Long Term Follow up of Patients with Advanced Cancers after Chemotherapy and Traditional Medicine (82 Cases)
Affiliation
The Institute of Oncology, Tehran University of Medical Science, Tehran
Corresponding Author
George Zhu, The Institute of Oncology, Tehran University of Medical Science, Tehran, E-mail: sansan4240732@163.com
Citation
Zhu, G. Long Term Follow up of Patients with Advanced Cancers after Chemotherapy and Traditional Medicine (82 Cases). (2018) J Clin trials Pathol Case Stud 3(1): 13- 21.
Copy rights
© 2018 Zhu, G. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Cancer chemotherapy;Traditional medicine;Target therapy
Abstract
Traditional systems of medicine all over the world even traditional medicine and cancer have been using plants and plants products for therapeutic intention. The purpose of these retrospective trials is to evaluate the clinical efficacy of chemotherapy in conjunction with traditional medicine (TCM) for a broad variety of cancers.
Methods: 82 patients with available cancers were concluded in the study during September 1993 - May 2018. The sex ratio of male: female was 55:27 respectively. The mean age at onset was 46.4 years (range 10 - 79 years). All patients were treated with different dosage of various chemotherapy in combination with TCM or traditional medicine alone. The basic chemotherapeutic regimen consisted of vincristine (VCR, 1 - 2 mg / week), cyclophosphamide (CTX, 200 - 1,000 mg / week), mitomycin C(MMC, 2 - 4 mg / week) and 5-fluorouracil (5-FU, 250 - 500 mg / day). In addition, according to patient condition, the additional drug doxorubicin (ADM, 20 mg / week) in lymphoma and metastatic breast cancer, demethylcantharidin in liver cancer and cisplatin (DDP) or interleukin-2(PHA) or gefitinib in lung cancer. The detail prescription of TCM varied among a broad variety of carcinomas (see full text case reports). The criteria of complete remission(CR) and/or partial remission(PR)is according to the rules where physicians have in common with in clinics.
Results: In 82 cases, the CR was obtained in 37 (45.1%) advanced cancers, a short CR in 13 (15.9%) cases, PR in 26 (31.7%) cancers, Stable disease in 6 cases. In differential types of 20 patients over ten years, lymphoma occupied 8 cases (40%). As to approach to the schedule of drug administration, 11 lymphoma obtained CR via CVP or COMF/ COMA (CTX, VCR, MMC, ADM) regimen and TCM or antibiotics and immunotherapy. Five advanced gastric cancer were successfully treated using MFC and cinobufacini / cantharidin, and TCM. In follow up, one HCC accompanied with colon polyps obtained CR via hepatectomy and targeting oncogenic receptor tyrosine kinase inhibitor sorafenib. Among two lung cancers, one female with metastatic lung cancer was given targeting oncogenic receptor EGFR gefitinib therapy after the combination chemotherapy, which was stable disease for 8+ months. CR can also be achieved in one advanced cholangiocarcinoma and one advanced gallbladder cancer through major protocol of TCM and the addition of small dosage of chemotherapy. Two patients the breast has inflamed redness, swelling and an enlarged and firm mass palpable. Cures were obtained through the primary use of traditional medicine with antibiotics regimen. Traditional herbs consisted of taraxacum, honeysuckle, asparagus, cremastra appendiculata, coix lacryma, cordate houttuynia, scutellaria barbate d don, and oldenlandia diffusa roxb. Among those long-term survivors, 34 carcinomas obtained in disease-free survival over 5years, 22 cancers were survival over 10 years, the longest four patients over 25 years.
Conclusion: In this study, I experienced that a CR was a pivotal influencing factor in those longest survival patients, and traditional medicine was also recommended. Down regulating oncogenic receptors may be useful paradigm and perspective in currently the third line setting of clinical target therapy and in rendering our better understanding of cancer biology.
Introduction
Chemotherapy is a major skillful of cancer therapy. one of the most important advances in oncology has been increased acceptance of evidence that most patients with disseminated tumors were setted to the protocol of chemotherapy in conjunction with recent targeting oncogenic receptor[1-20], traditional medicine(TCM) and / or adoptive immunotherapy (LAK cells, TIL therapy)[21,22]. The experience in Ugandan children with Hodgkin’s disease has been excellent[23] and in a study of 14 adults with stage I and II Hodgkin’s disease, mostly clinically staged, 13 patients (93%) achieved CR with combination chemotherapy and all were in CR 11 to 94 months after the completion of treatment[24]. Another, a disease-free survivors of 5 years (56.5 - 59.3% versus 22 - 24.3%) and 10 years (48.9%) was remarkedly higher rate in those breast cancers with stage III following surgery plus chemotherapy than only surgery. More promising, in large trials of 48 HER2 - positive early breast cancer patients, targeting the adjuvant trastuzumab treatment demonstrated highly favorable outcome. Five year overall survival rates and disease-free survival rates were 95.8% and 93.8% respectively[25]. Recently, neratinib was recently approved by FDA for extended adjuvant treatment of ER+ / HER2+ breast cancer[26]. Others advanced or metastatic gastric cancer constitutes the majority of patients in clinical practice. Systemic chemotherapies and combined regimens are currently available, provides palliation and prolongs survival. In particular, high-quality clinical trials on TCM in cancer are generally lacking, except for Kampo medication for Japanese cancer patients[27,28], arsenic trioxide (As2O3) in the role of acute promyelocytic leukemia (Zhang TD,1973;Sun HD, 1992; Zhang P, 1996)[29], and cantharidin in treatment of liver cancer [Yang BY, 1970s;[30]. This paper will attempt to place in proper interpretative review from those patients with cancers under remission in this group.
Materials and Methods
82 patients with available cancers were concluded in the study during September 1993- May 2018. The sex ratio of male: female was 55: 27 respectively. The mean age at onset was 46.4 years ranging from 10 to 79 years. Among age distribution, although there is some uncertain about the type distribution of cancers, it was found 38.1 years as the mean age at onset for lymphoma; 44.0 years for liver cancer, while higher mean age at 60.1 years has been shown in lung cancer in this group. The clinical diagnoses in a broad variety of carcinomas consisted of metastatic nasopharyngeal cancer 5 cases, metastatic breast cancer 4, inflammatory breast tumor 2, lung tumors 12, hepatocellular carcinoma (HCC) 12, stomach cancer 5, hematological malignancies 27 cases (acute leukemias FAB M1 type 2, M2 type 1, acute promyelocytic leukemia 1, chronic myeloid leukemia CML 2, chronic lymphocytic leukemia CLL 1, refractory anemia 1, multiple myeloma 2, lymphoma 16), thyroid cancer 2, maxillary sinus carcinoma 1, carcinoma of mandibular sinus 2, laryngeal carcinoma 1, esophagus cancer 1, gallbladder cancer 1, cholangiocarcinoma 1, metastatic oral cancer 1, epidermoid carcinoma 1,metastatic melanoma 1, replapsed vulvar cancer 1, and other metastatic sternal and spinal (T12) tumor 1, myxoid chondrosarcoma 1 (figure 1) respectively. all other benign neoplasias were not statistically included. The basic chemotherapeutic regimen consisted of vincristine(VCR, 1-2 mg / wk), cyclophosphamide (CTX, 200 - 1,000 mg / wk), mitomycin C (MMC, 2 - 4 mg / wk) and 5-fluorouracil (5-FU, 250 - 500 mg / day). In addition, the additional drug adriamycin (ADM, 20 mg / wk) in lymphoma and metastatic breast cancer, demethylcantharidin in liver cancer and cisplatin (DDP) or interleukin-2 (PHA) /gefitinib in lung cancer. The detail prescription of TCM varied among a broad variety of carcinomas (see full text case reports).The criteria of complete remission (CR) and / or partial remission (PR) is according to the rules where physicians have in common with in clinics. Complete remission (CR): there was no more tumor or tumor complete regressed in patients for at least 1 month; Parttial remission: the tumor decreased by more than 50% in patients for at least 1 month; Stable disease: the tumor decreased by less than 50% or increased by no more than 25% in patients; Disease progression: the tumor increased by more than 25% in patients, or new lesions emerged. The efficacy was evaluated according to the survival time from the day when patients were at onset. The clinical data for liver cancer[31,32] and lung cancer[33,34] were previously described.
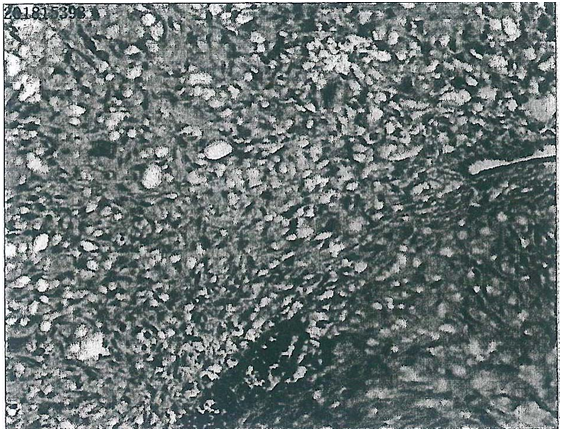
Figure 1: A patient with myxoid chondrosarcoma. A biopsy specimen stain: CK - S-100+ EMA - Ki-67 ~50 %
Results
In 82 cancers, the rate of complete remission(CR) was achieved in 37 (45.1%) advanced cancers. All CR patients with advanced cancers was survival over 5 years, 18 cancers was survival 10 years. Another, a short CR was obtained in 13 (15.9%) advanced cancers, the survival time varied from 20 months to 4 years. PR was obtained in 26 (31.7%) patients with a broad variety of carcinoma, while three patients (1 malignant lymphoma,1 carcinoma of mandibular sinus, 1 metastatic tumor of bone) had survival 12, 18+ and 11+ years respectively, implicating a longer survivor in patients the survival with tumours. Otherwise, stable disease was 6 cases. Basic characteristics of studied population were summarized in table 1.
Table 1: Patient’s characteristics.
| Cancer types | Cases No | Sex | Mean ages (years) | Treatment Protocol |
Response following therapy |
Duration of remission (years) < 1 1-3 > 3-5 >5-10 >10 |
|---|---|---|---|---|---|---|
| lymphoma | 17 | M14, F2 | 38.1 (13 - 66) |
CVP or COMA(11)*, Radiotherapy**(1), prednisone(1), Immunotherapy(4) |
CR(10)*, short CR(2), PR(5) |
1 3 2 1 9 |
| HCC | 12 | M10, F2 | 44.0 (26 - 63) |
a. 5-Fu (250-1,000 mg/day), VCR, CTX, MMC, TCM b. Cantharidin, TCM c. hepatectomy, sorafenib |
CR(8), Short CR(2), PR(1), stable disease(1) |
1 4 1 3 3 |
| Lung tumors | 12 | M8,F4 | 60.1 (40 - 79) |
a. COMF, TCM; b. DDP, etoposide, gefitinib c. CTX, 5-FU, antitumor capsule; d. TCM alone |
CR(2), short CR(2), PR(3), stable disease(5) |
4 3 3 1 |
| NPC | 5 | M3, F2 | 51.4 (38 - 75) |
a. VCMF,TCM b. CTX, 5-FU, TCM |
CR(1), short CR(1), PR(3) |
1 3 1 |
| MBC | 4 | F4 | 31.3 (25 - 41) |
a. COMF, TCM b. COP, TCM |
CR(3), PR(1) | 1 2 |
| IBT | 2 | F2 | 31,35 | Antibiotics, TCM | CR(2) | 2 |
| Stomach cancer | 5 | M2, F3 | 42.3 (35 - 50) |
a. MFC, TCM; b. CTX, 5-FU, antitumor capsule |
CR(1), short CR(2), PR(2) |
1 3 1 |
| AML | 3 | M2, F1 | 4, 18, 20 | DA**, HA, TCM | PR(3) | 3 |
| APL | 1 | M | 31 | ATRA 80 mg/day; H 1 mg x 5 days; TCM |
CR | 1 died of relapsed APL |
| CML | 2 | M1, F1 | 33, 62 | Busulfan, TCM | CR(1), short CR(1) | 1 1 |
| CLL | 1 | M | 58 | Chlorambucil, TCM | CR | 1 died of stomach cancer |
| RA | 1 | M | 43 | Vitamin B12, folate acid, TCM | CR | 1 |
| MM | 2 | M1, F1 | 60, 63 | Thalidomide, pred, TCM | Short CR(1), PR(1) | 1 1 |
| Epidermoid cancer | 1 | F | 72 | 5 % FU of retinoic acid ointment |
PR | 1 |
| Thyroid cancer | 2 | F2 | 54, 60 | TCM alone | CR(2) | 1 1 |
| bile cancer | 1 | F | 65 | CTX, 5-FU, TCM | CR | 1 |
| Cholangio -carcinoma | 1 | M | 72 | MFC (MMC, 5-FU, CTX), TCM | CR | 1 died of intestinal cancer |
| Others*** | 10 | M8,F2 | 57.5 (44 - 69) |
COFP, COMMB, TCM |
CR(2), short CR(2), PR(6) |
3 2 1 3 |
Note: HCC: hepatocellular carcinoma; NPC: metastatic nasopharyngeal cancer; MBC: metastatic breast cancer; IBT: inflammatory breast tumor; AML: acute myeloid leukemia; APL: acute promyelocytic leukemia; CML: chronic myeloid leukemia; CLL: chronic lymphocytic leukemia; RA: refractory anemia; MM: multiple myeloma; COMA: CTX,VCR,MMC,ADM; COMF:CTX,VCR,MMC/ADM,5-FU; VCMF:VCR,CTX,MMC/DDP; 5-FU; COP:CTX,VCR,pred; COFP:CTX,VCR,5-FU,PHA, Pred; DA:DNR,45mg/m2, Ara-c 100mg/m2; HA: homoharringtone 1mg x 5days, ara-c 50 mg, intramuscle, twice a day; MFC:MMC,5-FU, Ara-c / H,CTX; COMMB: CTX,VCR, MMC, MTX, Bleomycin; ATRA: all-trans retinoic acid; Pred: prednisone; TCM: traditional medicine; M:male; F: female: *cases number; **:treatment in another hospital; ***include oral cancer 1, metastatic melanoma 1, relapsed vulva cancer 1, laryngeal cancer 1, esophagus cancer 1, maxillary sinus carcinoma 1, carcinoma of mandibular sinus 2, and metastatic bone tumor 2.
During the schedule of drug administration, all patients were treated with the different dosage of 1 to 4 courses of various combination chemotherapy in conjunction with traditional medicine. In statistically analysis, one patient with nasopharyngeal cancer, the diplopia and unable version in his eye were recovered to “normal” visual acuity following the combination chemotherapy of VCMF(VCR, CTX, MMC and 5-FU) plus traditional medicine. A patient with rodent ulcer (8 x 5 cm) once obtained complete response as to an approach of 5% Fu of retinoic acid ointment. A short CR was achieved by the protocol of MFC(MMC; 5-Fu; Ara-C / homoharringtonine, CTX) plus cantharidin or cinobufacini drug in 5 advanced gastric cancers. One of them was a long-term survivor for 6 years via mass incision and the combination of MFC with herbs Scutellaria barbata d.don.
In view of cancer types, 10 lymphoma was setted to the major protocol of the combination conventional chemotherapy (COMF, CTX, VCR, MMC, 5-FU; COMA, CTX, VCR, MMC or ADM) in conjunction with traditional medicine which to relieve the chemotherapeutic toxicity, and reinforced the efficacy of chemotherapy. One lymphoma was regressed only by prednisone (200 #). Another 4 patients with thumb lymphadenopathy was treated by the use of antibiotics regimen in full dose with anti-inflammatory herbal tablets or immunotherapy lymphocyte transfer factor. In 12 HCC, 6 HCC were treated mainly by 5- FU (500 - 1000 mg / day) and TCM. 2 patients obtained CR through cantharidin and traditional medicine. The main protocol of TCM with adjuvant antibiotics regimen and low dose of dexamethasone was given in a primary liver cancer (AFP+, ascites +++, jaundice +++, liver tumor 3.2 x 3.0 cm). One acute promyelocytic leukemia complicated with metastatic liver cancer (7 x 4.5 cm) was in CR with all-trans retinoic acid (ATRA) and TCM. The detail prescription of TCM was mentioned before[25,26]. In the follow up, one HCC accompanied with colon polyps obtained complete remission via hapatectomy and targeting oncogenic receptor tyrosine kinase inhibitor sorafenib. Dose intensity has proven to be critical in maximizing chemotherapeutic efficacy for numerous human cancers. Eight other patients with cancers were in remission through small dosage of chemotherapy and TCM or traditional medicine (TCM) alone. There were 4 lung cancers, 1 gallbladder cancer, 1 cholangiocarcinoma and 2 thyroid cancers. Among targeting two metastatic lung cancers, one female with lung cancer was given the combination chemotherapy plus targeting oncogenic receptor EGFRvIII gefitinib, which was stable disease for 8+ months. Thyroid cancer was placed on the primary use of traditional medicine. The crude herbs consisted of sargassum, tangle, Oyster (mussels), Poriacocos, Ophiopogonjaponicus, Prunella vulgaris, Taraxacum, Scrophularia ningpoensis, Cremastra appendiculata, Trichosanthes Kirilowii, Sophora subprostrata, Houttuynia cordata, Scutellaria barbata d. don and Oldenlandia diffusa roxb.
A novel approach to the study of traditional medicine antitumor capsule was treated in 8 patients with advanced cancers. The initial results of antitumor capsule is an effective benefits to cancer treatment especially brain tumors through down regulation of inflammation, attenuated pain symptom and blocking convulsion. EGFR+++ was found in one glioma. This adjuvant agent is undergoing to clinical trials.
The survival times in those patients with remission were less than 1 years 10 cases, 1 to 3 years 22 cases, over 3 to 5 years 12 cases, over 5 to 10 years 12 cases, over 10 to 20 years 15 cases, and over 20 years 7 cases. In differential types of 20 patients with over 10 year’s survivors, lymphoma occupied 8 cases (40%). Among 7 patients with over 20 years, lymphoma occupied 3 cases, metastatic breast cancer 2 cases and hepatocellular carcinoma 2 cases.
Discussion
In this study, a series of the long follow up of patients with cancers were reported. I experienced that a CR was a pivotal influencing factor in those longest survival patients, and traditional medicine was also recommended.
The traditional combination chemotherapy program for lymphomas of favorable histologic type has been CVP (CTX, VCR, Pred) given at 21-days intervals[35]. Cyclophosphamide, vincristine, procarbazine, and prednisone (COPP) were used in the NCI study for patients with nodular mixed and nodular histocytic lymphomas[36]. More intensive CVP programs with the addition of adriamycin or bleomycin, or both, known as BACOP or CHOP- bleo, resulted in overall complete remission rates for patients with diffuse lymphomas ranging from 48% to 89%[37-40]. The NCI program of this 5-drug program, complete remission rates with this approaches has ranged from 48% to 94%[39]. In this study, the CR rates was 63% in 16 lymphomas, 6 CR were used by CVP or COMA regimens.
The use of chemotherapy to treat stomach cancer has no firmly established standard of care[41]. Some drugs used in stomach cancer treatment have included:5- FU (fluorouracil), doxorubicin (adriamycin), mitomycin C and most recently oxaliplatin, irinotecan in various combination. The relative benefits of these different drugs, alone or in combination,are unclear[42]. There are evidence supporting that clinical researches are exploring the benefits of giving chemotherapy as adjuvant therapy for surgery to destroy remaining cancer cells[43]. In recent analyses of definitive surgery followed by adjuvant radio chemotherapy (5-FU / leucovorin LV regimens) for patients with gastric cancer, Liu and Ahmed[44] reported that 59.3% (48 / 81) patients survived > 3 years,18.5% (15 / 81) patients survived 5 or more years. Eighteen out of 81 (22.2%) patients are still alive with a medium survival of 142 months (57 - 196 months). In this study, 5 patients with gastric cancer obtained a short CR through MFC regimen plus cinobufacini and cantharidin drugs. One relapsed gastric cancer survived over 6 years after surgery and adjuvant chemotherapy. More recent, treatment with HER2 inhibitor, trastuzumab, has been demonstrated to improve overall survival in inoperable locally advanced or metastatic gastric carcinoma overexpressing the HER2[43]. Oncogenic receptor HER2[45] is over expressed in 13 - 22% of patients with gastric cancer[46,47]. Tanz and colleagues[48] reported two HER2 - positive metastatic gastric adenocarcinoma who favorably responded to second line chemotherapy (FOLFIRI, irinotecan plus 5-Fu) with trastuzumab continuation following progressive disease to first line treatment containing trastuzumab, implicating trastuzumab continuation in metastatic HER positive gastric cancer is safe, practical and improve survival.
Oncogenic EGFR mutations are found in 10% to 35% of lung adenocarcinomas, with predominants in a subset of patients with non-small cell lung cancer (NSCLC[49-55]. These mutations, which commonly occur as either small in-frame deletions in exon 19 or point mutations T790M or L858R in exon 21 within the EGFR tyrosine kinase domain, confer constitutive activity and sensitivity to EGFR tyrosine kinase inhibitor (TKI[55,56]. Konduri and colleagues[57] reported five patients with metastatic lung cancer whose tumors harbored EGFR fusion, most commonly RAD5, are recurrent in lung cancer. Four of whom were treated with EGFR TKI erlotinib with documented antitumor response for 5, 6, 8, and 20 months respectively. An early EGFR TKI trial randomized patients with EGFR mutation positive stage IIIb or IV adenocarcinoma to treatment with afatinib or gemcitabine and cisplatin, treatment with afatinib prolonged progression free survival to 11.0 months as opposed to 5.6 months with gemcitabine and cisplatin[7]. In a total of 65 lung cancers with EGFR- mut (exon19 del / L858R, no T790M), after INC 280 plus gefitinib, partial remission (PRs) were obtained in 12 / 65 evaluable patients (ORR 18%) and 40 / 65 (62%) patients had stable disease[59]. Central nervous system (CNS) metastases are common in patients with non-small-cell lung cancer (NSCLC). Osimertinib has shown systemic efficacy in patients with CNS metastases, and early clinical evidence shows efficacy in the CNS. In the phase II trials of 50 patients with T790M-positive advanced NSCLC, confirmed CNS ORR (objective response rate) and DCR (disease control rate) were 54% (27 / 50) and 92% (46 / 50) respectively. Median follow-up for CNS PFS (progression-free survival) was 11 months. Osimertinib (80 mg) demonstrated clinically meaningful efficacy against CNS metastases[59]. In the phase III trial of 419 patients with advanced T790M positive NSCLC with osimertinib vs platinum based therapy, progression free survival in the osimertinib group was 8.5 months, compared to the platinum-based therapy group at 4.2 months[6]. Otherwise, targeting oncogenic ALK inhibitors Crizotinib (250 mg, twice a day)[60], and Alectinib (600 mg,orally twice daily, second-generation ALK inhibitor, better efficacy and better tolerability) also prevented lung cancer progression and delayed the time to brain metastases according to the results of the phase III ALEX trial presented at the 2017 ASCO Annual Meeting[61]. Serra[62] reported the clinical response of a lapatinib-based therapy in lung metastatic lesions of a Li-Fraumeni syndrome patient with oncogenic HER2V659E mutation and an EGFR-exon 20 insertion. A symptomatic and radiologic clinical response was achieved using oral daily lapatinib at a dose of 1,000 mg in combination with intravenous weekly paclitaxel 80 mg / m2, lately, trastuzumab initial dose of 8 mg / kg intravenously, and then followed by 6 mg / kg every three weeks. In total, the clinical benefits lasted over 9 months. In Cuba, CimaVax-EGF, promising, an active vaccine targeting EGF as the major ligand of oncogenic EGFR, it is in use as a cancer therapy against non-small cell lung cancer (NSCLC[63,64]. In this study, we use gefitinib in keeping stable disease for 8+ months in a woman with lung adenocarcinoma, and using gefitinib in more patients is under investigation.
Interesting, in an APL complicated with secondary HCC, it has been demonstrated previously that nuclear RARB has been shown to be rearranged as a result of insertion of HBV sequences[65]. Recent promising, these oncogenic receptor derivatives[28,66-68], in addition to oncogenic pml / RARA, oncogenic TBL1XR1-RARB[69], and NUP98 / RARG[70,71], and oncogenic PML-RARG[72] were also detected in APL rare cases. The involvement of RARB may explain why the disappearance of malignant hepatic tumour cells via the use of ATRA agent. In this case, ATRA (80 - 100 mg / day) was resistance to the relapse episode. In the presence of genetic mutation in RARA LBD and the PML-B2 domain of PML-RARA, one explanation for ATRA resistance is that the N-CoR / SMAT- corepressor complex tightly interact with pml / RARA or PLZF, even under pharmacological concentration of ATRA, so that transcriptional de-repression cannot occur at RARA target gene promoter, ATRA binding LBD impaired, degradation of pml / RARA by proteasome pathway are inhibited[29,73]. In addition, new emerging aberrant pml / RARA in relapsed APL returned to act as a constitutive transcriptional repressor[1,2,29,73-89] by pertubing normal retinoid signaling and RAR function, suppressing (the blockade of ) differentiation [see figure[29], possessing an altered specify for DNA response element, these DNA recognition changes target a distinct set of “neoplastic” genes that differ from the genes normally targeted by normal RARA. Previous studies uncovered that the ATRA and 13-cis forms of retinoic acid, two isomers of RA, are equally effective inhibiting proliferation[90]. In literature alternative strategy, an APL obtained CR after treatment with 13-cis retinoic acid first and repeated CR with ATRA in relapse[91]. And more, 80% (4 / 5) CR in newly APL and 33% (4 / 12) CR in relapsed APL were achieved after treatment with 9-cis retinoic acid(LGD1057) alone[92]. Nowadays, a lot of cohort trials, 61.5% (24 / 39) achieved CR using tamibarotene including 5 newly APL and 13 relapse APL twice or more[93-99]. Among 269 APL with CR underwent maintenance random, four year relapse-free survival rate was 84% (ATRA) and 91% (Tamibarotene). In 52 high risk patients, this became significant (50% for ATRA, 87% for tamibarotene)[98]. In comparative analysis among those relapsed APL[100], 80% (28 / 35) achieved CR and 22.86% CRm in tamibarotene - ATO versus 54.2% (19 / 35) CR with only 2.86 - 3.7% CRm in ATRA and ATO regimen[100]. In particular, appreciable benefits of tamibarotene- ATO regimen might occur at significantly lower frequency of leukocytosis with development of retinoic acid syndrome, an important adverse reaction during treatment of APL. Thus, Tamibarotene demonstrated more efficacy in both untreated APL patients and relapsed who have been treated ATRA and chemotherapy, especially as novel strategy in relapsed APL in Japan and others[100-102]. This is encouraging perspective.
Conflicts of Interest: The author declares that there is no conflict of interest regarding the publication of this paper.
References
- 1. Zhu, G., Saboor-Yaraghi, A.A., Yarden, Y., et al. Downregulating oncogenic receptor: From bench to clinic. (2016) Hematol Med Oncol 1(1): 30-40.
- 2. Zhu, G., Saboor-Yaraghi, A.A., Yarden, Y. Targeting oncogenic receptor:from molecular physiology to currently the standard of target therapy. (2017) Adv Pharma J 2(1): 10-28.
Pubmed|| Crossref|| Others
- 3. Das, A.I., de Bitter, T., Simmer, F., et al. Quantification and localization of oncogenic receptor tyrosine kinase variant transcripts using molecular inversion probes. (2018) Sci Reports 8(1): 7072.
- 4. Toledo, R. A., Garralda, E., Mitsi, M., et al. Exome sequencing of plasma DNA portrays the mutation landscape of colorectal cancer and discovers mutated VEGFR2 receptors as modulators of anti-angiogenic therapies. (2018) Clin Cancer Res March 24(15): 3550-3559.
- 5. Cross, D.A., Ashton, S.E., Ghiorghlu, S., et al. AZD9291,an irreversible EGFR TKI, overcomes T790M mediated resistance to EGFR inhibitors in lung cancer. (2014) Cancer Discov 4(9): 1046-1061.
- 6. Conterato, A.J., Belanger, A.R.., Yarmus, L.B., et al. Update on NSCLC tissue acquisition, processing, and profiling in the molecular age. (2017) Hematology Med Oncol 2(2): 1-8.
Pubmed|| Crossref|| Others
- 7. Pal, S.K., Rosenberg, J.E., Hoffman-Censits, J.H., et al. Efficacy of BGJ398,a fibroblast growth factor receptor 1-3 inhibitor,in patients with previously treated advanced urothelial carcinoma with FGFR3 alteration. (2018) Cancer Discov 8(7): 812-821.
- 8. Liu, J., Sareddy, G.R., Zho, M., et al. Differential effects of estrogen receptor beta isoforms on glioblastoma progression. (2018) Cancer Res 78(12): 3176-3189.
- 9. Weir, H.M., Bradbury, R.H., Lawson, M., et al. AZD9496.An oral estrogen receptor inhibitor that block the growth of ER-positive and ESR1-mutant breast tumors in preclinical models. (2016) Cancer Res ٧٦(١١): ٣٣٠٧-٣٣١٨.
- 10. West, D.C., Kocherginsky, M., Tonsing-Carter, E.Y., et al. Discovery of a glucocorticoid receptor (GR) activity signature using selective GR antagonism in ER-negative breast cancer. (2018) Clin Cancer Res 24(14): 3433-3446.
- 11. Reddy, J.A., Allagadda, V.M., Leamon, C.P. Targeting therapeutic and imaging agents to folate receptor positive tumors. (2005) Curr Pharm Biotechnol 6(2): 131-150.
- 12. Kalli, K.R., Block, M.S., Kasi, P.M., et al. Folate receptor alpha peptide vaccine generates immunity in breast and ovarian cancer patients. (2018) Clin Cancer Res
- 13. Zhu, G., Saboor-Yaraghi, A., Dharmadhikari, D., et al. A pilot study of chemotherapy and traditional plant medicine in hematology malignancy: report of thirty-four cases. (2017) Hematology Med Oncol 2(2): 1-3.
Pubmed|| Crossref|| Others
- 14. Zhu, G. EpCAM-an old cancer antigen, turned oncogenic receptor and its targeting immunotherapy. (2018) Uni J Pharma Res 3(2): 43-48.
- 15. Martowicz, A., Bobowski, K., Klocker, H., et al. EpCAM is over expressed in local and metastatic prostate cancer, suppressed by chemotherapy and modulated by MET-associated miRNA-200C/205. (2014) Br J Cancer 111(5): 955-964.
- 16. Mohtar, M.A., Hernychova, L., O’Neill, R., et al. The sequence-specific peptide-binding activity of the protein sulfide isomerase AGR2 directs its stable binding to the oncogenic receptor EpCAM. (2018) Mol Cell Proteomics 17(4): 737-763.
- 17. Wong, F., Coban, O., Weitsman, G., et al. Integrating imaging,exosome and protein network rewiring information to track early tumor evolution of resistance mechanisms. (2017) Converg Sci Phys Oncol 3: 013004.
Pubmed|| Crossref|| Others
- 18. Bacus, S., Spector, N.L., Yarden, Y. The era of ErbB-receptor-targeted therapies: advances toward personalized medicine. (2005) Per Med 2(4): 301-315.
- 19. Duarte, H.O., Bolmafia, M., Mereiter, S., et al. Gastric cancer cell glycosylation as a modulator of the ErbB2 oncogenic Receptor. (2017) Int J Mol Sci 18(11): 2262.
- 20. Wang, L.K., Hsiao, T.H., Hong, T.M., et al. MicroRNA-133a suppresses multiple oncogenic membrane receptors and cell invasion in non-small cell lung carcinoma. (2014) Plos one 9(5): 96765.
- 21. Rosenberg, S.A., Lotze, M.T., Muul, L.M. et al. Observation on the sysyemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 in patients with metastatic cancer. (1985) N Engl J Med 313(23): 1485-1492.
Pubmed|| Crossref|| Others
- 22. Forget, M.A., Haymakere, C., Hess, K.R., et al. Prospective analysis of adoptive TIL therapy in patients with metastatic melanoma: response, impact of anti-CTLA4, and biomarkers to predict clinical outcome. (2018) Clin Cancer Res 24(18): 4416-4428.
- 23. Olweny, C.L., Magrath, I., Ziegler, J.L., et al. Childhood Hodgkin’s disease in uganda: a ten-year experience. (1978) Cancer 42(2): 787-792.
- 24. Launa, F., Baccarani, M., Fiacchini, M., et al. Combination chemotherapy in stage I or II Hodgkin’s disease. (1979) Lancet 314(8151): 1072-1073.
- 25. Kato M, Sakuyama A, Matsutani T, Minato H. Efficacy of Trastuzumab therapy in HER2-positive early breast cancer patients in our clinic. (2015) Proceedings of BIT’s 8th Annual World Cancer Congress: 301.
Pubmed|| Crossref|| Others
- 26. Singh, H., Walker, A.J., Amiri-Kordestani, L., et al. US Food and Drug Administration Approval: Neratinib for extended adjuvant treatment of early stage HER2-positive breast cancer. (2018) Clin Cancer Res 24(15): 3486-3491.
- 27. Takeda, T., Yamaguchi, T., Yaegashi, N. perceptions and attitudes of Japanese gynecologic cancer patients to Kampo (Japanese herbal) medicines. (2012) Int J Clin Oncol 17(2): 143-149.
- 28. Ito, A., Munakata, K., Imazu, Y., et al. First nationwide attitude survey of Japanese physicians on the use of traditional Japanese medicine (Kampo) in cancer treatment. Evidence-Based Com Alternative Med: 8.
- 29. Zhu, G., Mische, S.E., Seigneres, B. Novel treatment of acute promyelocytic leukemia: As2O3, retinoic acid and retinoid pharmacology. (2013) Curr Phar Biotechnol, 14(9): 849-858.
Pubmed|| Crossref|| Others
- 30.Zhang, Q., Zhu, G. The pathological pattern of seven malignant cancers following Demethylcantharidin. (2017) Adv Pharma J 2(6): 243-247.
Pubmed|| Crossref|| Others
- 31. Zhu, G., Musumecci, F., Byrne, P., et al. Treatment of advanced hepayocelluar carcinoma(HCC) with the combined protocol of chemotherapy 5-fluorouracil and traditional medicine:report of ten cases. (2017) Clin Trials Pathol Case Stud 2(2): 61-65.
Pubmed|| Crossref|| Others
- 32. Zhu, G., Musumecci, F., Byrne, P., et al. Role of traditional herbal medicine in the treatment of advanced hepatocellular carcinoma(HCC: past and future ongoing. (2017) Adv P J 2(3): 115-120.
Pubmed|| Crossref|| Others
- 33. Zhu, G., Musumecci, F., Byrne, P., et al. A pilot study of lung cancer following chemotherapy and traditional medicine: report of 12 cases. (2017) Lungs and Breathing 1(3): 1-4.
Pubmed|| Crossref|| Others
- 34. Zhu, G., Musumecci, F., Byrne, P., et al. Clinical trials of lung cancer after chemotherapy and traditional medicine (12 cases). (2017) Adv Pharma J 2(5): 199-203.
Pubmed|| Crossref|| Others
- 35. Bonadonna, G., Lattuada, A., Monfardini, S., etal. Combined radiotherapy-chemotherapy in localized non-Hodgkin’s lymphomas:five-year results of a randomized study. (1979) In Adjuvant Therapy of Cancer II: 145-153.
Pubmed|| Crossref|| Others
- 36. Anderson, T., Bender, R.A., Fisher, R.I., et al. Combination chemotherapy in non-Hodgkin’s lymphoma: results of long-term follow up. (1977) Cancer Treat Rep 61(6): 1057-1066.
Pubmed|| Crossref|| Others
- 37. Skarin, A.T., Rosenthal, D.S., Moloney, W.C., et al. Combination chemotherapy of advanced non-Hodgkin’s lymphoma with bleomycin, Adriamycin, cyclophosphamide, vincristine, and prednisone (BACOP). (1977) Blood 49(5): 759-770.
Pubmed|| Crossref|| Others
- 38. Rodriguez, V., Cabanillas, F., Burgess, M.A., et al. Combination chemotherapy(‘CHOP-bleo’) in advanced (non-Hodgkin’s) malignant lymphoma. (1977) Blood 49(3): 325-333.
Pubmed|| Crossref|| Others
- 39. Schein, P.S., DeVita, V.T., Hubbard, S., et al. Bleomycin, Adriamycin, Cyclophosphamide, Vincristine, and Prednisone (BACOP) combination chemotherapy in the treatment of advanced diffuse histocytic lymphoma. (1976) Ann Intern Med 85(4): 417-422.
Pubmed|| Crossref|| Others
- 40. Case Jr, D.C. Combination chemotherapy of advanced diffuse non-Hodgkin’s lymphoma: results of cyclophosphamide, Adriamycin, vincristine, prednisone, and bleomycin (CHOP-bleo). (1979) J Maine Med Assoc 70(9): 348-352.
Pubmed|| Crossref|| Others
- 41. Wagner, A.D., Syn, N.L., Moehler, M., et al. Chemotherapy for advanced gastric cancer. (2017) Cochrane Database Syst Rev (8): CD004064.
- 42. Scartozzi, M., Galizia, E., Verdecchia, L., et al. Chemotherpay for advanced gastric cancer: across the years for a standard of care. (2007) Expert Opin Pharmacother 8(6): 797-808.
- 43. Orditura, M., Galizia, G., Sforza, V., et al. Treatment of gastric cancer. (2014) World J Gastroenterol 20(7): 1635-1649.
- 44. Liu, J.L., Ahme, S. Long term survival of patients with gastric cancer treated with adjuvant radio-chemotherapy: proposal of a prognostic index with implication for treatment modification. (2018) Oncol Res Rev 1(2): 1-5.
- 45. Skrypek, N., Vasseur, R., Vincent, A., et al. The oncogenic receptor ErbB2 modulates gemcitabine and irinotecin/ SN-38 chemoresistance of human pancreatic cancer cells via hCNT1 transporter and multidrug resistance associated protein MRP-2. (2015) Oncotarget 6(13): 10853-10867.
- 46. Meza-Junco, J., Au, H.J., Sawyer, M.B. Critical appraisal of trastuzumab in treatment of advanced stomach cancer. (2011) Cancer Manag Res 3: 57-64.
- 47. Fusco, N., Rocco, E.G., Del Conte, C., et al. HER2 in gastric cancer: a digital image analysis in pre- neoplastic, primary and metastatic lesions. (2013) Mol Pathol 26(6): 816-824.
- 48. Tanz, R., Mahfoud, T., Alami, E.I., et al. Is there any advantage from continuation of trastuzumab beyond progression in metastatic her positive gastric cancer? (2018) Hematol Med Oncol 3(2): 1-3.
- 49. Gabitova, L., Gorin, A., Astsaturov, I. Molecular pathways: sterols and receptor signaling in cancer. (2014) Clin Cancer Res 20(1): 28-34.
- 50. Shimizu, N., Kondo, I. Hyper production of EGF receptor in human A431 cell is regulated by a translocation chromosome. (1982) Cytog Cell Gen 32: 316-317.
Pubmed|| Crossref|| Others
- 51. Merlino, G.T., Xu, Y.H., Ishii, S., et al. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. (1984) Science 224(4647): 417-419.
- 52. Ullrich, A., Coussens, J., Hayflick, J.S., et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. (1984) Nature 309(5967): 418-425.
- 53. Miltra, S., Han, S., Soderstram, K., et al. Preferential expression of an oncogenic receptor in brain tumor stem cells: identification and targeting using an engineered antibody approach. (2009) Assoc Cancer Res 69(9): 72.
Pubmed|| Crossref|| Others
- 54. Hembrough, T., Thyparambil, S., Liao, W.L., et al. Quantitative multiplexed SRM analysis of oncogenic receptors in FFPE colorectal carcinoma tissue. (2012) Cancer Res 72(8): 5537.
- 55. Lee, J.C., Vivanco, I., Beroukhim, R., et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in extracellular domain. (2006) PLoS Med 3(12): 485.
- 56. Godin-Heymann, N., Bryant, I., Rivera, M.N., et al. Oncogenic activity of epidermal growth factor receptor kinase mutant alleles is enhanced by the T790M drug resistance mutation. (2007) Cancer Res 67(15): 7319-7326.
- 57. Konduri, K., Gallant, J.N., Chae, Y.K., et al. EGFR fusions as Novel Therapeutic Targets in Lung Cancer. (2016) Cancer Discov 6(6): 601-61.
- 58. Wu, Y.L., Kim, D.W., Felip, E., et al. Phase II safety and efficacy results of a single-arm ph ib/II study of capmatinib(INC 280) + gefitinib in patients(pts) with EGFR-mutated(mut),cMET-positive(cMET+) non-small cell lung cancer(NSCLC). (2016) ASCO.
Pubmed|| Crossref|| Others
- 59. Goss, G., Tsai, C.M., Shepherd, F.A., et al. CNS response to Osimertinib in patients with T790M-positive advanced NSCLC:pooled data from two phase II trials. (2018) Ann Oncol, 29(3): 687-693.
- 60. Shun, L., Tony, M., You, L., et al. Phase 3 study of first-line crizotinib vs pemetrexed/cisplatin or carboplatin (PCC) in East Asian patients(pts) with ALK+ advanced non-squamous nonsmall cell lung cancer(NSCLC). (2016) J Clin Oncol 34(15): 9058.
- 61. Shaw, A.T., Peters, S., Mok, T, et al. Alectinib versus Crizotinib in treatment-naive advanced ALK positive non-small cell lung cancer (NSCLC): primary results of the Global Phase III ALEX Study. (2017) J Clin On 35(18): 9008.
- 62. Serra, V., Vivancos, A., Puente, X.S., et al. Clinical response to a lapatinib-based therapy in a Li-Fraumeni syndrome patient with a novel HER2V659E mutation. (2013) Cancer Discov 3: 1238-1244.
Pubmed|| Crossref|| Others
- 63. Rodriguez, P.C., Rodriguez, C., Gonzalez, G., et al. Clinical development and perspective of CIMAvax EGF,Cuban vaccine for non-small-cell lung cancer therapy. (2010) MEDICC Rev 12:17-23.
Pubmed|| Crossref|| Others
- 64. Gonzalez, G., Crombet, T., Lage A. Chronic vaccination with a therapeutic EGF-based cancer vaccine: a review of patients receiving long lasting treatment. (2011) Curr Cancer Drug Targets 11:103-110.
Pubmed|| Crossref|| Others
- 65. de The, H., Marchio, A., Tiollais, P., Dejean, A. A novel steroid thyroid hormone receptor- related gene inappropriately expressed in human hepatocellular carcinoma. (1987) Nature 330: 667.
Pubmed|| Crossref|| Others
- 66. Marinelli, A., Bossi, D., Pellicci, P.G., et al. A redundant oncogenic potential of the retinoic acid receptor (RAR) alpha,beta and gamma isoforms in acute promyelocytic leukemia. (2007) Leukemia 21: 647-650.
- 67. Rietveld, L.E., Caldenhoven, E., Stunnenberg, H.G. Avian erythroleukemia: a model for corepressor function in cancer. (2001) Oncogene 20: 3100-3109.
Pubmed|| Crossref|| Others
- 68. Hauksdotti, H., Privalsky, M.L. DNA recognition by the aberrant retinoic acid receptors implicated in human acute promyelocytic leukemia. (2001) Cell Growth Differ 12: 85-98.
Pubmed|| Crossref|| Others
- 69. Osumi, T., Tsujimoto, S.I., Tamura, M., et al. Recurrent RARB-translocations in acute promyelocytic leukemia lacking RARA translocation. (2018) Cancer Res 78(16).
- 70. , E., Cervera, J., Valencia, A., et al. A novel NUP98/RARG fusion in acute myeloid leukemia resembling acute promyelocytic leukemia. (2011) Blood 117(1): 242-245.
- 71. Such, E., Cordon, L., Sempere, A., et al. In vitro all-trans retinoic acid sensitivity of acute myeloid leukemia blasts with NUP98/RARG fusion gene. (2014) Ann Hematol 93(11): 1931-1933.
- 72. Ha, J.S., Do, Y.R., Ki, C.S., et al. Identification of a novel PML-RARG fusion in acute promyelocytic leukemia. (2017) Leukemia 31(9): 1992-1995.
- 73. Tomita, A., Kiyoi, H., Naoe T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide(As2O3) in acute promyelocytic leukemia. (2013) Int J Hematol 97(6): 717-725.
- 74. Grignani, F., Ferrucci, P., Testa, U., et al. The acute promyelocytic leukemia-specific PML-RARa fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. (1993) Cell 74(3): 423-431.
- 75. Rousselot, P., Hardas, B., Patel, A., et al. The PML-RARa gene product of the t (15;17) translocation inhibits retinoic acid-induced granulocytic differentiation and mediated transactivation in human myeloid cells. (1994) Oncogene 9(2): 545-551.
- 76. He, L.Z., Guidez, F., Tribioli, C., et al. Distinct interactions of PML-RARa and PLZF-RAR alpha with co-repressors determine differential response to RA in APL. (1998) Nat Genet 18(2): 126-135.
- 77. Kitareewan, S., Pitha-Rowe, I., Sekula, D., et al. UBEIL is a retinoid target that triggers PML/RARalpha degradation and apoptosis in acute promyelocytic leukemia. (2002) Proc Natl Acad Sci 99(6): 3806-3811.
- 78. Segalla, S., Rinaldi, L., Kalstrup-Nielsen, C., et al. Retinoic acid receptor alpha fusion to PML affects its transcriptional and chromatin-remodeling properties. (2003) Mol Cell Biol 23(23): 8795-8808.
Pubmed|| Crossref|| Others
- 79. Jing, Y. The PML-RARa fusion protein and target therapy for acute promyelocytic leukemia. (2004) Leuk Lymphoma 45(4): 639-648.
- 80. Carbone, R., Botrugn, O.A., Ronzoni, S., et al. Recruitment of the histone methyltransferase SUV39H1 and it’s the oncogenic properties of the leukemia-associated PML-retinoic acid receptor fusion protein. (2006) Mol Cell Bio 26(4): 1288-1296.
- 81. Nasr, R., Guillemin, M.C., Ferhi, O., etal. The eradication of acute promyelocytic leukemia- initiating cells through PML-RARA degradation. (2008) Nat Med 14(12): 1333-1342.
- 82. Marstrand, T.T. A conceptual framework for the identification of candidate drugs and drug targets in acute promyelocytic leukemia. (2010) Leukemia 24(7): 1265-1275.
- 83. Lallemand, B.V., Hugues, T. A new oncoprotein catabolism pathway. (2010) Blood 116: 2200-2201.
Pubmed|| Crossref|| Others
- 84. Rosen, M., Privalsky, M.L. Thyroid hormone receptor mutations in cancer and resistance to thyroid hormone: perspective and prognosis. (2011) J Thyroid Res.
- 85. Podhorecka, M., Macheta, A. Acute promyelocyticleukemia-modern approach to disease pathogenesis and differentiation treatment. (2013) Postepy Hig Med Dosw 67: 1083-1089.
Pubmed|| Crossref|| Others
- 86. Dos Santos, G.A., Kats, L., Pandolfi, P.P. Synergy against PML-RARa targeting transcription, proteolysis, differentiation, and self-renewal in acute promyelocytic leukemia. (2013) J Exp Med 210(13): 2793-2802.
- 87. Humbert, M. The tumor suppressor gene DAPK2 is induced by myeloid transcription factor pu.1 and c.EBPa during granulocytic differentiation but repressed by PML-RARa in APL. (2014) J Leuk Biol 95: 83-93.
Pubmed|| Crossref|| Others
- 88. De Braekeleer, E., Dout-Guilbert, N., De Braekeleer, M. RARA fusion genes in acute promyelocytic leukemia:A review. (2014) Expert Rev Hematol 7(3): 347-357.
- 89. Noguera, N.I., Piredda, M.L., Taulli, R., et al. PML/RARa inhibits PTEN expression in hematopoietic cells by competing with PU.1 transcriptional activity. (2016) Oncotarget 7(41): 66386-66397.
- 90. Chomienne, C., Ballerini, P., Balitrand, N., et al. All-trans retinoic acid in acute promyelocytic leukemia II.In vitro studies, structure-function relationship. (1990) Blood 76(9): 1710-1717.
- 91. Haferiach, T., Loffler, H., Glass, B., et al. Repeated complete remission in a patient with acute promyelocytic leukemia after treatment with 13-cis-retinoic acid first and with all-trans-retinoic acid in relapse. (1993) Clin Investig 71(10): 774-779.
- 92. Soignet, S.L., Benedetti, F., Fleischauer, A., et al. Clinical study of 9-cis retinoic acid (LGD) in acute promyelocytic leukemia. (1998) Leukemia 12: 1518-1521.
Pubmed|| Crossref|| Others
- 93. Tobita, T., Takeshita, A., Kitamura, K., et al. Treatment with a new synthetic retinoid, Am80, of acute promyelocytic leukemia relapsed from complete remission induced by all-trans retinoic acid. (1997) Blood 90(3): 967-973.
- 94. Takeuchi, M., Yano, T., Omoto, E., et al. Relapsed acute promyelocytic leukemia previously treated with ATRA: clinical experience with a new synthetic retinoid, Am80. (1998) Leuk lymphoma 31(5-6): 441-451.
- 95. Takeuchi, M., Yoshida, I., Takahashi, K. Long-term follow up of re-induction with a new synthetic retinoid, Am-80,for relapse of acute promyelocytic leukemia previously treated with all-trans retinoic acid: results of 7 cases from a single institute. (2003) Rinsho Ketsueki 44 (11): 1069-1073.
- 96. Takeshita, A., Shinagawa, K., Adachi, M., et al. Tamibarotene for the treatment of acute promyelocytic leukemia. (2014) Expert opinion orphan drugs 2(9): 961-969.
- 97. Naoe, T. Tamibarotene for the treatment of acute promyelocytic leukemia Hematology. (2014) 71(9): 96-69.
Pubmed|| Crossref|| Others
- 98. Shinagawa, K., Yanada, M., Sakura, T., et al. Tamibarotene as maintenance therapy for acute promyelocytic leukemia: results from a randomized controlled trial. (2014) J Clin Oncol 32(33): 3729-3735.
- 99. Sanford, D., Lo-coco, F., Sanz, M.A., et al. Tamibarotene in patients with acute promyelocytic leukemia relapsing after treatment with all-trans retinoic acid and arsenic trioxide. (2015) Br J Hematol 171(4): 471-477.
- 100. Wang, J.X., Mi, Y.C., Jiang, B., et al. Tamibarotene compared to all-trans retinoic acid (ATRA) as add-on to arsenic trioxide (ATO) in subjects with relapsed acute promyelocytic leukemia(APL). (2015) Blood.
Pubmed|| Crossref|| Others
- 101. Kojima, M., Ogiya, D., Ichiki, A., et al. Refractory acute promyelocytic leukemia successfully treated with combination therapy of arsenic trioxide and tamibarotene. (2016) Leukemia Res Rep 5: 11-13.
Pubmed|| Crossref|| Others
- 102. Asou, N. Retinoic acid, all-trans retinoic acid (ATRA) and Tamibarotene. (2017) Chemotherapy for leukemia: 183-211.
Pubmed|| Crossref|| Others
Chemotherapy is a major skillful of cancer therapy. one of the most important advances in oncology has been increased acceptance of evidence that most patients with disseminated tumors were setted to the protocol of chemotherapy in conjunction with recent targeting oncogenic receptor[1-20], traditional medicine(TCM) and / or adoptive immunotherapy (LAK cells, TIL therapy)[21,22]. The experience in Ugandan children with Hodgkin’s disease has been excellent[23] and in a study of 14 adults with stage I and II Hodgkin’s disease, mostly clinically staged, 13 patients (93%) achieved CR with combination chemotherapy and all were in CR 11 to 94 months after the completion of treatment[24]. Another, a disease-free survivors of 5 years (56.5 - 59.3% versus 22 - 24.3%) and 10 years (48.9%) was remarkedly higher rate in those breast cancers with stage III following surgery plus chemotherapy than only surgery. More promising, in large trials of 48 HER2 - positive early breast cancer patients, targeting the adjuvant trastuzumab treatment demonstrated highly favorable outcome. Five year overall survival rates and disease-free survival rates were 95.8% and 93.8% respectively[25]. Recently, neratinib was recently approved by FDA for extended adjuvant treatment of ER+ / HER2+ breast cancer[26]. Others advanced or metastatic gastric cancer constitutes the majority of patients in clinical practice. Systemic chemotherapies and combined regimens are currently available, provides palliation and prolongs survival. In particular, high-quality clinical trials on TCM in cancer are generally lacking, except for Kampo medication for Japanese cancer patients[27,28], arsenic trioxide (As2O3) in the role of acute promyelocytic leukemia (Zhang TD,1973;Sun HD, 1992; Zhang P, 1996)[29], and cantharidin in treatment of liver cancer [Yang BY, 1970s;[30]. This paper will attempt to place in proper interpretative review from those patients with cancers under remission in this group.
Materials and Methods
82 patients with available cancers were concluded in the study during September 1993- May 2018. The sex ratio of male: female was 55: 27 respectively. The mean age at onset was 46.4 years ranging from 10 to 79 years. Among age distribution, although there is some uncertain about the type distribution of cancers, it was found 38.1 years as the mean age at onset for lymphoma; 44.0 years for liver cancer, while higher mean age at 60.1 years has been shown in lung cancer in this group. The clinical diagnoses in a broad variety of carcinomas consisted of metastatic nasopharyngeal cancer 5 cases, metastatic breast cancer 4, inflammatory breast tumor 2, lung tumors 12, hepatocellular carcinoma (HCC) 12, stomach cancer 5, hematological malignancies 27 cases (acute leukemias FAB M1 type 2, M2 type 1, acute promyelocytic leukemia 1, chronic myeloid leukemia CML 2, chronic lymphocytic leukemia CLL 1, refractory anemia 1, multiple myeloma 2, lymphoma 16), thyroid cancer 2, maxillary sinus carcinoma 1, carcinoma of mandibular sinus 2, laryngeal carcinoma 1, esophagus cancer 1, gallbladder cancer 1, cholangiocarcinoma 1, metastatic oral cancer 1, epidermoid carcinoma 1,metastatic melanoma 1, replapsed vulvar cancer 1, and other metastatic sternal and spinal (T12) tumor 1, myxoid chondrosarcoma 1 (figure 1) respectively. all other benign neoplasias were not statistically included. The basic chemotherapeutic regimen consisted of vincristine(VCR, 1-2 mg / wk), cyclophosphamide (CTX, 200 - 1,000 mg / wk), mitomycin C (MMC, 2 - 4 mg / wk) and 5-fluorouracil (5-FU, 250 - 500 mg / day). In addition, the additional drug adriamycin (ADM, 20 mg / wk) in lymphoma and metastatic breast cancer, demethylcantharidin in liver cancer and cisplatin (DDP) or interleukin-2 (PHA) /gefitinib in lung cancer. The detail prescription of TCM varied among a broad variety of carcinomas (see full text case reports).The criteria of complete remission (CR) and / or partial remission (PR) is according to the rules where physicians have in common with in clinics. Complete remission (CR): there was no more tumor or tumor complete regressed in patients for at least 1 month; Parttial remission: the tumor decreased by more than 50% in patients for at least 1 month; Stable disease: the tumor decreased by less than 50% or increased by no more than 25% in patients; Disease progression: the tumor increased by more than 25% in patients, or new lesions emerged. The efficacy was evaluated according to the survival time from the day when patients were at onset. The clinical data for liver cancer[31,32] and lung cancer[33,34] were previously described.
Figure 1: A patient with myxoid chondrosarcoma. A biopsy specimen stain: CK - S-100+ EMA - Ki-67 ~50 %
Results
In 82 cancers, the rate of complete remission(CR) was achieved in 37 (45.1%) advanced cancers. All CR patients with advanced cancers was survival over 5 years, 18 cancers was survival 10 years. Another, a short CR was obtained in 13 (15.9%) advanced cancers, the survival time varied from 20 months to 4 years. PR was obtained in 26 (31.7%) patients with a broad variety of carcinoma, while three patients (1 malignant lymphoma,1 carcinoma of mandibular sinus, 1 metastatic tumor of bone) had survival 12, 18+ and 11+ years respectively, implicating a longer survivor in patients the survival with tumours. Otherwise, stable disease was 6 cases. Basic characteristics of studied population were summarized in table 1.
Table 1: Patient’s characteristics.
| Cancer types | Cases No | Sex | Mean ages (years) | Treatment Protocol |
Response following therapy |
Duration of remission (years) < 1 1-3 > 3-5 >5-10 >10 |
|---|---|---|---|---|---|---|
| lymphoma | 17 | M14, F2 | 38.1 (13 - 66) |
CVP or COMA(11)*, Radiotherapy**(1), prednisone(1), Immunotherapy(4) |
CR(10)*, short CR(2), PR(5) |
1 3 2 1 9 |
| HCC | 12 | M10, F2 | 44.0 (26 - 63) |
a. 5-Fu (250-1,000 mg/day), VCR, CTX, MMC, TCM b. Cantharidin, TCM c. hepatectomy, sorafenib |
CR(8), Short CR(2), PR(1), stable disease(1) |
1 4 1 3 3 |
| Lung tumors | 12 | M8,F4 | 60.1 (40 - 79) |
a. COMF, TCM; b. DDP, etoposide, gefitinib c. CTX, 5-FU, antitumor capsule; d. TCM alone |
CR(2), short CR(2), PR(3), stable disease(5) |
4 3 3 1 |
| NPC | 5 | M3, F2 | 51.4 (38 - 75) |
a. VCMF,TCM b. CTX, 5-FU, TCM |
CR(1), short CR(1), PR(3) |
1 3 1 |
| MBC | 4 | F4 | 31.3 (25 - 41) |
a. COMF, TCM b. COP, TCM |
CR(3), PR(1) | 1 2 |
| IBT | 2 | F2 | 31,35 | Antibiotics, TCM | CR(2) | 2 |
| Stomach cancer | 5 | M2, F3 | 42.3 (35 - 50) |
a. MFC, TCM; b. CTX, 5-FU, antitumor capsule |
CR(1), short CR(2), PR(2) |
1 3 1 |
| AML | 3 | M2, F1 | 4, 18, 20 | DA**, HA, TCM | PR(3) | 3 |
| APL | 1 | M | 31 | ATRA 80 mg/day; H 1 mg x 5 days; TCM |
CR | 1 died of relapsed APL |
| CML | 2 | M1, F1 | 33, 62 | Busulfan, TCM | CR(1), short CR(1) | 1 1 |
| CLL | 1 | M | 58 | Chlorambucil, TCM | CR | 1 died of stomach cancer |
| RA | 1 | M | 43 | Vitamin B12, folate acid, TCM | CR | 1 |
| MM | 2 | M1, F1 | 60, 63 | Thalidomide, pred, TCM | Short CR(1), PR(1) | 1 1 |
| Epidermoid cancer | 1 | F | 72 | 5 % FU of retinoic acid ointment |
PR | 1 |
| Thyroid cancer | 2 | F2 | 54, 60 | TCM alone | CR(2) | 1 1 |
| bile cancer | 1 | F | 65 | CTX, 5-FU, TCM | CR | 1 |
| Cholangio -carcinoma | 1 | M | 72 | MFC (MMC, 5-FU, CTX), TCM | CR | 1 died of intestinal cancer |
| Others*** | 10 | M8,F2 | 57.5 (44 - 69) |
COFP, COMMB, TCM |
CR(2), short CR(2), PR(6) |
3 2 1 3 |
Note: HCC: hepatocellular carcinoma; NPC: metastatic nasopharyngeal cancer; MBC: metastatic breast cancer; IBT: inflammatory breast tumor; AML: acute myeloid leukemia; APL: acute promyelocytic leukemia; CML: chronic myeloid leukemia; CLL: chronic lymphocytic leukemia; RA: refractory anemia; MM: multiple myeloma; COMA: CTX,VCR,MMC,ADM; COMF:CTX,VCR,MMC/ADM,5-FU; VCMF:VCR,CTX,MMC/DDP; 5-FU; COP:CTX,VCR,pred; COFP:CTX,VCR,5-FU,PHA, Pred; DA:DNR,45mg/m2, Ara-c 100mg/m2; HA: homoharringtone 1mg x 5days, ara-c 50 mg, intramuscle, twice a day; MFC:MMC,5-FU, Ara-c / H,CTX; COMMB: CTX,VCR, MMC, MTX, Bleomycin; ATRA: all-trans retinoic acid; Pred: prednisone; TCM: traditional medicine; M:male; F: female: *cases number; **:treatment in another hospital; ***include oral cancer 1, metastatic melanoma 1, relapsed vulva cancer 1, laryngeal cancer 1, esophagus cancer 1, maxillary sinus carcinoma 1, carcinoma of mandibular sinus 2, and metastatic bone tumor 2.
During the schedule of drug administration, all patients were treated with the different dosage of 1 to 4 courses of various combination chemotherapy in conjunction with traditional medicine. In statistically analysis, one patient with nasopharyngeal cancer, the diplopia and unable version in his eye were recovered to “normal” visual acuity following the combination chemotherapy of VCMF(VCR, CTX, MMC and 5-FU) plus traditional medicine. A patient with rodent ulcer (8 x 5 cm) once obtained complete response as to an approach of 5% Fu of retinoic acid ointment. A short CR was achieved by the protocol of MFC(MMC; 5-Fu; Ara-C / homoharringtonine, CTX) plus cantharidin or cinobufacini drug in 5 advanced gastric cancers. One of them was a long-term survivor for 6 years via mass incision and the combination of MFC with herbs Scutellaria barbata d.don.
In view of cancer types, 10 lymphoma was setted to the major protocol of the combination conventional chemotherapy (COMF, CTX, VCR, MMC, 5-FU; COMA, CTX, VCR, MMC or ADM) in conjunction with traditional medicine which to relieve the chemotherapeutic toxicity, and reinforced the efficacy of chemotherapy. One lymphoma was regressed only by prednisone (200 #). Another 4 patients with thumb lymphadenopathy was treated by the use of antibiotics regimen in full dose with anti-inflammatory herbal tablets or immunotherapy lymphocyte transfer factor. In 12 HCC, 6 HCC were treated mainly by 5- FU (500 - 1000 mg / day) and TCM. 2 patients obtained CR through cantharidin and traditional medicine. The main protocol of TCM with adjuvant antibiotics regimen and low dose of dexamethasone was given in a primary liver cancer (AFP+, ascites +++, jaundice +++, liver tumor 3.2 x 3.0 cm). One acute promyelocytic leukemia complicated with metastatic liver cancer (7 x 4.5 cm) was in CR with all-trans retinoic acid (ATRA) and TCM. The detail prescription of TCM was mentioned before[25,26]. In the follow up, one HCC accompanied with colon polyps obtained complete remission via hapatectomy and targeting oncogenic receptor tyrosine kinase inhibitor sorafenib. Dose intensity has proven to be critical in maximizing chemotherapeutic efficacy for numerous human cancers. Eight other patients with cancers were in remission through small dosage of chemotherapy and TCM or traditional medicine (TCM) alone. There were 4 lung cancers, 1 gallbladder cancer, 1 cholangiocarcinoma and 2 thyroid cancers. Among targeting two metastatic lung cancers, one female with lung cancer was given the combination chemotherapy plus targeting oncogenic receptor EGFRvIII gefitinib, which was stable disease for 8+ months. Thyroid cancer was placed on the primary use of traditional medicine. The crude herbs consisted of sargassum, tangle, Oyster (mussels), Poriacocos, Ophiopogonjaponicus, Prunella vulgaris, Taraxacum, Scrophularia ningpoensis, Cremastra appendiculata, Trichosanthes Kirilowii, Sophora subprostrata, Houttuynia cordata, Scutellaria barbata d. don and Oldenlandia diffusa roxb.
A novel approach to the study of traditional medicine antitumor capsule was treated in 8 patients with advanced cancers. The initial results of antitumor capsule is an effective benefits to cancer treatment especially brain tumors through down regulation of inflammation, attenuated pain symptom and blocking convulsion. EGFR+++ was found in one glioma. This adjuvant agent is undergoing to clinical trials.
The survival times in those patients with remission were less than 1 years 10 cases, 1 to 3 years 22 cases, over 3 to 5 years 12 cases, over 5 to 10 years 12 cases, over 10 to 20 years 15 cases, and over 20 years 7 cases. In differential types of 20 patients with over 10 year’s survivors, lymphoma occupied 8 cases (40%). Among 7 patients with over 20 years, lymphoma occupied 3 cases, metastatic breast cancer 2 cases and hepatocellular carcinoma 2 cases.
Discussion
In this study, a series of the long follow up of patients with cancers were reported. I experienced that a CR was a pivotal influencing factor in those longest survival patients, and traditional medicine was also recommended.
The traditional combination chemotherapy program for lymphomas of favorable histologic type has been CVP (CTX, VCR, Pred) given at 21-days intervals[35]. Cyclophosphamide, vincristine, procarbazine, and prednisone (COPP) were used in the NCI study for patients with nodular mixed and nodular histocytic lymphomas[36]. More intensive CVP programs with the addition of adriamycin or bleomycin, or both, known as BACOP or CHOP- bleo, resulted in overall complete remission rates for patients with diffuse lymphomas ranging from 48% to 89%[37-40]. The NCI program of this 5-drug program, complete remission rates with this approaches has ranged from 48% to 94%[39]. In this study, the CR rates was 63% in 16 lymphomas, 6 CR were used by CVP or COMA regimens.
The use of chemotherapy to treat stomach cancer has no firmly established standard of care[41]. Some drugs used in stomach cancer treatment have included:5- FU (fluorouracil), doxorubicin (adriamycin), mitomycin C and most recently oxaliplatin, irinotecan in various combination. The relative benefits of these different drugs, alone or in combination,are unclear[42]. There are evidence supporting that clinical researches are exploring the benefits of giving chemotherapy as adjuvant therapy for surgery to destroy remaining cancer cells[43]. In recent analyses of definitive surgery followed by adjuvant radio chemotherapy (5-FU / leucovorin LV regimens) for patients with gastric cancer, Liu and Ahmed[44] reported that 59.3% (48 / 81) patients survived > 3 years,18.5% (15 / 81) patients survived 5 or more years. Eighteen out of 81 (22.2%) patients are still alive with a medium survival of 142 months (57 - 196 months). In this study, 5 patients with gastric cancer obtained a short CR through MFC regimen plus cinobufacini and cantharidin drugs. One relapsed gastric cancer survived over 6 years after surgery and adjuvant chemotherapy. More recent, treatment with HER2 inhibitor, trastuzumab, has been demonstrated to improve overall survival in inoperable locally advanced or metastatic gastric carcinoma overexpressing the HER2[43]. Oncogenic receptor HER2[45] is over expressed in 13 - 22% of patients with gastric cancer[46,47]. Tanz and colleagues[48] reported two HER2 - positive metastatic gastric adenocarcinoma who favorably responded to second line chemotherapy (FOLFIRI, irinotecan plus 5-Fu) with trastuzumab continuation following progressive disease to first line treatment containing trastuzumab, implicating trastuzumab continuation in metastatic HER positive gastric cancer is safe, practical and improve survival.
Oncogenic EGFR mutations are found in 10% to 35% of lung adenocarcinomas, with predominants in a subset of patients with non-small cell lung cancer (NSCLC[49-55]. These mutations, which commonly occur as either small in-frame deletions in exon 19 or point mutations T790M or L858R in exon 21 within the EGFR tyrosine kinase domain, confer constitutive activity and sensitivity to EGFR tyrosine kinase inhibitor (TKI[55,56]. Konduri and colleagues[57] reported five patients with metastatic lung cancer whose tumors harbored EGFR fusion, most commonly RAD5, are recurrent in lung cancer. Four of whom were treated with EGFR TKI erlotinib with documented antitumor response for 5, 6, 8, and 20 months respectively. An early EGFR TKI trial randomized patients with EGFR mutation positive stage IIIb or IV adenocarcinoma to treatment with afatinib or gemcitabine and cisplatin, treatment with afatinib prolonged progression free survival to 11.0 months as opposed to 5.6 months with gemcitabine and cisplatin[7]. In a total of 65 lung cancers with EGFR- mut (exon19 del / L858R, no T790M), after INC 280 plus gefitinib, partial remission (PRs) were obtained in 12 / 65 evaluable patients (ORR 18%) and 40 / 65 (62%) patients had stable disease[59]. Central nervous system (CNS) metastases are common in patients with non-small-cell lung cancer (NSCLC). Osimertinib has shown systemic efficacy in patients with CNS metastases, and early clinical evidence shows efficacy in the CNS. In the phase II trials of 50 patients with T790M-positive advanced NSCLC, confirmed CNS ORR (objective response rate) and DCR (disease control rate) were 54% (27 / 50) and 92% (46 / 50) respectively. Median follow-up for CNS PFS (progression-free survival) was 11 months. Osimertinib (80 mg) demonstrated clinically meaningful efficacy against CNS metastases[59]. In the phase III trial of 419 patients with advanced T790M positive NSCLC with osimertinib vs platinum based therapy, progression free survival in the osimertinib group was 8.5 months, compared to the platinum-based therapy group at 4.2 months[6]. Otherwise, targeting oncogenic ALK inhibitors Crizotinib (250 mg, twice a day)[60], and Alectinib (600 mg,orally twice daily, second-generation ALK inhibitor, better efficacy and better tolerability) also prevented lung cancer progression and delayed the time to brain metastases according to the results of the phase III ALEX trial presented at the 2017 ASCO Annual Meeting[61]. Serra[62] reported the clinical response of a lapatinib-based therapy in lung metastatic lesions of a Li-Fraumeni syndrome patient with oncogenic HER2V659E mutation and an EGFR-exon 20 insertion. A symptomatic and radiologic clinical response was achieved using oral daily lapatinib at a dose of 1,000 mg in combination with intravenous weekly paclitaxel 80 mg / m2, lately, trastuzumab initial dose of 8 mg / kg intravenously, and then followed by 6 mg / kg every three weeks. In total, the clinical benefits lasted over 9 months. In Cuba, CimaVax-EGF, promising, an active vaccine targeting EGF as the major ligand of oncogenic EGFR, it is in use as a cancer therapy against non-small cell lung cancer (NSCLC[63,64]. In this study, we use gefitinib in keeping stable disease for 8+ months in a woman with lung adenocarcinoma, and using gefitinib in more patients is under investigation.
Interesting, in an APL complicated with secondary HCC, it has been demonstrated previously that nuclear RARB has been shown to be rearranged as a result of insertion of HBV sequences[65]. Recent promising, these oncogenic receptor derivatives[28,66-68], in addition to oncogenic pml / RARA, oncogenic TBL1XR1-RARB[69], and NUP98 / RARG[70,71], and oncogenic PML-RARG[72] were also detected in APL rare cases. The involvement of RARB may explain why the disappearance of malignant hepatic tumour cells via the use of ATRA agent. In this case, ATRA (80 - 100 mg / day) was resistance to the relapse episode. In the presence of genetic mutation in RARA LBD and the PML-B2 domain of PML-RARA, one explanation for ATRA resistance is that the N-CoR / SMAT- corepressor complex tightly interact with pml / RARA or PLZF, even under pharmacological concentration of ATRA, so that transcriptional de-repression cannot occur at RARA target gene promoter, ATRA binding LBD impaired, degradation of pml / RARA by proteasome pathway are inhibited[29,73]. In addition, new emerging aberrant pml / RARA in relapsed APL returned to act as a constitutive transcriptional repressor[1,2,29,73-89] by pertubing normal retinoid signaling and RAR function, suppressing (the blockade of ) differentiation [see figure[29], possessing an altered specify for DNA response element, these DNA recognition changes target a distinct set of “neoplastic” genes that differ from the genes normally targeted by normal RARA. Previous studies uncovered that the ATRA and 13-cis forms of retinoic acid, two isomers of RA, are equally effective inhibiting proliferation[90]. In literature alternative strategy, an APL obtained CR after treatment with 13-cis retinoic acid first and repeated CR with ATRA in relapse[91]. And more, 80% (4 / 5) CR in newly APL and 33% (4 / 12) CR in relapsed APL were achieved after treatment with 9-cis retinoic acid(LGD1057) alone[92]. Nowadays, a lot of cohort trials, 61.5% (24 / 39) achieved CR using tamibarotene including 5 newly APL and 13 relapse APL twice or more[93-99]. Among 269 APL with CR underwent maintenance random, four year relapse-free survival rate was 84% (ATRA) and 91% (Tamibarotene). In 52 high risk patients, this became significant (50% for ATRA, 87% for tamibarotene)[98]. In comparative analysis among those relapsed APL[100], 80% (28 / 35) achieved CR and 22.86% CRm in tamibarotene - ATO versus 54.2% (19 / 35) CR with only 2.86 - 3.7% CRm in ATRA and ATO regimen[100]. In particular, appreciable benefits of tamibarotene- ATO regimen might occur at significantly lower frequency of leukocytosis with development of retinoic acid syndrome, an important adverse reaction during treatment of APL. Thus, Tamibarotene demonstrated more efficacy in both untreated APL patients and relapsed who have been treated ATRA and chemotherapy, especially as novel strategy in relapsed APL in Japan and others[100-102]. This is encouraging perspective.
Conflicts of Interest: The author declares that there is no conflict of interest regarding the publication of this paper.
References
- 1. Zhu, G., Saboor-Yaraghi, A.A., Yarden, Y., et al. Downregulating oncogenic receptor: From bench to clinic. (2016) Hematol Med Oncol 1(1): 30-40.
- 2. Zhu, G., Saboor-Yaraghi, A.A., Yarden, Y. Targeting oncogenic receptor:from molecular physiology to currently the standard of target therapy. (2017) Adv Pharma J 2(1): 10-28.
Pubmed|| Crossref|| Others
- 3. Das, A.I., de Bitter, T., Simmer, F., et al. Quantification and localization of oncogenic receptor tyrosine kinase variant transcripts using molecular inversion probes. (2018) Sci Reports 8(1): 7072.
- 4. Toledo, R. A., Garralda, E., Mitsi, M., et al. Exome sequencing of plasma DNA portrays the mutation landscape of colorectal cancer and discovers mutated VEGFR2 receptors as modulators of anti-angiogenic therapies. (2018) Clin Cancer Res March 24(15): 3550-3559.
- 5. Cross, D.A., Ashton, S.E., Ghiorghlu, S., et al. AZD9291,an irreversible EGFR TKI, overcomes T790M mediated resistance to EGFR inhibitors in lung cancer. (2014) Cancer Discov 4(9): 1046-1061.
- 6. Conterato, A.J., Belanger, A.R.., Yarmus, L.B., et al. Update on NSCLC tissue acquisition, processing, and profiling in the molecular age. (2017) Hematology Med Oncol 2(2): 1-8.
Pubmed|| Crossref|| Others
- 7. Pal, S.K., Rosenberg, J.E., Hoffman-Censits, J.H., et al. Efficacy of BGJ398,a fibroblast growth factor receptor 1-3 inhibitor,in patients with previously treated advanced urothelial carcinoma with FGFR3 alteration. (2018) Cancer Discov 8(7): 812-821.
- 8. Liu, J., Sareddy, G.R., Zho, M., et al. Differential effects of estrogen receptor beta isoforms on glioblastoma progression. (2018) Cancer Res 78(12): 3176-3189.
- 9. Weir, H.M., Bradbury, R.H., Lawson, M., et al. AZD9496.An oral estrogen receptor inhibitor that block the growth of ER-positive and ESR1-mutant breast tumors in preclinical models. (2016) Cancer Res ٧٦(١١): ٣٣٠٧-٣٣١٨.
- 10. West, D.C., Kocherginsky, M., Tonsing-Carter, E.Y., et al. Discovery of a glucocorticoid receptor (GR) activity signature using selective GR antagonism in ER-negative breast cancer. (2018) Clin Cancer Res 24(14): 3433-3446.
- 11. Reddy, J.A., Allagadda, V.M., Leamon, C.P. Targeting therapeutic and imaging agents to folate receptor positive tumors. (2005) Curr Pharm Biotechnol 6(2): 131-150.
- 12. Kalli, K.R., Block, M.S., Kasi, P.M., et al. Folate receptor alpha peptide vaccine generates immunity in breast and ovarian cancer patients. (2018) Clin Cancer Res
- 13. Zhu, G., Saboor-Yaraghi, A., Dharmadhikari, D., et al. A pilot study of chemotherapy and traditional plant medicine in hematology malignancy: report of thirty-four cases. (2017) Hematology Med Oncol 2(2): 1-3.
Pubmed|| Crossref|| Others
- 14. Zhu, G. EpCAM-an old cancer antigen, turned oncogenic receptor and its targeting immunotherapy. (2018) Uni J Pharma Res 3(2): 43-48.
- 15. Martowicz, A., Bobowski, K., Klocker, H., et al. EpCAM is over expressed in local and metastatic prostate cancer, suppressed by chemotherapy and modulated by MET-associated miRNA-200C/205. (2014) Br J Cancer 111(5): 955-964.
- 16. Mohtar, M.A., Hernychova, L., O’Neill, R., et al. The sequence-specific peptide-binding activity of the protein sulfide isomerase AGR2 directs its stable binding to the oncogenic receptor EpCAM. (2018) Mol Cell Proteomics 17(4): 737-763.
- 17. Wong, F., Coban, O., Weitsman, G., et al. Integrating imaging,exosome and protein network rewiring information to track early tumor evolution of resistance mechanisms. (2017) Converg Sci Phys Oncol 3: 013004.
Pubmed|| Crossref|| Others
- 18. Bacus, S., Spector, N.L., Yarden, Y. The era of ErbB-receptor-targeted therapies: advances toward personalized medicine. (2005) Per Med 2(4): 301-315.
- 19. Duarte, H.O., Bolmafia, M., Mereiter, S., et al. Gastric cancer cell glycosylation as a modulator of the ErbB2 oncogenic Receptor. (2017) Int J Mol Sci 18(11): 2262.
- 20. Wang, L.K., Hsiao, T.H., Hong, T.M., et al. MicroRNA-133a suppresses multiple oncogenic membrane receptors and cell invasion in non-small cell lung carcinoma. (2014) Plos one 9(5): 96765.
- 21. Rosenberg, S.A., Lotze, M.T., Muul, L.M. et al. Observation on the sysyemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 in patients with metastatic cancer. (1985) N Engl J Med 313(23): 1485-1492.
Pubmed|| Crossref|| Others
- 22. Forget, M.A., Haymakere, C., Hess, K.R., et al. Prospective analysis of adoptive TIL therapy in patients with metastatic melanoma: response, impact of anti-CTLA4, and biomarkers to predict clinical outcome. (2018) Clin Cancer Res 24(18): 4416-4428.
- 23. Olweny, C.L., Magrath, I., Ziegler, J.L., et al. Childhood Hodgkin’s disease in uganda: a ten-year experience. (1978) Cancer 42(2): 787-792.
- 24. Launa, F., Baccarani, M., Fiacchini, M., et al. Combination chemotherapy in stage I or II Hodgkin’s disease. (1979) Lancet 314(8151): 1072-1073.
- 25. Kato M, Sakuyama A, Matsutani T, Minato H. Efficacy of Trastuzumab therapy in HER2-positive early breast cancer patients in our clinic. (2015) Proceedings of BIT’s 8th Annual World Cancer Congress: 301.
Pubmed|| Crossref|| Others
- 26. Singh, H., Walker, A.J., Amiri-Kordestani, L., et al. US Food and Drug Administration Approval: Neratinib for extended adjuvant treatment of early stage HER2-positive breast cancer. (2018) Clin Cancer Res 24(15): 3486-3491.
- 27. Takeda, T., Yamaguchi, T., Yaegashi, N. perceptions and attitudes of Japanese gynecologic cancer patients to Kampo (Japanese herbal) medicines. (2012) Int J Clin Oncol 17(2): 143-149.
- 28. Ito, A., Munakata, K., Imazu, Y., et al. First nationwide attitude survey of Japanese physicians on the use of traditional Japanese medicine (Kampo) in cancer treatment. Evidence-Based Com Alternative Med: 8.
- 29. Zhu, G., Mische, S.E., Seigneres, B. Novel treatment of acute promyelocytic leukemia: As2O3, retinoic acid and retinoid pharmacology. (2013) Curr Phar Biotechnol, 14(9): 849-858.
Pubmed|| Crossref|| Others
- 30.Zhang, Q., Zhu, G. The pathological pattern of seven malignant cancers following Demethylcantharidin. (2017) Adv Pharma J 2(6): 243-247.
Pubmed|| Crossref|| Others
- 31. Zhu, G., Musumecci, F., Byrne, P., et al. Treatment of advanced hepayocelluar carcinoma(HCC) with the combined protocol of chemotherapy 5-fluorouracil and traditional medicine:report of ten cases. (2017) Clin Trials Pathol Case Stud 2(2): 61-65.
Pubmed|| Crossref|| Others
- 32. Zhu, G., Musumecci, F., Byrne, P., et al. Role of traditional herbal medicine in the treatment of advanced hepatocellular carcinoma(HCC: past and future ongoing. (2017) Adv P J 2(3): 115-120.
Pubmed|| Crossref|| Others
- 33. Zhu, G., Musumecci, F., Byrne, P., et al. A pilot study of lung cancer following chemotherapy and traditional medicine: report of 12 cases. (2017) Lungs and Breathing 1(3): 1-4.
Pubmed|| Crossref|| Others
- 34. Zhu, G., Musumecci, F., Byrne, P., et al. Clinical trials of lung cancer after chemotherapy and traditional medicine (12 cases). (2017) Adv Pharma J 2(5): 199-203.
Pubmed|| Crossref|| Others
- 35. Bonadonna, G., Lattuada, A., Monfardini, S., etal. Combined radiotherapy-chemotherapy in localized non-Hodgkin’s lymphomas:five-year results of a randomized study. (1979) In Adjuvant Therapy of Cancer II: 145-153.
Pubmed|| Crossref|| Others
- 36. Anderson, T., Bender, R.A., Fisher, R.I., et al. Combination chemotherapy in non-Hodgkin’s lymphoma: results of long-term follow up. (1977) Cancer Treat Rep 61(6): 1057-1066.
Pubmed|| Crossref|| Others
- 37. Skarin, A.T., Rosenthal, D.S., Moloney, W.C., et al. Combination chemotherapy of advanced non-Hodgkin’s lymphoma with bleomycin, Adriamycin, cyclophosphamide, vincristine, and prednisone (BACOP). (1977) Blood 49(5): 759-770.
Pubmed|| Crossref|| Others
- 38. Rodriguez, V., Cabanillas, F., Burgess, M.A., et al. Combination chemotherapy(‘CHOP-bleo’) in advanced (non-Hodgkin’s) malignant lymphoma. (1977) Blood 49(3): 325-333.
Pubmed|| Crossref|| Others
- 39. Schein, P.S., DeVita, V.T., Hubbard, S., et al. Bleomycin, Adriamycin, Cyclophosphamide, Vincristine, and Prednisone (BACOP) combination chemotherapy in the treatment of advanced diffuse histocytic lymphoma. (1976) Ann Intern Med 85(4): 417-422.
Pubmed|| Crossref|| Others
- 40. Case Jr, D.C. Combination chemotherapy of advanced diffuse non-Hodgkin’s lymphoma: results of cyclophosphamide, Adriamycin, vincristine, prednisone, and bleomycin (CHOP-bleo). (1979) J Maine Med Assoc 70(9): 348-352.
Pubmed|| Crossref|| Others
- 41. Wagner, A.D., Syn, N.L., Moehler, M., et al. Chemotherapy for advanced gastric cancer. (2017) Cochrane Database Syst Rev (8): CD004064.
- 42. Scartozzi, M., Galizia, E., Verdecchia, L., et al. Chemotherpay for advanced gastric cancer: across the years for a standard of care. (2007) Expert Opin Pharmacother 8(6): 797-808.
- 43. Orditura, M., Galizia, G., Sforza, V., et al. Treatment of gastric cancer. (2014) World J Gastroenterol 20(7): 1635-1649.
- 44. Liu, J.L., Ahme, S. Long term survival of patients with gastric cancer treated with adjuvant radio-chemotherapy: proposal of a prognostic index with implication for treatment modification. (2018) Oncol Res Rev 1(2): 1-5.
- 45. Skrypek, N., Vasseur, R., Vincent, A., et al. The oncogenic receptor ErbB2 modulates gemcitabine and irinotecin/ SN-38 chemoresistance of human pancreatic cancer cells via hCNT1 transporter and multidrug resistance associated protein MRP-2. (2015) Oncotarget 6(13): 10853-10867.
- 46. Meza-Junco, J., Au, H.J., Sawyer, M.B. Critical appraisal of trastuzumab in treatment of advanced stomach cancer. (2011) Cancer Manag Res 3: 57-64.
- 47. Fusco, N., Rocco, E.G., Del Conte, C., et al. HER2 in gastric cancer: a digital image analysis in pre- neoplastic, primary and metastatic lesions. (2013) Mol Pathol 26(6): 816-824.
- 48. Tanz, R., Mahfoud, T., Alami, E.I., et al. Is there any advantage from continuation of trastuzumab beyond progression in metastatic her positive gastric cancer? (2018) Hematol Med Oncol 3(2): 1-3.
- 49. Gabitova, L., Gorin, A., Astsaturov, I. Molecular pathways: sterols and receptor signaling in cancer. (2014) Clin Cancer Res 20(1): 28-34.
- 50. Shimizu, N., Kondo, I. Hyper production of EGF receptor in human A431 cell is regulated by a translocation chromosome. (1982) Cytog Cell Gen 32: 316-317.
Pubmed|| Crossref|| Others
- 51. Merlino, G.T., Xu, Y.H., Ishii, S., et al. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. (1984) Science 224(4647): 417-419.
- 52. Ullrich, A., Coussens, J., Hayflick, J.S., et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. (1984) Nature 309(5967): 418-425.
- 53. Miltra, S., Han, S., Soderstram, K., et al. Preferential expression of an oncogenic receptor in brain tumor stem cells: identification and targeting using an engineered antibody approach. (2009) Assoc Cancer Res 69(9): 72.
Pubmed|| Crossref|| Others
- 54. Hembrough, T., Thyparambil, S., Liao, W.L., et al. Quantitative multiplexed SRM analysis of oncogenic receptors in FFPE colorectal carcinoma tissue. (2012) Cancer Res 72(8): 5537.
- 55. Lee, J.C., Vivanco, I., Beroukhim, R., et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in extracellular domain. (2006) PLoS Med 3(12): 485.
- 56. Godin-Heymann, N., Bryant, I., Rivera, M.N., et al. Oncogenic activity of epidermal growth factor receptor kinase mutant alleles is enhanced by the T790M drug resistance mutation. (2007) Cancer Res 67(15): 7319-7326.
- 57. Konduri, K., Gallant, J.N., Chae, Y.K., et al. EGFR fusions as Novel Therapeutic Targets in Lung Cancer. (2016) Cancer Discov 6(6): 601-61.
- 58. Wu, Y.L., Kim, D.W., Felip, E., et al. Phase II safety and efficacy results of a single-arm ph ib/II study of capmatinib(INC 280) + gefitinib in patients(pts) with EGFR-mutated(mut),cMET-positive(cMET+) non-small cell lung cancer(NSCLC). (2016) ASCO.
Pubmed|| Crossref|| Others
- 59. Goss, G., Tsai, C.M., Shepherd, F.A., et al. CNS response to Osimertinib in patients with T790M-positive advanced NSCLC:pooled data from two phase II trials. (2018) Ann Oncol, 29(3): 687-693.
- 60. Shun, L., Tony, M., You, L., et al. Phase 3 study of first-line crizotinib vs pemetrexed/cisplatin or carboplatin (PCC) in East Asian patients(pts) with ALK+ advanced non-squamous nonsmall cell lung cancer(NSCLC). (2016) J Clin Oncol 34(15): 9058.
- 61. Shaw, A.T., Peters, S., Mok, T, et al. Alectinib versus Crizotinib in treatment-naive advanced ALK positive non-small cell lung cancer (NSCLC): primary results of the Global Phase III ALEX Study. (2017) J Clin On 35(18): 9008.
- 62. Serra, V., Vivancos, A., Puente, X.S., et al. Clinical response to a lapatinib-based therapy in a Li-Fraumeni syndrome patient with a novel HER2V659E mutation. (2013) Cancer Discov 3: 1238-1244.
Pubmed|| Crossref|| Others
- 63. Rodriguez, P.C., Rodriguez, C., Gonzalez, G., et al. Clinical development and perspective of CIMAvax EGF,Cuban vaccine for non-small-cell lung cancer therapy. (2010) MEDICC Rev 12:17-23.
Pubmed|| Crossref|| Others
- 64. Gonzalez, G., Crombet, T., Lage A. Chronic vaccination with a therapeutic EGF-based cancer vaccine: a review of patients receiving long lasting treatment. (2011) Curr Cancer Drug Targets 11:103-110.
Pubmed|| Crossref|| Others
- 65. de The, H., Marchio, A., Tiollais, P., Dejean, A. A novel steroid thyroid hormone receptor- related gene inappropriately expressed in human hepatocellular carcinoma. (1987) Nature 330: 667.
Pubmed|| Crossref|| Others
- 66. Marinelli, A., Bossi, D., Pellicci, P.G., et al. A redundant oncogenic potential of the retinoic acid receptor (RAR) alpha,beta and gamma isoforms in acute promyelocytic leukemia. (2007) Leukemia 21: 647-650.
- 67. Rietveld, L.E., Caldenhoven, E., Stunnenberg, H.G. Avian erythroleukemia: a model for corepressor function in cancer. (2001) Oncogene 20: 3100-3109.
Pubmed|| Crossref|| Others
- 68. Hauksdotti, H., Privalsky, M.L. DNA recognition by the aberrant retinoic acid receptors implicated in human acute promyelocytic leukemia. (2001) Cell Growth Differ 12: 85-98.
Pubmed|| Crossref|| Others
- 69. Osumi, T., Tsujimoto, S.I., Tamura, M., et al. Recurrent RARB-translocations in acute promyelocytic leukemia lacking RARA translocation. (2018) Cancer Res 78(16).
- 70. , E., Cervera, J., Valencia, A., et al. A novel NUP98/RARG fusion in acute myeloid leukemia resembling acute promyelocytic leukemia. (2011) Blood 117(1): 242-245.
- 71. Such, E., Cordon, L., Sempere, A., et al. In vitro all-trans retinoic acid sensitivity of acute myeloid leukemia blasts with NUP98/RARG fusion gene. (2014) Ann Hematol 93(11): 1931-1933.
- 72. Ha, J.S., Do, Y.R., Ki, C.S., et al. Identification of a novel PML-RARG fusion in acute promyelocytic leukemia. (2017) Leukemia 31(9): 1992-1995.
- 73. Tomita, A., Kiyoi, H., Naoe T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide(As2O3) in acute promyelocytic leukemia. (2013) Int J Hematol 97(6): 717-725.
- 74. Grignani, F., Ferrucci, P., Testa, U., et al. The acute promyelocytic leukemia-specific PML-RARa fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. (1993) Cell 74(3): 423-431.
- 75. Rousselot, P., Hardas, B., Patel, A., et al. The PML-RARa gene product of the t (15;17) translocation inhibits retinoic acid-induced granulocytic differentiation and mediated transactivation in human myeloid cells. (1994) Oncogene 9(2): 545-551.
- 76. He, L.Z., Guidez, F., Tribioli, C., et al. Distinct interactions of PML-RARa and PLZF-RAR alpha with co-repressors determine differential response to RA in APL. (1998) Nat Genet 18(2): 126-135.
- 77. Kitareewan, S., Pitha-Rowe, I., Sekula, D., et al. UBEIL is a retinoid target that triggers PML/RARalpha degradation and apoptosis in acute promyelocytic leukemia. (2002) Proc Natl Acad Sci 99(6): 3806-3811.
- 78. Segalla, S., Rinaldi, L., Kalstrup-Nielsen, C., et al. Retinoic acid receptor alpha fusion to PML affects its transcriptional and chromatin-remodeling properties. (2003) Mol Cell Biol 23(23): 8795-8808.
Pubmed|| Crossref|| Others
- 79. Jing, Y. The PML-RARa fusion protein and target therapy for acute promyelocytic leukemia. (2004) Leuk Lymphoma 45(4): 639-648.
- 80. Carbone, R., Botrugn, O.A., Ronzoni, S., et al. Recruitment of the histone methyltransferase SUV39H1 and it’s the oncogenic properties of the leukemia-associated PML-retinoic acid receptor fusion protein. (2006) Mol Cell Bio 26(4): 1288-1296.
- 81. Nasr, R., Guillemin, M.C., Ferhi, O., etal. The eradication of acute promyelocytic leukemia- initiating cells through PML-RARA degradation. (2008) Nat Med 14(12): 1333-1342.
- 82. Marstrand, T.T. A conceptual framework for the identification of candidate drugs and drug targets in acute promyelocytic leukemia. (2010) Leukemia 24(7): 1265-1275.
- 83. Lallemand, B.V., Hugues, T. A new oncoprotein catabolism pathway. (2010) Blood 116: 2200-2201.
Pubmed|| Crossref|| Others
- 84. Rosen, M., Privalsky, M.L. Thyroid hormone receptor mutations in cancer and resistance to thyroid hormone: perspective and prognosis. (2011) J Thyroid Res.
- 85. Podhorecka, M., Macheta, A. Acute promyelocyticleukemia-modern approach to disease pathogenesis and differentiation treatment. (2013) Postepy Hig Med Dosw 67: 1083-1089.
Pubmed|| Crossref|| Others
- 86. Dos Santos, G.A., Kats, L., Pandolfi, P.P. Synergy against PML-RARa targeting transcription, proteolysis, differentiation, and self-renewal in acute promyelocytic leukemia. (2013) J Exp Med 210(13): 2793-2802.
- 87. Humbert, M. The tumor suppressor gene DAPK2 is induced by myeloid transcription factor pu.1 and c.EBPa during granulocytic differentiation but repressed by PML-RARa in APL. (2014) J Leuk Biol 95: 83-93.
Pubmed|| Crossref|| Others
- 88. De Braekeleer, E., Dout-Guilbert, N., De Braekeleer, M. RARA fusion genes in acute promyelocytic leukemia:A review. (2014) Expert Rev Hematol 7(3): 347-357.
- 89. Noguera, N.I., Piredda, M.L., Taulli, R., et al. PML/RARa inhibits PTEN expression in hematopoietic cells by competing with PU.1 transcriptional activity. (2016) Oncotarget 7(41): 66386-66397.
- 90. Chomienne, C., Ballerini, P., Balitrand, N., et al. All-trans retinoic acid in acute promyelocytic leukemia II.In vitro studies, structure-function relationship. (1990) Blood 76(9): 1710-1717.
- 91. Haferiach, T., Loffler, H., Glass, B., et al. Repeated complete remission in a patient with acute promyelocytic leukemia after treatment with 13-cis-retinoic acid first and with all-trans-retinoic acid in relapse. (1993) Clin Investig 71(10): 774-779.
- 92. Soignet, S.L., Benedetti, F., Fleischauer, A., et al. Clinical study of 9-cis retinoic acid (LGD) in acute promyelocytic leukemia. (1998) Leukemia 12: 1518-1521.
Pubmed|| Crossref|| Others
- 93. Tobita, T., Takeshita, A., Kitamura, K., et al. Treatment with a new synthetic retinoid, Am80, of acute promyelocytic leukemia relapsed from complete remission induced by all-trans retinoic acid. (1997) Blood 90(3): 967-973.
- 94. Takeuchi, M., Yano, T., Omoto, E., et al. Relapsed acute promyelocytic leukemia previously treated with ATRA: clinical experience with a new synthetic retinoid, Am80. (1998) Leuk lymphoma 31(5-6): 441-451.
- 95. Takeuchi, M., Yoshida, I., Takahashi, K. Long-term follow up of re-induction with a new synthetic retinoid, Am-80,for relapse of acute promyelocytic leukemia previously treated with all-trans retinoic acid: results of 7 cases from a single institute. (2003) Rinsho Ketsueki 44 (11): 1069-1073.
- 96. Takeshita, A., Shinagawa, K., Adachi, M., et al. Tamibarotene for the treatment of acute promyelocytic leukemia. (2014) Expert opinion orphan drugs 2(9): 961-969.
- 97. Naoe, T. Tamibarotene for the treatment of acute promyelocytic leukemia Hematology. (2014) 71(9): 96-69.
Pubmed|| Crossref|| Others
- 98. Shinagawa, K., Yanada, M., Sakura, T., et al. Tamibarotene as maintenance therapy for acute promyelocytic leukemia: results from a randomized controlled trial. (2014) J Clin Oncol 32(33): 3729-3735.
- 99. Sanford, D., Lo-coco, F., Sanz, M.A., et al. Tamibarotene in patients with acute promyelocytic leukemia relapsing after treatment with all-trans retinoic acid and arsenic trioxide. (2015) Br J Hematol 171(4): 471-477.
- 100. Wang, J.X., Mi, Y.C., Jiang, B., et al. Tamibarotene compared to all-trans retinoic acid (ATRA) as add-on to arsenic trioxide (ATO) in subjects with relapsed acute promyelocytic leukemia(APL). (2015) Blood.
Pubmed|| Crossref|| Others
- 101. Kojima, M., Ogiya, D., Ichiki, A., et al. Refractory acute promyelocytic leukemia successfully treated with combination therapy of arsenic trioxide and tamibarotene. (2016) Leukemia Res Rep 5: 11-13.
Pubmed|| Crossref|| Others
- 102. Asou, N. Retinoic acid, all-trans retinoic acid (ATRA) and Tamibarotene. (2017) Chemotherapy for leukemia: 183-211.
Pubmed|| Crossref|| Others