.B. Cicatricial pemphigoid: immunopathogenesis and treatment. (2002) Dermatologic Therapy 15(4): 382-388.
- 6. Xu, H.H., Werth, V.P., Parisi, E., et al. Mucous membrane pemphigoid. (2013) Dent Clin North Am 57(4): 611-630.
- 7. Ciarrocca, K.N., Greenberg, M.S. A retrospective study of the management of oral mucous membrane pemphigoid with dapsone. (1999) Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 88(2): 159-163.
- 8. Darling, M.R., Daley, T. Blistering mucocutaneous diseases of the oral mucosa--a review: part 1. (2005) J Can Dent Assoc 71(11): 851-854.
- 9. Boedeker, C.C., Termeer, C.C., Staats, R., et al. Cicatricial pemphigoid in the upper aerodigestive tract: diagnosis and management in severe laryngeal stenosis. (2003) Ann Otol Rhinol Laryngol 112(3): 271-275.
- 10. Scully, C., Lo Muzio, L. Oral mucosal diseases: mucous membrane pemphigoid. (2008) Br J Oral Maxillofac Surg 46(5): 358-66.
- 11. Radulescu, M. The Pharmacologic Management of Common Lesions of the Oral Cavity. (2016) Dent Clin North Am 60(2): 407-420.
- 12. Rogers, R.S., Seehafer, J.R., Perry, H.O. Treatment of cicatricial (benign mucous membrane) pemphigoid with dapsone. (1982) J Am Acad Dermatol 6(2): 215-223.
- 13. Rogers, R.S., Mehregan, D.A. Dapsone therapy of cicatricial pemphigoid. (1988) Semin Dermatol 7(3): 201-205.
- 14. Matthews, R.W., Pinkney, R.C., Scully, C. The management of intransigent desquamative gingivitis with Dapsone. (1989) Ann Dent 48(1): 41-43.
- 15. Al-Shehhi, F., Balakirski, G., Baratli, J., et al. Localized oral mucous membrane pemphigoid: successful topical treatment with 1% tacrolimus solution as steroid-sparing therapy. (2016) J Eur Acad Dermatol Venereol.
- 16. Le Roux-Villet, C., Prost-Squarcioni, C., Alexandre, M., et al. Rituximab for patients with refractory mucous membrane pemphigoid. (2011) Arch Dermatol 147(7): 843-849.
- 17. Kasperkiewicz, M., Shimanovich, I., Ludwig, R.J., et al. Rituximab for treatment-refractory pemphigus and pemphigoid: a case series of 17 patients. (2011) J Am Acad Dermatol 65(3): 552-558.
- 18. Taverna, J.A., Lerner, A., Bhawan, J., et al. Successful adjuvant treatment of recalcitrant mucous membrane pemphigoid with anti-CD20 antibody rituximab. (2007) J Drugs Dermatol 6(7): 731-732.
- 19. Gea-Banacloche, J.C., Opal, S.M., Jorgensen, J., et al. Sepsis associated with immunosuppressive medications: an evidence-based review. (2004) Critical care medicine 32(11): S578-S590.
- 20. Yeh, S.W., Usman, A.Q., Ahmed, A.R. Profile of autoantibody to basement membrane zone proteins in patients with mucous membrane pemphigoid: long-term follow up and influence of therapy. (2004) Clin Immunol 112(3): 268-272.
- 21. Letko, E., Miserocchi, E., Daoud, Y.J., et al. A nonrandomized comparison of the clinical outcome of ocular involvement in patients with mucous membrane (cicatricial) pemphigoid between conventional immunosuppressive and intravenous immunoglobulin therapies. (2004) Clin Immunol 111(3): 303-310.
- 22. Iaccheri, B., Roque, M., Fiore, T., et al. Ocular cicatricialpemphigoid, keratomycosis, and intravenous immunoglobulin therapy. (2004) Cornea 23(8): 819-822.
- 23. Reiche, L., Wojnarowska, F., Mallon, E. Combination therapy with nicotinamide and tetracyclines for cicatricialpemphigoid: further support for its efficacy. (1998) Clin Exp Dermatol 23(6): 254-257.
- 24. Sakamoto, K., Mori, K., Hashimoto, T.,et al. Antiepiligrin cicatricial pemphigoid of the larynx successfully treated with a combination of tetracycline and niacinamide. (2002) Arch Otolaryngol Head Neck Surg 128(12): 1420-1423.
- 25. Carrozzo, M., Arduino, P., Bertolusso, G., et al. Systemic minocycline as a therapeutic option in predominantly oral mucous membrane pemphigoid: a cautionary report. (2009) Int J Oral Maxillofac Surg 38(10): 1071-1076.
- 26. Knudson, R.M., Kalaaji, A.N., Bruce, A.J. The management of mucous membrane pemphigoid and pemphigus. (2010) Dermatol Ther 23(3): 268-280.
- 27. Alkan, A., Gunhan, O., Alkan, A., et al. A clinical study of oral mucous membrane pemphigoid. (2003) J Int Med Res 31(4): 340-344.