Long-Term Stability Study of Complex Darunavir: β-Cyclodextrin
Jaqueline Nakau Mendonça , Norberto Peporine Lopes , Selma Gutierrez Antonio , Hérida Regina Nunes Salgado
Affiliation
- 1Department of Pharmaceutics, School of Pharmaceutical Sciences of Araraquara, Univ Estadual Paulista - UNESP, Araraquara, São Paulo, Brazil
- 2Center for Research in Natural and Synthetic Products, Department of Physics and Chemistry, School of Pharmaceutical Sciences of Ribeirão Preto, Universidade de São Paulo, Ribeirão, Preto, Brazil
- 3Department of Physical Chemistry, Institute of Chemistry, Univ Estadual Paulista - UNESP, Araraquara, São Paulo, Brazil
Corresponding Author
Ana Carolina Kogawa, Postgraduate Program in Pharmaceutical Sciences, School of Pharmaceutical Sciences of Araraquara, UNESP, Road CEP 14801-902, Araraquara, SP, Brazil, Tel: +55 16 3301 4681; Fax: +55 16 3301 6967; E-mail: ac_kogawa@yahoo.com.br
Citation
Kogawa, A.C., et al. Long-Term Stability Study of Complex Darunavir: β-Cyclodextrin. (2016) J Pharm Pharmaceutics 3(1): 35-39.
Copy rights
© 2016 Kogawa, A.C. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
β-cyclodextrin; Darunavir; Degradation products; LC-MS; Long-term stability
Abstract
Darunavir, a protease inhibitor most widely used in the treatment of HIV infection, was complexed with β-cyclodextrin due to the low solubility in water and its poor bioavailability. This research describes the study the long-term stability of the complex darunavir: β-cyclodextrin which was kept in a climatic chamber for 24 months at 30°C ± 2°C and 75 % UR ± 5 %. The samples were analysed using LC-MS, 250 mm × 4.6 mm CN Luna column, water + 0.1 % glacial acetic acid: acetonitrile + 0.1 % glacial acetic acid 60:40 (v/v) as mobile phase, flow rate of 1.0 mL min-1, UV detection at 268 nm and ambient room temperature (25°C) before the start of study and thereafter 3, 6, 9, 12, 18 and 24 months. The data obtained associated with the infrared, TG, DSC and X-ray diffraction analysis was sufficient to study the behavior of complex darunavir: β-cyclodextrin. The results of this research indicate that the stability of the complex darunavir: β-cyclodextrin is high under conditions associated of temperature and humidity.
Introduction
Darunavir is a protease inhibitor used in the treatment of Human Immunodeficiency Virus (HIV) infection. It is a pillar of therapy cocktail for patients with the virus[1]. Like most new drugs it presents low water solubility and poor bioavailability, leading to poor absorption in the gastrointestinal tract[2]. These new drugs require frequent administration at relatively high doses, being the major cause of non-adherence to treatment and an obstacle for compliance of pharmacotherapy[3]. For this reason the complexation of darunavir to β-ciclodextrina was performed.
The interactions of drugs with cyclodextrin is an important feature in the pharmaceutical field since these systems can modifying their chemical stability as well as other properties such as solubility, dissolution rate and bioavailability[4]. β-cyclodextrins are the most used for the development of pharmaceuticals, particularly because of their complexing properties, which provide increased solubility and consequent increased dissolution rate of poorly soluble drugs[5].
There is no long-term stability method described in the literature for complex darunavir: β-cyclodextrin. Its behavior under conditions of temperature and humidity are unknown and, therefore, the development of a formulation and study of this new presentation becomes blind.
Stability is a required quality for all drugs and consists of the period from the date of manufacture and packaging of the formulation until their chemical and biological activity is not less a predetermined level of labeled content and its physical properties have not changed appreciably and deleterious fashion[6].
The study of the stability of drugs and medicines is mandated by regulatory agencies throughout the world. The parameters used in the study of accelerated and long-term stability are indicated by the ICH[7], the guide to conducting stability studies of ANVISA[8] and by the guidance of the World Health Organization[9].
The stability of pharmaceuticals depends on environmental factors such as temperature, humidity and light, factors related to the product itself and factors related to packaging materials[8].
Monitoring the stability of drugs is one of the most effective methods for evaluation, prediction and prevention of problems related to the quality of the product during the validity.
The long-term stability study checks the physical, chemical, biological and microbiological characteristics of a pharmaceutical product during and, optionally, after the expiration date expected[8].
This research describes the long-term stability study of complex darunavir: β-cyclodextrin maintained in a climatic chamber for 24 months at 30°C ± 2°C and 75 % UR ± 5 %. Analyzes were performed by LC and LC-MS at times 0, 3, 6, 9, 12, 18 and 24 months to evaluate the content of darunavir and appearance of degradation products. The time 0 and 24 months were also compared through the infrared, TG, DSC and X-ray diffraction techniques.
Experimental
Instrumentation and reagents
Equipments: The samples were degraded in a climatic chamber (Marconi™). LC analysis was performed on a Waters™ LC system equipped with Waters 1525 binary gradient chromatography pump, Rheodyne Breeze 7725i manual injector and Waters 2487 UV-Vis detector, Phenomenex™ CN Luna (250 mm × 4.6 mm, 5.0 μm particle size) column. LC-MS analysis was performed on Shimazdu™ HPLC system connected to AmaZon SL Bruker™ ion trap mass spectrometer operating in positive ion electrospray ionization mode. Analytical balance model 410 Kern™, ultrasonic bath Ultrasonic Cleaner Unique™, water purification system Millipore™ and membranes of polytetrafluoroethylene (PTFE) hydrophilic with pore 0.45 μm and diameter 47.0 mm were used.
In IR analysis was used spectrophotometer IR Prestige- 21 Shimadzu™. The TG curves were obtained from the SDT Q600 V8.3 Build 101 and the DSC curves with heat flow were obtained from the DSC Q100 TA Instruments™. In the X-ray diffraction was used Rigaku rotating anode RINT2000 equipment, with curved graphite monochromator in the diffracted beam, divergence slit and scattering of 0.25°, receiving slit of 0.3 mm and Soller slit of 2.5° of divergence in CuKα radiation (λ = 1.5406 Å).
Chemicals and reagents: The chemical used were HPLC grade acetonitrile (J.T.Baker™), HPLC grade glacial acetic acid (Synth™), analytical reagent grade ethyl alcohol (Synth™), deionised Milli Q water (Millipore™). The complex darunavir: β-cyclodextrin in powder[9] developed by our research group was used. Mobile phase was prepared by mixing water + 0.1 % glacial acetic acid and acetonitrile + 0.1 % glacial acetic acid in the ratio 60:40 (v/v) filtered through 0.45 μm membrane filter. Diluent was ethyl alcohol. A stock standard solution equivalent to 1000 μg mL-1 darunavir was prepared by dissolving an accurately weighed amount of pure drug in the diluents.
Materials and Methods
Long-term stability study protocol
To study long-term stability of complex darunavir: β-ciclodextrin in powder was used climatic chamber 835/UR MA (Marconi™) with controlled temperature of 30ºC ± 2ºC and 75% UR ± 5% UR. In times of 3, 6, 9, 12, 18 and 24 months, samples were taken to assess the behavior of the drug to complexed β-cyclodextrin. Identify possible degradation products by LC-MS and evaluate the content of darunavir by LC. The chromatograms were compared together and with an analysis of time zero (drug was prepared and immediately analyzed) with the objective of monitoring and evaluation of degradation.
Sample: A quantity of complex darunavir: β-cyclodextrin equivalent to 10 mg of darunavir was weighed accurately into a 10 mL calibrated flask; 5 mL of diluents solution was added and sonicated for 10 min to complete dissolution of the darunavir; then the mixture was diluted to the mark with the diluents to obtain a concentration of 1000 μg mL-1. 1 ml of this solution was diluted in ethyl alcohol into a 25 mL calibrated flask to obtain a concentration of 40 μg mL-1. A portion of the resulting mixture was withdrawn and filtered through a 0.45 μm filter to ensure the absence of particulate matter. Thus, the filtrate was injection onto the column.
Chromatographic conditions: Chromatographic analysis was carried out at ambient temperature (25°C) on a Phenomenex™ CN Luna (250 mm × 4.6 mm, 5.0 μm particle size) column. The mobile phase was a mixture of water + 0.1 % glacial acetic acid and acetonitrile + 0.1 % glacial acetic acid (60:40, v/v). Flow rate was 1.0 mL min-1. The detector wavelength was set at 268 nm with injection volume at 20 μL. The same conditions were used to LC-MS, but without addition of acid in mobile phase. Mass spectrometry conditions: Before samples were injected into the LC-MS they were applied to preparative plate thin layer chromatography (TLC) to remove the β-cyclodextrin, using purified water and methanol 70:30 (v/v) adjusted to pH 2.4 with glacial acetic acid as mobile phase. Mass spectral analyses were performed on AmaZon SL (Bruker™) provided with ESI ion source and ion trap mass analyzer. Analyses were carried out using nitrogen as nebulizing (60 psi) and drying gas (10 L min-1, 320ºC). The capillary high voltage was set to 3500 V.
Infrared conditions: In analytical balance model H51 Mettler Toledo™ were weighed 2.5 mg of sample diluted in potassium bromide, previously dried to constant weight in an oven Nova Ética™, to form pellets of 150 mg each. The analyzes and comparisons of the spectra were carried out in transmittance.
Conditions of thermal analysis: 1 to 3 mg of sample was weighed and placed in an alumina crucible. The temperature range used in the TG was 30 to 600ºC and DSC was 25 to 150°C, both under a dynamic nitrogen atmosphere (flow rate 100 ml/min) and a heating rate of 2°C/min.
Conditions of X-ray diffraction: The X-ray diffractometer operated at 40 KV of voltage and 70 mA of tube current and. The samples were placed on a sample port of 2 cm diameter and 1 mm depth.
Results
Degradation products
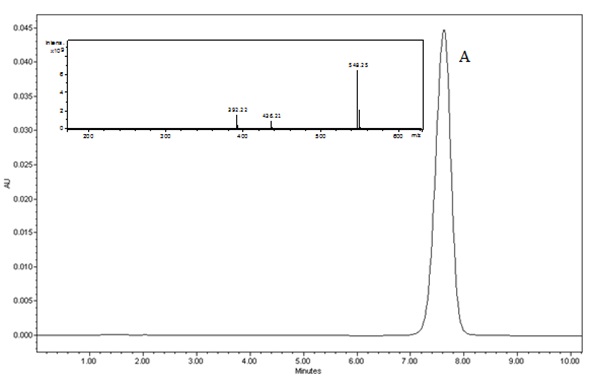
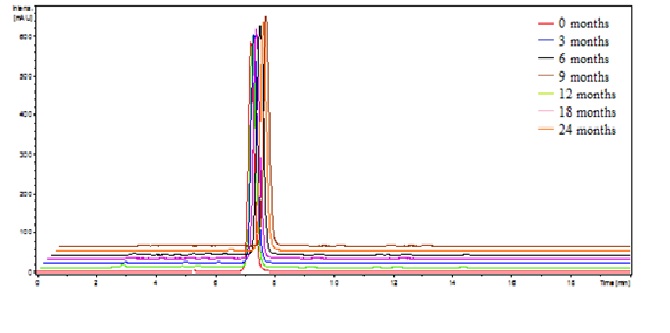
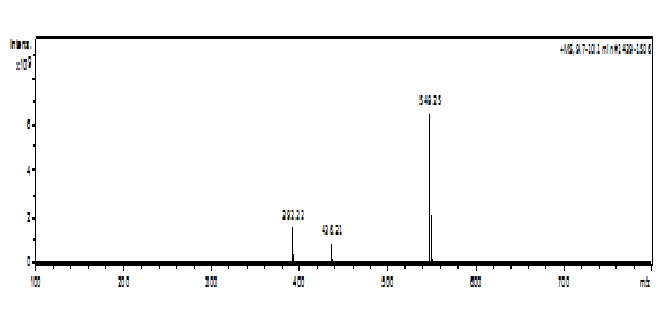
The chromatograms of darunavir complexed obtained at zero time, ie before being placed in a climatic chamber showed retention time of 7.2 minutes. (Figure 1) illustrates the chromatogram of complexed drug at time zero.(Figure 2) shows the overlap of the complex chromatograms at times 3, 6, 9, 12, 18 and 24 months and (Figure 3) shows signal of the mass spectra obtained in the chromatograms in these times.
Figure 1:
Figure 2:
Figure 3:
Analysis with degraded complex darunavir: β-cyclodextrin 24 months in climatic chamber
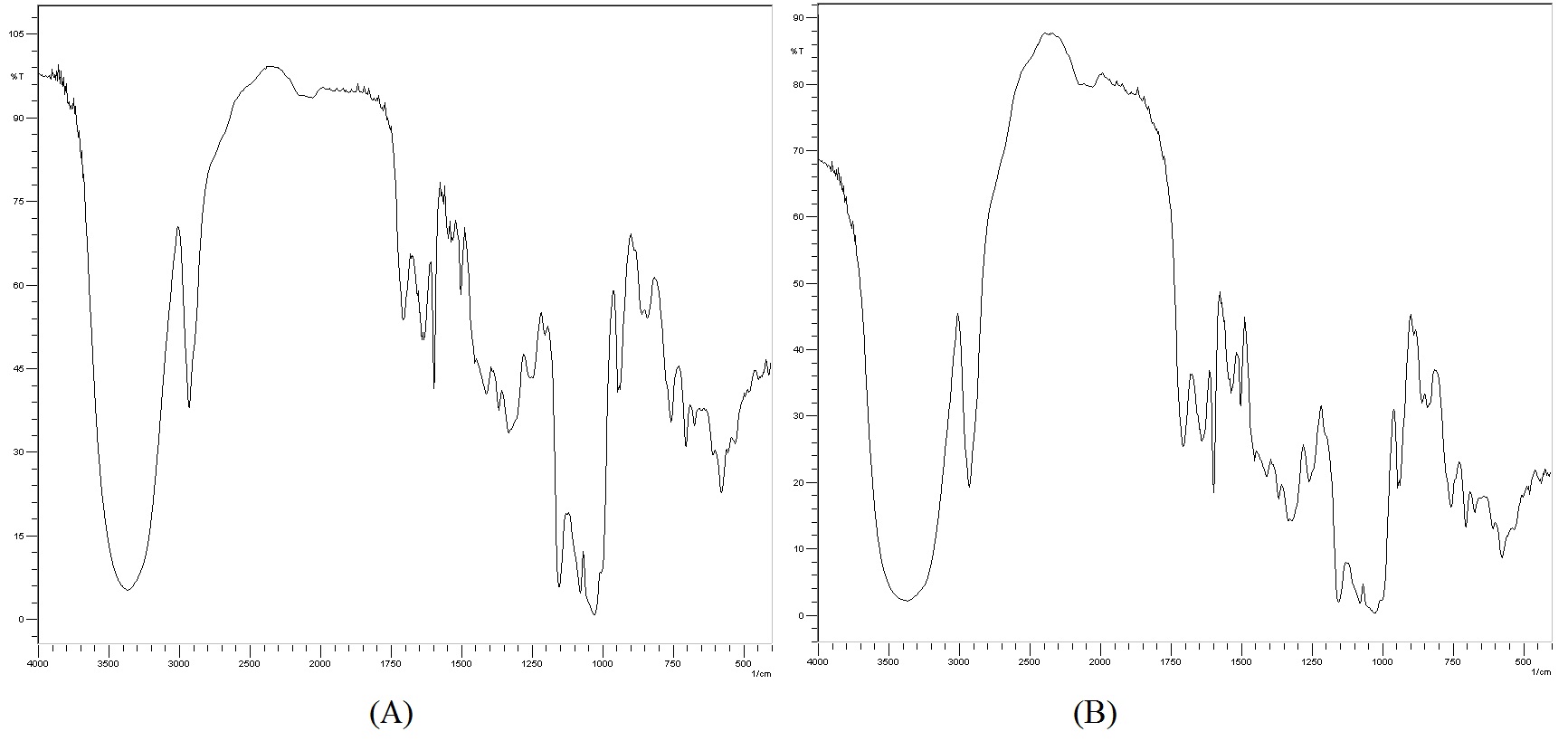
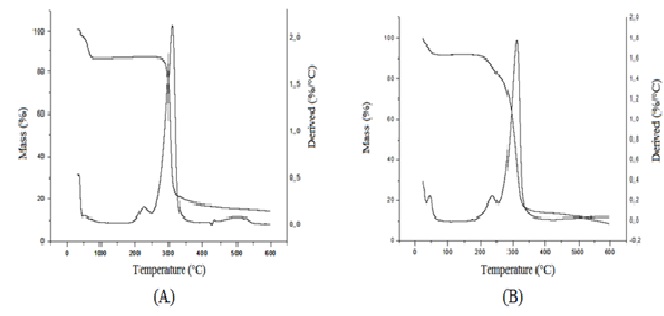
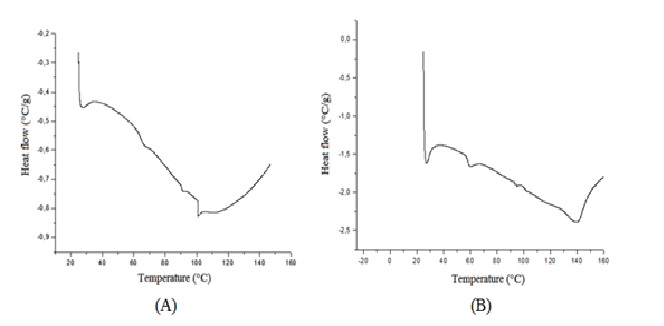
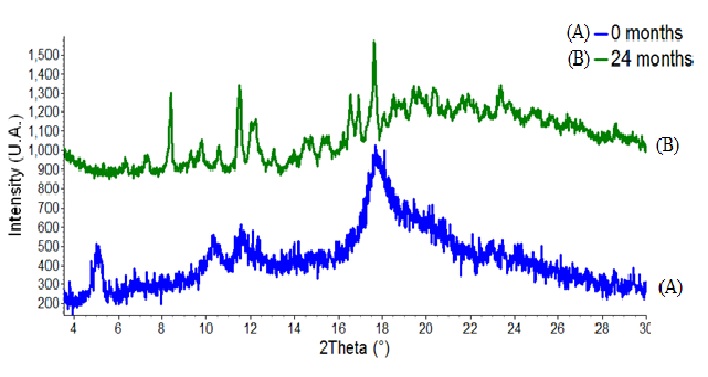
After 24 months in climate chamber the complex darunavir: β-cyclodextrin in powder was analyzed by techniques of infrared, TG, DSC and X-ray diffraction and its results were compared with zero time analyzes (no degradation), according to ( Figures 4 to7).
Figure 4:
Figure 5:
Figure 6:
Figure 7:
Discussion
Stability is defined as the ability of the drug or pharmaceutical product to remain within the specifications established for maintaining its identity, potency, purity and quality during the entire retest period or expiration date[7,10,11]. Stability is an essential factor of quality, safety and efficacy of the pharmaceutical product. A pharmaceutical product that is not stable enough, can result in physical changes, which can affect the appearance, melting point, clarity and color of solution, loss or absorption of water, the crystal modification (polymorphism) or particle size etc. as well as changes in chemical properties, which can be observed as an increase in degradation products or the formation of substances at high risk due to the decomposition potentially toxic to patients, or the reduction of the content due to hydrolysis, oxidation, isomerization, decarboxylation[10,12]. The complex in powder when subjected to heat combined with the humidity of the climate chamber (30°C ± 2°C and 75 % RH ± 5 % RH) after 9 months, presented physical change. The color of the powder previously white or almost white passed to slightly yellowish color. The darunavir content, calculated by peak area were not exactly the same in all the degradation times. There was little variation among them. However, it was not detected the appearance of new peaks in the chromatogram. But the no exact mass balance could be due to the early or late eluting impurities, absence of appropriate chromophore, difference to UV response for active and impurities, presence of volatile degradation products or complex degradation pathways that provide a large number of low-level impurities[13]. After 24 months in the climatic chamber the only sign still observed in the chromatogram was darunavir with m/z 548, as demonstrated by the mass spectrum. Stability is defined as the time during which the medicinal product or even the raw material considered in isolation, remains within the specified limits and throughout the period of storage and use, the same conditions and characteristics it had when the time of its manufacture. It may also be defined as the time between the moment in which the product is being manufactured to that its power is reduced to not more than 10 %, provided that the alteration products are all safely recognized previously and identified their effects[14].
Thus, the IC under conditions of 30 ± 2°C and 75 % RH ± 5 % RH after 24 months is stable in powder form. For future pharmaceutical form new stability tests for the finished product would be needed. The resources of pharmaceutical technology will be required to protect the powder of the physical change, for the finished product of darunavir complexed not present color difference within 2 years or more. Another proof of the stability of the complex after the climatic chamber conditions, are the results of infrared, TG, DSC and X-ray diffraction performed on the sample submitted after 24 months at 30 ± 2°C and 75 % RH ± 5 % UR. The results are similar to those obtained at time zero analysis, without any degradation. In infrared analysis, the inclusion complex presents a broad band between 3000 and 3750 cm-1 as β-CD, but it keeps all the characteristics bands of darunavir (primary amine, alcohol, alkanes, benzene ring, ester, amide and sulphoxide secondary), proving that the drug is present in the complex[9,15]. The TG curve exhibits two mass loss events, the first at 50°C and the second at 300°C. In DSC still no thermal event can be observed, which should be due to changes in the crystalline solid form or by complexation with β-cyclodextrin[9]. In the analysis of X-ray diffraction the darunavir: β-cyclodextrin complex zero time is semi-crystalline, in which we can observe some broad peaks indicating a small average size of crystalline and the characteristic halo of amorphous. In the darunavir: β-cyclodextrin complex 24 months showed an increase in crystallinity with the definition of some peaks of X-ray diffraction but also with semi-crystalline aspect. The peaks could not be attributed to the drug or cyclodextrin or indicating that darunavir: β-cyclodextrin complex is more crystalline in 24 months.
Conclusion
The study of the long-term stability of the complex darunavir: β-cyclodextrin in powder was possible through the techniques of HPLC and LC-MS. Infrared, TG, DSC and X-ray diffraction analysis confirmed the stability of the complex after 24 months at 30 ± 2°C and 75 % RH ± 5 % UR in climatic chamber.
Acknowledgment
The authors acknowledge CNPq (Brasília, Brazil), FAPESP (São Paulo, Brasil), CAPES (São Paulo, Brasil) and PADC/FCF/UNESP (Araraquara, Brazil).
Conflict of Interest:
The authors report no conflict of interest.
References
- 1. Reddy, B.V., Jyothi, G., Reddy, B.S., et al. Stability-indicating HPLC method for the determination of darunavir ethanolate. (2013) J Chromatogr Sci 51(5): 471-476.
- 2. Sinha, S., Ali, M., Baboota, S., et al. Solid dispersion as an approach for bioavailability enhancement of poorly water-soluble drug ritonavir. (2010) AAPS Pharm Sci Tech 11(2): 518-527.
- 3. Sosnik, A., Chiappetta, D.A., Carcaboso, A.M. Drug delivery systems in HIV pharmacotherapy: What has been done and the challenges standing ahead. (2009) J Control Release 138(1): 2-15.
- 4. Valero, M., Pérez-Revuelta, B.I., Rodríguez, L.J. Effect of PVP K-25 on the formation of the naproxen:β-cyclodextrin complex. (2003) Int J Pharm 253(1-2): 97-110.
- 5. Lyra, M.A.M., Alves, L.D.S., Fontes, D.A.F., et al. Ferramentas analíticas aplicadas à caracterização de complexos de inclusão fármaco-ciclodextrina. (2010) Rev Ciênc Farm Básica Apl 31(2): 117-124.
- 6. Vadas, E.B. stability of pharmaceuticals. (2000) In the science and practice of pharmacy.
- 7. Food and Drug Administration, H.H.S. International Conference on Harmonisation; Stability data package for registration applications in climatic zones III and IV; Stability testing of new drug substances and products. (2003) Fed Regist 68(225): 65717-65718.
- 8. Brasil. RE nº 01, de 29 de julho de 2005. Guia para a realização de estudos de estabilidade. (2005) Brasilia: Agência Nacional de Vigilância Sanitaria.
- 9. Kogawa, A.C., Zoppi, A., Quevedo, M.A., et al. Complexation between darunavir ethanolate and β-cyclodextrin experimental and theoretical studies. (2014) WJPPS 3(6): 298-309.
- 10. Stability testing of active pharmaceutical ingredients and finished pharmaceutical products. (2009) WHO Technical Report Series Annex 2: 953.
- 11. Guidance for industry, Stability testing of drug substances and drug products. (1998) Food and Drug Administration.
- 12. Markens, U. Conducting stability studies-recent changes to climatic zone IV. Pharma Times (2009).
- 13. Kogawa, A.C., Salgado, H.R.N. Impurities and forced degradation studies: a review. (2016) Curr Pharm Anal 12(1): 18-24.
- 14. Silva, K.E.R., Alves, L.D.S., Soares, M.F.R., et al. Models for assessing the stability of drugs and medicines for the pharmaceutical industry. (2009) Rev Farm Ciênc Basic Appl 30(2): 129-135.
- 15. Kogawa, A.C., Salgado, H.R.N. Development and validation of infrared spectroscopy method for the determination of darunavir in tablets. (2013) Physical Chemistry 3(1): 1-6.