Major clinical application of adipose derived stem cells
Sanjay Gottipamula, Kumar Chokalingam
Affiliation
Sri Research for Tissue Engineering Pvt. Ltd, Sri Shankara Research Centre, Rangadore Memorial Hospital, Bangalore, India.
Corresponding Author
Sridhar, K.N. M.B.B.S, L.R.C.P (London), M.R.C.S (England), F.R.C.S. (Ireland, Edinburg, England, Canada), F.R.C.P.S. (Glasgow), Sri Research for Tissue Engineering Pvt. Ltd, Sri Shankara Research Centre, Rangadore Memorial Hospital, Bangalore, India, Tel: +91-080-41076759; Email: knsridhar@sr-te.com
Citation
Sridhar K.N., et al. Major Clinical Application of Adipose-Derived Stromal Cells. (2018) J Stem Cell Regen Biol 4(1): 4- 19.
Copy rights
© 2018 Sridhar K.N. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License
Keywords
Adipose-derived stromal cells; Cell therapy; Clinical translation; Ischemia; Sclerosis; Crohn and Osteoarthritis based indications
Abstract
In recent years, Adipose-Derived Stromal Cells (ADSCs) have emerged as a potent source of therapeutic cells for the treatment of various clinical indications. This review focusses on ADSCs as a product and the current clinical trial landscape of ADSCs. In-depth emphasis has been given for the clinical and conceptual framework of ADSCs to treat ischemic based indications such as Inflammatory Bowel Dieseases, Cerebrovascular Diseases, Chronic Obstructive Pulmonary Disease, sclerosis based indications such as Amyotrophic Lateral Sclerosis, Multiple Sclerosis, Systemic Sclerosis, Crohn based Fistula, and Osteoarthritis.
Introduction
Drugs modulate the human system to get cure from the diseases, but they rarely have the capacity to regenerate or restore the entire tissues. Many pharmaceutical drugs generally address only the relief from debilitating symptoms, but not the root cause of disease. Even when drugs are designed to address the root cause, their efficacy rate is not satisfactory. For example the efficacy rate of drugs ranges from 30% to 62 % in diseases like Alzheimer’s, Cardiac disease, Diabetes, Oncology, Osteoporosis, Rheumatoid arthritis, Schizophrenia etc[1]. Furthermore, for new drugs, one in five has a chance of causing serious Adverse Drug Reactions (ADRs) and these reactions rank as the fourth main cause of death[2-4]. Only 11 % to 15 % of newly approved drugs have a clinical advantage over existing prescription. Hence there is a need for alternatives to pharmaceutical drugs with better safety and efficacy.
One such alternative is cellular therapy which has the capacity to regenerate or restore the entire tissues. While drugs address a single target or disease-causing mechanism, cells by integrating multiple inputs and by acting through multiple, coordinated signals can regenerate or restore the entire tissues. Cells exert therapeutic effects because of their ability to hone to the site of injury, of their secretory activity, of their ability to differentiate into the required needed tissues and are safe. These properties of cells give them the ability to treat diseases which were previously untreatable. Hence attention is currently devoted to cell therapy to leverage its natural self-renewal and regenerative potential to develop as cell biologics.
Adipose derived uncultured cells as Stromal Vascular Fraction (SVF) and purified and expanded cells as Adipose-Derived Stromal Cells (ADSCs) are rapidly evolving in the field of regenerative medicine. Adipose tissue can be harvested easily with minimal discomfort to patients as opposed to bone marrow and with very few ethical concerns[5]. The therapeutic mechanism of ADSCs lies in adjusting its functionality based on the microenvironment of diseased tissue, to repair, replace and regenerate for a targeted outcome. ADSCs have a tremendous self-renewal capacity and also secrete cytokines, exosomes and extracellular vesicles that aid in regeneration process[6-8]. ADSCs demonstrate multi-lineage differentiation capabilities in both in-vitro and modules[9-11]. ADSCs also exhibit powerful hypoimmunogenic and immunomodulatory properties, suppress T cell proliferation in mixed lymphocyte reactions[12]. This regenerative and immunotolerant profile of ADSCs make them an ideal candidate for various clinical applications.
This review is focussed on using cultured ADSCs to address 4 major disease indications- Ischemia, Sclerosis, Fistula and Osteoarthritis where ADSCs can provide significant therapeutic benefits than traditional drugs. This review identifies the major gap and challenges in classical pharmaceutical drugs in clinics for these indications and emerging opportunity for the use of culture expanded ADSCs.
ADSCs as a product
Fat is an extremely promising rich source of stem cells. Since a small volume of liposuction is required for ADSCs isolation and expansion, it is relatively easy to perform without any complications compared to bone marrow aspiration. Various liposuction procedures like surgical resection, Power-Assisted Liposuction (PAL) and Laser-Assisted Liposuction (LAL) methodologies can be employed for harvesting adipose tissue. PAL provides higher yield, viability, proliferation ability, stable expression and prolonged culture of ADSCs compared to others[13,14]. The ultrasound assisted liposuction procedures also provide similar ADSCs viability and yield without compromising on differentiation potential[15]. The cost of PAL equipment is prohibitive for it to be used for adipose tissue harvesting in developing nations. Research is ongoing for the development of semi-automated or syringe based micro-lipoaspiration procedures for world-wide usability for small volume adipose tissue harvesting.
There are no standard isolation procedures and clear unified phenotypic marker to identify the therapeutic ADSCs from adipose tissue. Various enzymatic and non-enzymatic approaches have been utilized. The most common enzymes used for the adipose tissue digestion are collagenase, trypsin or dispase[16]. Various enzymes, enzyme concentration, number of washing steps, centrifugation parameters, erythrocyte removal steps and incubation time period are utilized for the isolation of ADSCs. Enzyme digestion increases the cost and presents the risk of residuals in finished products. Non-enzymatic methodologies have shown high variability in cell yield and viability because of shear force, centrifugal, and pressure force[17]. The manual enzymatic or non-enzymatic isolation procedures are tedious, non-scalable and prone to human error and contamination risk.
The automated and semi-automated whole cell isolation system were primarily designed for autologous fat grafting and later upgraded to isolation of cells from adipose tissue. There are several patented commercially available cell isolation systems that automate the SVF isolation process like AdiStem™ Sepax 2, Cellthera Kit I, Celution® 800 / CRS and 820 / CRS, GID SVF-1™ CHA STATION™ Icellator. These systems have a process time of around 65 to 120 mins[18-19]. The SVF can be used as point-of-care cellular therapeutics and as potential to be used as a bio-surgical adjuvant.
From the SVF, the ADSCs are allowed to attach onto tissue culture flasks. The ADSCs are sequentially cultured in tissue culture flasks or in spinner flasks /bioreactors using micro carriers to get the required clinical dose[20-22]. The feasibility of expanding ADSCs in controlled environment is clinically relevant to get consistent therapeutic effects and to maintain cGMP compliant production system.
While SVF from lipoaspirated fat have been utilized as a therapeutic product in various diseases, cultured ADSCs are superior because of the reasons hence forth. The cellular subunit within the SVF depends on donor characteristics and the site of aspiration varies from donor to donor and also from the site to site of the aspiration likely resulting in variable clinical outcome. Scale-up of purified, cultured, homogenous ADSCs from SVF generates quality tested, validated safe and efficacious products. Inability of obtaining sufficient lipoaspirate compromises on the cell numbers per dose in SVF especially in lean donors. Further for multiple doses, repeat lipoaspirates are likely required. Expanded ADSCs allows for constant cell dose availability and requires only single small volume lipoaspirate even for repeat doses. Cultured ADSCs can be primed to increase the intended therapeutic efficacy. For example in-vivo chondrogenic potential can be improved through hypoxic preconditioning of ADSCs, which is not possible in SVF[23]. Cryopreserved SVF for repeat doses, has significantly reduced stability and post-thaw proliferation efficiency. Expanded ADSCs can be cryopreserved which maintains stability and viability. Pure population of ADSCs allow us to perform controlled in-vitro and pre-clinical safety, mechanistic and efficacy studies in support of therapeutic in-vivo applications[24-25]. Variation in SVF poses limitation for such studies. Expanded ADSCs generates off-the-shelf allogeneic products, while still allowing for autologous procedures. Multiple expanded ADSCs can be screened to select one with maximum therapeutic potential. SVF cannot be used for allogenic purpose.
The screening and selection framework to find therapeutic ADSCs is similar to screening and selection of therapeutic candidates in biopharmaceutical sector[26,27]. Hence ADSCs can easily adopt the industrial scale-up and commercialization techniques from biopharmaceutical sectors. Expanded ADSCs can be used in various tissue engineered medicinal products. In addition, ADSCs / differentiated ADSCs can be used as in-vitro toxicological platforms for safety / drug testing[28]. On the other hand, expanded ADSCs can be used to produce products similar to Progenza (allogeneic ADSCs from Regeneous). The process of generating similar cell therapeutics allows shortening of clinical testing phase, time and investment.
Globally, Mesenchymal Stem Cells (MSCs) have been used as approved products as well as in several clinical trials. The approved products include Autologous modalities such as CupiStem (ADSCs), Hearticellgram AMI (BM - MSCs), CardioRel (BM - MNCs) and Allogeneic modalities like CARTISTEM (UB - MSCs), Prochymal (BM - MSCs), AlloStem (BM - MSCs + Bone Matrix), Trinity Evolution (MSCs + Bone matrix). Other MSCs based products are in near approval and some are in late stage clinical trials[29]. The above standard MSCs based products provide a clear road-map for the development and commercialization of ADSCs based products.
Landscape of ADSCs clinical trials
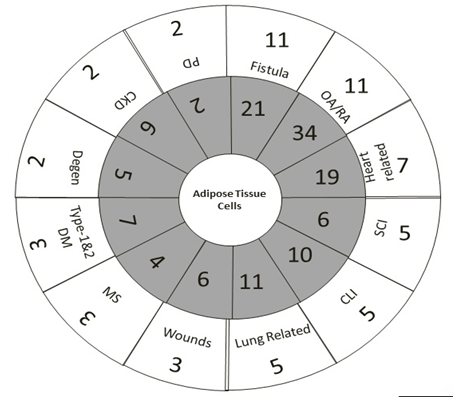
Search on Clinicaltrials.gov reveals that SVF and ADSCs have been used in 253 registered trials. Among the 253 trials, only 64 trials (25 %) were completed. The 61 (24.01 %) clinical trials are industry sponsored trials and rest are non-industry sponsored trials. The 11 trials (4.34 %) were withdrawn or terminated for unknown reasons. Active clinical trials are 136 in number (53.75 %) and the remaining 42 clinical trials (16.6 %) are of unknown status. The major clinical indications of ADSCs used by industry and non-industry group was plotted in [Figure. 1] and the status of industry sponsored trial for development of adipose based product in clinical trials was tabulated in [Table.1] Non-industry sponsors like clinics, academics, hospitals, university hospitals contributed to the majority stake (82) in using ADSCs for clinical translation compared to industry-sponsored trials (26 firms). The majority of the clinical trials were ischemic based indications (Inflammatory bowel diseases, cerebrovascular diseases, chronic obstructive pulmonary disease), sclerosis based indications (Amytrolateral sclerosis, multiple sclerosis, systemic sclerosis), Crohn based fistula, Osteoarthritis. Here we have attempted to review clinical applications of adipose cell-based therapy for these indications based on rationale, and conceptual approach.
Figure 1: Various clinical trial indications based on industry priority scale from 5 min to 60 min clock. Inner circle indicates a total number of clinical trials of particular indications, whereas outer circle provides industry sponsored clinical trials for particular indications.
Abbreviations: CKD-Chronic Kidney Disease; CLI-Critical Limb Ischemia; Degen-Degenerative Diseases; MS-Multiple Sclerosis; OA: Osteoarthritis; D-Pulmonary Disease; RA-Rheumatoid Arthritis; SCI-Spinal Cord Injuries.
Table 1: Industry sponsored clinical trials using adipose-based cells as biologics. Abbreviations: ADSCs-Adipose Derived Stromal Cells; Allo-Allogeneic; AMI-Acute Myocardial Infarction; Auto-Autologous; AZ-Alzheimer’s Disease; CLI-Critical Limb Ischemia; COPD-Chronic Obstructive Pulmonary Disease; DM-Diabetes Mellitus; ED-Erectile Dysfunction; MS-Multiple Sclerosis; OA-Osteoarthritis; PD-Parkinson’s Disease; SCI-Spinal Cord Injury; SVF-Stromal Vascular Fractions; P1-Phase 1; P2-Phase2; NA-Not Available; UK-Status Unknown;
| SL | Company Name | Biologicals | Indications | Phase |
|---|---|---|---|---|
| 1 | AdiSave Inc. | ADSc-SVF | Abnormally Healing Wounds | Scars | Soft Tissue Defects | P1 |
| 2 | Adistem Ltd | Auto-ADSCs (SVF) | Type-1 DM | P1 |
| Auto-ADSCs (SVF) | Type-2 DM | P1 | ||
| 3 | Ageless Regenerative Institute | Auto-AD-SVF | CLI, PD,ED, COPD, Type-2 DM, MS, AZ, OA | P1 |
| Auto-AD-SVF | FISTULA, STROKE, AMI | P1 | ||
| 4 | American Cryo Stem Corporation | Auto-ADSCs | MS | P1 |
| 5 | Anterogen Co., Ltd. | Auto-ADSCs(low dose) | Complex Perianal Fistula | P2 |
| ADIPOPLUS | Crohn's Fistula, Crohn;s Disease, Fistula | P2 | ||
| Allo-ADSCs | Crohn, Tennis Elbow | P1 | ||
| Auto-ADSCs(ANT-SM) | Fecal Incontinence | P1 | ||
| Auto-ADSCs | Perianal Fistula | Primary; Complex | P1 | ||
| Allo-ADSC-DFU | Burn | P1 | ||
| Allo-ADSC-DFU | Crohn's Disease | P1 | ||
| Allo-ADSC-DFU | Diabetic Foot Ulcer | P1 | ||
| Adipocell | Depressed Scar | P1 | ||
| Repaircell | Healthy | P2 / P3 | ||
| 6 | Antria | SVF | Lipoatrophy | Aging | Wrinkles | P1 |
| Liposuction | Soft Tissue Mass Removal | P1 | ||
| SVF | Facial Atrophy | P2 | ||
| 7 | Bioheart, Inc. | Auto-ADSCs | Dry Macular Degeneration | NA |
| Auto-ADSCs | Degenerative Disc Disease | NA | ||
| Auto-ADSCs | COPD | NA | ||
| 8 | Biostar | RNL-Vascostem® | Buerger's Disease | P1 / P2 |
| Auto-ADSCs | SCI | P1 | ||
| Auto-ADSCs | Degenerative Arthritis | P1 / P2 | ||
| Auto-ADSCs | Degenerative Arthritis | Knee OA | UK | ||
| Auto-ADSCs(JOINTSTEM) | Degenerative Arthritis | Knee OA | P2 | ||
| Auto-ADSCs | Progressive Hemifacial Atrophy | Romberg's Disease | P2 | ||
| Auto-ADSCs | SCI | P1 / P2 | ||
| Auto-ADSCs | Lumbar Intervertebral Disc Degeneration | P1 / P2 | ||
| Auto-ADSCs | CLI | P1 / P2 | ||
| Auto-ADSCs | Avascular Necrosis of the Femoral Head | P1 / P2 | ||
| 9 | Bukwang Pharmaceutical | Auto-ADSCs | SCI | P1 |
| 10 | Cellerix | Auto-ASCs (Cx401)+Fibrin addisive | Anal Fistula | P3 |
| Auto-ADSCs | Complex Perianal Fistula | Crohn Disease | P3 | ||
| Auto-ADSCs (Cx601) | Crohn's Disease | Anal Fistula | P1 / P2 | ||
| Auto-ASCs (Cx401)+Fibrin addisive | Complex Perianal Fistula | |||
| Non-surgical autologous implant of ASCs | Anal Fistula | P2 | ||
| 11 | Cellular Biomedicine Group Ltd | Auto-ADSCs ( ReJoinTM) | Drug: Sodium Hyaluronate | Defect of Articular Cartilage | Knee Osteoarthritis | P2 |
| 12 | Elliot Lander | Cell Surgical Network Inc. | SVF | Neurodegenerative Diseases | OA | ErectileDysfunction | AutoimmuneDiseases | Cardiomyopathies | Emphysema | |
| 13 | Ferrer Internacional S.A. | Histocell S.L. | Allo-ADSCs (FAB117-HC) | Acute Traumatic Spinal Cord Injury | P1 / P2 |
| 14 | Gwo Xi Stem Cell Applied Technology Co., Ltd. | Auto-ADSCs | Stroke | P1 |
| Auto-ADSCs | Liver Cirrhosis | P1 | ||
| 15 | Healeon Medical Inc(Device: Centricyte) | SVF | RSD (Reflex Sympathetic Dystrophy) | CRPS - Complex Regional Pain Syndrome Type I | Fibromyalgia | P1 / P2 |
| SVF | Inflammatory Bowel Diseases | P1 / P2 | ||
| SVF | Lung Disease | P1 / P2 | ||
| SVF | Multiple Sclerosis | Autoimmune | |||
| SVF | Hair Disease | P1 / P2 | ||
| 16 | InGeneron, Inc. | Auto-ADSCs | Rotator Cuff Tear - Partial Thickness | P1 |
| 17 | JKastrup | Rigshospitalet, Denmark | Allo-ADSCs | Heart Failure | P2 |
| Allo-ADSCs | Heart Failure | P1 | ||
| 18 | Kasiak Research Pvt. Ltd. | SVF; Auto-ADSCs | Idiopathic Pulmonary Fibrosis | P1 / P2 |
| SVF; Auto-ADSCs | CLI | P1 / P2 | ||
| 19 | Nature Cell Co. Ltd. | KCRN Research, LLC | Auto-ADSCs (JointStem); Drug: Synvisc-One | OA | P2 |
| 20 | StemGenex | SVF | Osteoarthritis | Uk |
| SVF | Rheumatoid Arthritis | |||
| SVF | Chronic Obstructive Pulmonary Disease | |||
| SVF | Multiple Sclerosis | |||
| SVF | Parkinson's Disease | |||
| 21 | Steminent Biotherapeutics Inc. | Auto-ADSCs | OA | P1 |
| 22 | TiGenix S.A.U. | Cellerix | Allo-ADSCs | Crohn's Disease | P3 |
| Allo-ADSCs | Sepsis | P1 | ||
| Allo-ADSCs | Localized Adverse Reaction to Administration of Drug | P1 | ||
| Allo-ADSCs | Rheumatoid Arthritis Aggravated | P1 / P2 | ||
| 23 | Tissue Genesis, LLC (Cell Iso system device) | Auto-ADSCs; Device: ASC coated ePTFE vascular graft | Device: Propaten graft | Lower Limb Ischemia | P1 |
| 24 | Translational Biosciences | SVF | Osteoarthritis | P1 |
| SVF | Rheumatoid Arthritis | P1 | ||
| 25 | Tri Phuoc Biotechnology., JSC | Device: Laminectomy | Device: Intraduralspace | Device: Intrathecal | Device: Intravenous | Acute Spinal Cord Injury | P1 |
| 26 | Unico Cell Biomed CO. LTD | Allo-ADSCs( Elixcyte) | Moderate to Severe Chronic Kidney Disease | P1 |
| Allo-ADScs; Drug: Elixcyte | Drug: Hya Joint Plus | Knee Osteoarthritis | P1 |
ADSCs Application for Major Disease Indications
Ischemia, Sclerosis, Fistula and Osteoarthritis are 4 major disease indications, where ADSCs can provide significant therapeutic benefits than traditional drugs.
Ischemic based indications
The shortage of blood supply and oxygen to any tissue due to narrowing of the local / regional blood vessels is termed as Ischemia. Applicability of ADSCs for some ischemic conditions namely Inflammatory Bowel Disease, CVD are reviewed.
Inflammatory Bowel Disease (IBD)
The IBD refers to a group of intestinal disorders arising due to the interaction of environmental and genetic factors that result in immunological responses and inflammation. The sub-group of IBD are Intestinal ischemia or mesenteric ischemia or infarction is the result of blockage of arteries supplying the intestine. Intestinal ischemia leads to transforms to bowel necrosis and perforation.
Incidences and prevalence
The Crohn’s Disease (CD) and Ulcerative Colitis (UC) are two major types of IBD that occur in industrialized urban societies and have been considered as global emergence disease[30]. Incidence of these disease in previously considered low risk countries like India and Japan are rising due to changes in lifestyle, diet, life stress, smoking etc[31]. There is a 50 % increase in mortality rate in CD compared to UC and 25 % - 50 % deaths are due to intestinal cancer, postoperative complications and malnutrition[32].
Pathophysiology
The various pathophysiological events in IBD includes activation of lymphocyte, phagocytes and pro-inflammatory cytokine leading to tissue distruction. The multifactorial cascading events following ischemia leads to oxygen depletion, abnormal epithelial barrier function leading to translocation of microbes and their products and inflammatory responses. Reperfusion to re-establish the blood flow generates the reperfusion injuries and ROS; thereby ROS-mediated apoptosis and inflammatory cascade which further trigger multi-organ failure and death[33].
Current treatment challenges
Current treatments involves induction and maintenance of remission using anti-inflammatory amniosalicylates and corticosterioids, immunosuppressive agents, antibiotics, direct peritoneal resuscitation, and biologic agents. Established anti-inflammatory agents, steroids and anticoagulation treatments after surgical resection of acute mesenteric ischemia (AMI) results in decrease in necrotic nature. The long term result does not show satisfactory improvement[33]. Conventional corticosteroid and new generation formulations have modest efficacy and are slow acting. The direct peritoneal resuscitation improved the survival rate after intestinal ischemia in animal models[34]. On contrary, the mortality rate for AMI was high as 40 %, when progressed towards surgical interventions[35,36]. New biologics do not rise response rate to above 45 % to 60 % and the patients who respond to biologic treatment can lose response over repeat dose due to development of anti-drug antibodies[37].
Mechanisms of Action of ADSCs for IBD
ADSCs promote functional recovery after injury, suppress inflammatory immunological cascade and secrete various paracrine and autocrine bio-factors to repair and regenerate the damaged site and hence would be ideal for IBD[6,33,38,39]. ADSCs decreases apoptosis of injured cells, decreases intestinal permeability, increases the recovery of the gut mucosal barrier and increases neovascularization under hypoxic conditions[40]. Transplantation of ADSCs in an animal model showed increased survival and improved outcome compared to keratinocytes as differentiated controls[41]. Currently, there are very few clinical trials on IBD (NCT02952131) and are under recruiting phase. ADSCs application to IBD is an untapped therapeutic application and needs further proof of concept and rigorous dose finding studies in appropriate animal models.
Cerebrovascular disease
The cerebral circulatory arteries supplying oxygen to the brain are affected by cerebrovascular events such as stroke due to blockage or narrowing of arteries.
Incidences and prevalence
Cerebrovascular disease (CVD) is the second most leading cause of death after cancer[42]. The 85 % of strokes are non-haemorrhagic ischemic strokes. Globally, around 16 million new cases are registered contributing to about 9.7 % of global deaths and expected to raise around 7.8 million death by 2030. CVD accounts for 87 % disability adjusted life year. Incidence of CVD are rising due to change in diet habits, unchecked industrialization, smoking and alcohol consumptions[43].
Pathophysiology
Reduced blood flow causes low energy availability and results in rapid failure of membrane ion gradient, thereby heavy influx of calcium into cells within minutes after the onset of ischemia. The exotoxic neurotransmitters like glutamate are released due to increased intra-cellular calcium levels, causing damage to neuronal cells. Then the onset of infammatory cascade in peri-ischemic area with release of heat-shock proteins, proteinases, thereby causes delayed release of remodelling proteins for healing[44].
Current treatment challenges
Current treatment modalities include blood clot prevention and inhibition by heparin, warfarin, non-specific streptokinase, urokinase, and tissue plasminogen activators. Non-specific digestion of thrombolytic therapies resulted in an allergic reaction and systemic bleeding or vascular lesions have been reported[45]. Further, the plasma half-life of thrombolytic therapies including 3rd generation drugs like Tenecteplase, Reteplase is 15 to 20 minutes[45]. But these treatments modality have failed to replace the surgical intervention to remove thrombus. Thus currently there are no effective drugs available as therapy to cure, regenerate the damaged tissue.
Mechanisms of Action of ADSCs for CVD
ADSCs can protect the neurons from cell death through secretion of VEGF, thereby promoting brain repair damage[46]. ADSCs can promote functional recovery through neuronal differentiation by secreting several neurotrophic factors[47]. ADSCs provide engraftment, differentiation and paracrine support and systemically administered cells have a shelf-life that is longer than a week[48,49]. ADSCs have shown significant neural development and reversal of the cerebral ischemia in various animal studies. There are few clinical trials on acute ischemic stroke or stroke using adipose derived stromal cells and some are in phase-I (NCT01453829, NCT02813512) and phase-II (NCT01678534, NCT02849613) and the results are not yet published. ADSCs application to CVD is an untapped therapeutic application.
Chronic Obstructive Pulmonary Disease (COPD)
COPD is the development of progressive irreversible constriction of airways and destruction of lung parenchyma and lung elasticity without any pulmonary fibrosis.
Incidences and prevalence
Among lung diseases, COPD prevalence is increasing and they would be cause of world’s 3rd disease mortality by 2030[50]. Globally, COPD is the fourth leading cause of death affecting mainly people over 75 years and its prevalence range from 0.2 % to 37 % and varies from nation to nation.
Pathophysiology
The COPD is a debilitating lung condition produced by a variety of lesions in the large airways, bronchioles and lung parenchyma. Clinically present as inflammation of the air tubes called Bronchitis and increased mucous production resulting in airway obstruction. Histologically the obstruction is caused by activated inflammatory cells which produce bronchiolar breakdown of the structural proteins (collagen and elastin) along with cellular apoptosis, inappropriate production of Matrix Metalloproteinase-9 (MMP9) and lack of lung tissue repair function[51,52]. In a small group of cases there is enlargement of lung spaces with fibrosis and narrowing of bronchioles resulting in emphysema.
Current treatment challenges
The current treatment options include cessation of smoking, bronchodilators, inhaled corticosteroids, pulmonary re-habitation, oxygen therapy and surgery. The β-agonists type of bronchodilators work by relaxing bronchial smooth muscle and these β-agonists have twofold increased risk of causing cardiovascular disease as adverse event[53,54].
Mechanisms of Action of ADSCs for COPD
ADSCs showed improved regenerative and reparative function in mice with elastase-induced emphysema[55]. Further, intravenous injection of MSCs has a greater in-vivo cell distribution towards lungs as 83 % of cells are present at 1 hour after injection, 42 % till 24 hours and 1 % till 10 days than all other organs (heart, liver, kidney, spleen and gut) 1 %[56]. ADSCs treatment in COPD showed significant improvement in functional scores[57]. Preconditioned pioglitazone ADSCs had a more potent therapeutic effect than non-pretreated ADSCs in both elastase and smoke-induced emphysema models and have shown higher VEGF levels in mouse lungs[58]. Adiponectin secreted in serum enhances the functional role of epithelial and endothelial pulmonary cells[59]. The recently published result of clinical trial results of MSCs treatment for COPD reveals that MSCs administration of 3 intravenous doses (weekly interval) of 2 million per kg body weight is safe and may improve the quality-of-life[60]. Further large-scale conformational clinical trials are needed to evaluate efficacy.
Sclerosis based indications
The sclerosis indicates hardness due to inflammation, muscle stiffness and fibrosis. Applicability of ADSCs for some ischemic conditions namely Amyotrophic Lateral Sclerosis (ALS), Multiple Sclerosis (MS) and Systemic Sclerosis are reviewed here.
Amyotrophic Lateral Sclerosis (ALS) (motor neuron disease)
ALS is a rare group of progressive neuromuscular degenerative disease of motor neuron system.
Incidences and prevalence
The estimated global annual prevalence rate was 1.9 cases per 100,000 with lowest rates among African and Asian populations[60]. ALS is projected to increase at the rate of 69 % of ALS cases from 2015 to 2040 and is mainly due to aging population[61].
Pathophysiology
The various genetic abnormalities in chromosome 9 of C9ORF72 gene, SOD1, TDP-43, FUS/LTS, VCP/97 indicates the major cause for ALS[62-64]. The ALS is identified by degenerative changes in lower and upper motor neurons with progressive muscle atrophy, weakness typically leading to respiratory failure and death[65,66].
Current treatment challenges
The Riluzole (Rilutek) is only the USFDA approved disease modifying drug believed to reduce the motor neuron damage by decreasing the transport messenger glutamate between nerve cells and motor neurons.Currently several mechanisms are being explored to select novel therapeutic candidate for ALS using mouse model[67]. Majority of the pharmaceutical active substances are uni-functional and are in preclinical testing.
Mechanisms of Action of ADSCs for ALS
The therapeutic application of ADSCs to treat ALS is by direct cell replacement, trophic factor delivery, immunomodulation[68]. ADSCs administration to SOD1-mutant mice delayed the motor neuron degeneration for 4-6 weeks and promoted neuroprotection and prolonged the lifespan[69,70]. Autologous ADSCs administered intrathecally in Phase-I trials were proven safe and provided Class IV evidence for dose escalation in ALS patients[71]. Cells from ALS patients has reduced or altered therapeutic effects[72-75]. This problem may be overcome with the use of allogeneic cells. Several ongoing clinical trials are in the exploration of the intramuscular delivery route and cell doses to optimize the effective cell based treatment strategies for ALS. Exosomal based therapeutic strategy from ADSCs, ADSC conditioned media, Curcumin-INVITE micelles loaded MSCs are some of the advanced therapeutic strategies which have shown promising pre-clinical outcome[76-79]. The comparative study of disease modifying agent Riluzole with cell-therapeutics along with spike in in-vitro and in-vivo studies provide an opportunity to develop proof-of-concept for cell based therapies.
Multiple Sclerosis (MS)
Multiple Sclerosis is a chronic inflammatory, and autoimmune disease caused by the demyelination of nerve cells in the brain and spinal cord leading to irreversible damage to the central nervous system (CNS)[80].
Incidences
The prevalence of MS is high as 100 / 100,000 in North America and Europe and low as 2 / 100,000 population in Eastern Asia and Sub-Sharan Africa[81]. The most common type (85 %) of MS is Relapsing-remitting (RPMS) and other categories are secondary progressive, primary progressive, and progressive relapsing[82].
Pathophysiology
MS is a self-directed autoimmune reaction to myelin antigen, irreversible damage to the myelin sheath, oligodendrocytes, axons and neurons and thereby causing the neurological disabilities like visual disturbances, muscle weakness, trouble with coordination and balance, bowel and bladder incontinence etc[83].
Current treatment challenges
Current treatment modalites include immunosuppressive drugs like cyclosporine, Rapamycin for the inhibition of IL-2 transcription, mAbs like anti-CD4 for depletion of T-cells, anti-CD20 (Rituximab) for depletion of B-Cells. The drugs like anti-CD2, anti-CD45, anti-CD154 (CD40L) Natalizumab etc block the entry of inflammatory cells into the CNS. Lastly Anti-Histamines, Statins etc alter the immune response in CNS. Unfortunately, these therapies have a moderate success rate of 30 % in reducing the symptoms and most of these therapies are unable to stop the progression of disease[84]. Besides, these drugs induce several side effects that limit its usage for an extended period[85].
Mechanisms of Action of ADSCs
Advances in understanding the pathophysiology of MS endorses the development of ADSCs based therapeutic approaches[86]. The initial inflammatory phase occurs in the majority of cases (85 - 90 %) in RRMS. ADSCS can stimulate local specific immunomodulation, provide neuroprotection repair, and regeneration with the help of trophic factors. Transplantation of ADSCs reduces the inflammatory cell infiltration, demyelination and improves the neurological functions in encephalomyelitis (EAE) mice[87]. ADSCs transduction with the Interferon beta gene in EAE model of mice (ideal model for MS) showed decreased peripheral and central neuroinflammation[88]. IL-4 overexpressing ADSCs are potent anti-inflammatory agents in animal models[89]. Non-transduced ADSCs from obese donors showed reduced efficacy in EAE model suggesting donor demographics and qualification criteria are important for potency of therapeutic ADSCs[90]. However, the cell therapy clinical trials for MS are practically feasible, relatively safe procedure with immediate immunomodulatory functions, but the ADSCs based clinical trials to MS needs to be built based on pre-clinical data as well as upgrading advanced cell delivery strategies[80].
Systemic Sclerosis
Systemic Sclerosis (SSc) is a rare complex chronic systemic autoimmune disorder affecting blood vessels, and connective tissue.
Incidences and prevalence
SSc affect about 2.5 million worldwide and more likely to affect women than men[91]. The under estimated annual incidence was around 20 per million population and reasonable estimate was around 500 per million population[92]. Incidences and prevalence of SSc are higher in European nations compared to Asian nations[91]. The overall 10 year survival after diagnosis is below 70 %.
Pathophysiology
SSc is characterized by progressive fibrosis of the skin and visceral organs due to accumulation of collagen and Extracellular Matrix (ECM), systemic inflammation due to the generation of autoantibodies and pro-inflammatory cytokines and microvascular abnormalities[91]. The localized form of scleroderma / SSc are limited to skin, face hands and feet’s, but the systemic/diffuse form involves visceral organs. SSc based organ complications include Raynaud’s Phenomenon (RP), Pulmonary Arterial Hypertension (PAH), scleroderma renal crisis, gastrointestinal, skin and Digital Ulcers (DUs).
Current treatment challenges
Treatment modality include corticosteroids, and Immunosuppressants Nonsteroidal anti-inflammatory drugs alleviate the symptoms and do not slow down the progression of disease. Short-term usage of steroids has side effects like hyperglycemia, neuropsychological effects and its long term usage might cause osteoporosis, aseptic joint necrosis etc[93]. The monoclonal antibodies for PDGF/TGFβ1 and therapeutic molecules like Tyrosinkinase inhibitors, relaxin are well tolerated therapies for scleroderma, but lacks significant efficacy[94]. Autologous fat grafting has become a first choice to treat cutaneous lesions. Although, lipoaspirate and fat transplantation has advanced and attained attractive procedure for various reconstructions, it’s still associated with increased number of complications[95]. Typical complications includes minimal / no graft retention, oil cysts, calcification, infection, necrosis of grafted fat and surgery related complications[95]. Major disadvantage of fat harvesting is the requirement of large volume (about 300 to 600 ml) and subsequent transplantation which may not be easy in thin patients.
Mechanisms of Action of ADSCs for SSC
ADSCs exhibit immunomodulatory properties, suppression of collagen reactive T cells and anti-inflammatory properties[96]. The co-transplantation of autologous ADSCs along with hyaluronic acid has demonstrated a significant improvement without any complications in scleroderma patients[97]. Allogeneic ADSCs showed anti-fibrosis effects in HOCl-model of SSc crossing species barrier[98]. Safety and efficacy using SVF injections for systemic sclerosis further strengthen the application of ADSCs[99]. However processed SVF in IVD degeneration animal model showed destructive responses. These adverse event may be model specific or species specific triggers a caution for autologous SVF injections[100]. ADSCs should be considered for future clinical trials, as it is a promising therapeutic candidate for SSc.
Crohn’s based fistula
Crohn’s Disease (CD) is rapidly evolving chronic inflammatory digestive track disease that may affect anypart of gastro-intestinal tract from mouth to anus. Perianal fistula is a well-known complication of the Crohn’s disease.
Incidences and prevalence
Perianal fistula is a well known complication of CD and prevalence of a fistula-in-ano range from 1.1 to 2.2 per 10,000 population per year[101,102]. An increase of 4% year on year was reported. It was more prevalent in males than females (24:1) with grade 4 as a common type of fistula in India[103,104]. The unknown aetiology of complex perianal fistula results in origin of few hypothesis like deep penetration of ulcer; derivation from an anal gland abscess[105].
Pathophysiology
The epithelial cell defects and inflammation causes leukocyte recruitment leading to altered repair mechanism that enhances the migration of intestinal epithelial cells[106]. On the other hand, the inflammatory cytokines-like TGF-β, TNF-α, and IL-13 induces IEC to myofibroblasts via EMT pathway and become transitional cells of fistula tract[107]. Thus creating the transitional cell invasiveness leading to cascading events of over activation of Matrix Metalloproteinases (MMP) like MMP-3 and MMP-9 contributingin development of fistulas[107].
Current treatment challenges
Various treatments modulates such as antibiotics, immunomodulatory agent anti-TNF α, surgical interventions, fibrin glue and plug have been tried with varying degree of success. Use of antibiotics resulted in incomplete closure of fistula[108]. The closure rate with the anti-TNF-α antibodies are also unsatisfactory[109,110]. The shutdown of inflammatory response increases the risk of infections and malignancy[111]. The outcome of medical treatment with surgical management does not sufficiently improve the quality-of-life[112,113]. While most acute cases can be easily treated through surgery, treatment of complex perianal fistula is a formidable challenge for most surgeons[114-116]. Aggressive, repeated surgery results in fecal incontinence, intraoperative difficulties and limited surgical management leads to recurrence[117-119]. Fibrin glue while preserving anal sphincter function results in a clinical efficacy of about 38 % and needs long-term follow-up[120]. The reported success rate of Fistula plug (sphincter saving surgical closure technique using fistula plug insertion and made of porcine small intestinal submucosa called biosynthetic fistula plug from cook biotech) in non-CD and CD fistulas were 54.8 and 54.3 % respectively[121]. Hence there is a need for better regenerative products for the better clinical outcome.
Mechanisms of Action of ADSCs for Fistula
The anti-inflammatory, self-renewing, and differentiation property of ADSCs might play a critical role in the healing of anal fistulas. First successful clinical evidence of the effectiveness of ADSCs in rectovaginal fistula of CD was reported as a case study[122]. Symptoms did not improve for 11 years prior to enrolment, but the fistula was completely closed within one week after infusion of ADSCs at the site[122]. Thereafter several clinical trials were initiated across the globe for fistula. The SVF offers instant therapeutic materials for infusion or injection, but clinically, expanded ADSCs are more effective than SVF[123].
In Phase I trials application of Autologous ADSCs resulted in complete fistula closure in 75 % of cases by 8th week[124] and healing rate of 71 % by ADSCs and 16 % by placebo in Phase II trials by the same group[125]. Infusion of autologous ADSCs achieved fistula healing and remission in 88 % of cases at 1 year[126]. Long-term follow-up at 3 years demonstrated that the administration of ADSCs were safe and efficacious with no reoccurrence in fistula[127]. Phase II study with allogeneic ADSCs reported a 85.7% healing rate with ADSCS compared to 33.3% healing rate with placebo[128]. In addition, García-Arranz M et al., (2015) demonstrated the ADSCs safety and efficacy in phase-I-IIa by administration of 20 million ADSCs through intra-lesional injection and second dose of 40 million at 12 week to unhealed fistula and follow-up to 52 weeks[129]. Phase-III randomized double-blinded controlled trials using allogeneic ADSCs by TiGenix have shown safe and effective clinical outcome[130]. Based on these results, TiGenix applied for European Medicines Agency for approval of the treatment[131]. Gastroenterology based pharmaceutical leader of Takeda acquired the exclusive rights to commercialize the product outside the US. None of the clinical trials reported major adverse reactions. Currently, 21 of academic and 11 of industry-sponsored clinical trials on fistula with various combinations of cell delivery options are underway. The recent evidence of meta-analysis also support the therapeutic effects of MSCs on Crohn’s fistula[132]. Biodistribution studies of ADSCs using bioluminescence in animal model reveal the higher fistula closure rate through the local infusion of ADSCs[133]. The pre-clinical, clinical trial exploration, commercialization and further conceptual development in fistula indication suggest that ADSCs may be the possible alternative treatment, but confirmatory evidence-based long-term follow-up studies are required on a large population.
Osteoarthritis
Osteoarthritis (OA) is a degenerative joint disease characterized by progressive cartilage deterioration, subchondral bone alteration, osteophyte formation, and loss of joint space[134].
Incidence and prevalence
OA is the leading cause of disability in the elderly and affects approximately 10 % of those aged over 60 years[135]. OA incidence is rising due to increasing obesity and an ageing population[134-136]. OA is the fastest increasing major health condition. Between 2005 and 2015, there was a 32.9 % increase in prevalence and 34.8 % increase in years lived with disability[137].
Pathophysiology
OA also affects joint capsule, meniscus and the synovium[138-142]. In OA pro-inflammatory and catabolic cytokines are produced leading to cartilage degradation[142] which further induces inflammation leading to a vicious cycle of further cartilage degradation and inflammation. The clinical symptoms of OA are persistent joint pain, stiffness disability and loss of joint function[143-146].
Current treatment options
The current non-surgical treatment options including physical therapy, opioids, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids and lubricating supplements. Physical therapy can improve joint function and can provide pain relief but cannot regenerate cartilage[144,147]. The pain relief is often not enough[148-150]. Opioids provide small to moderate benefit but are associated with adverse events such as cardiovascular events, fractures, addiction[151-153]. While NSAIDS supress inflammation and provide pain relief, its therapeutic benefits are only palliative and do little to treat and prevent cartilage damage[147]. NSAIDs are associated with an increased risk of serious Gastrointestinal (GI), Cardiovascular (CV), renal injury, and liver damage[154-161]. Intra-articular corticosteroids can provide short-term relief from pain but repeated injections might lead to joint damage and increase the infection risk[144]. Lubricating substances such as hyaluronic injections can provide pain relief and improved joint function[161], but show varying efficacy[144,162].
Mechanisms of Action of ADSCs for OA
ADSCs can help in treating OA, primarily through the paracrine effects of the secretory factors which attenuates the inflammation, imparts chondroinductive and chondroprotective effects. ADSCs attenuate inflammation by lowering the production of catabolic and inflammatory factor[163-165]. Thus by targeting the inflammatory process and the catabolic factors ADSCs can break the progressive loop of OA and prevent the progression of cartilage degradation. ADSCs have the chondroinductive effect on chondrocytes, by promoting the expression of cartilage specific markers[166,167], by reducing the expression of hypertrophic chondrocytes markers and fibrotic markers[168]. ADSCs also provides chondroprotective effect by promoting chondrocyte proliferation and by reversing apoptosis[165,168]. ADSCs may be able to affect OA by directly differentiating into chondrocytes. Various in-vitro studies demonstrate ADSCs differentiation into chondrocytes. However some in-vivo studies show that ADSCs home to the synovium and not to the cartilage[164,165]. Hence the contribution of ADSCs in OA could be due to the inhibition of cartilage degeneration and might not be through ADSC differentiation into chondrocytes.
The beneficial effects of ADSCS on OA have been demonstrated in various OA induced animal models such as rabbit[139,169], dog[170-173], goat[174], mouse[175], horse[176], rat[177]. ADSCs have been administrated along with serum[139,167], hyaluronic acid[170,177], medium[169], PBS[171,176,177], PRP[173]. Efficacy was demonstrated when the cells were of autologous[171-173], allogeneic[170-172] or xenogeneic[171]. Various joints were used such as hip[171], elbow[170,172], knee[167,175-177], humero radial joints[173], middle carpal joint[176]. There are only a few clinical cases published on the use of ADSCs. Various dosages (Low-dose (1.0 x 107 cells), medium mid-dose (5.0 x 107 cells), and high-dose (1.0 x 108 cells) of autologous ADSCs in saline were injected in patients with knee OA. The high dose group demonstrated both pain improvement and increased volume of hyaline like cartilage at 6 months[178]. These clinical outcomes tended to deteriorate after 1 year in the low- and medium-dose groups, whereas those in the high-dose group plateaued at 2 years[179]. In another dose escalation study with three doses (low-dose (2 x 106 cells), medium mid-dose (10 x 106), and high-dose (50 x 106 ) lower dose of autologous ADSCs improved pain and knee function at 6 months in 18 patients[180].
Cell Therapies for OA
In the absence of disease modifying agents, cell-based therapies that regenerate cartilage is ideal for OA. There are a few approved cell-based therapies and more are in various stage of clinical trials. Carticel and MACI from Genzyme approved by the FDA and Chondrocelect approved by European Medicines Agency use autologous chondrocytes. CARTISTEM®, approved by Korean FDA use human umbilical cord blood-derived mesenchymal stem cells in sodium hyaluronate. Other products such as Chondrogen® MSC in HA from Osiris Therapeutics (NCT00225095, NCT00702741), BioCart® scaffold containing autologous chondrocytes from ProChon Biotech (NCT00729716), Mesoblast allogeneic mesenchymal precursor cells in Rheumatoid Arthritis (NCT01851070), Progenza Allogeneic ADSCS from Regeneus (ACTRN12615000439549), and Neocart autologous chondrocytes in collagen matrix from Histogenics (NCT01066702, NCT00548119) are in various stages of clinical trials.
Overall these few clinical studies demonstrate that ADSCs are capable of reducing pain and improving functions in OA. More randomized controlled trials are needed before ADSCs can come to the clinic.
Clinical Translational Challenges for ADSCs
The ADSCs are fascinating tools for regenerative medicine and its clinical translation exploration for various indications encounter ethical, non-specific/specific regulatory and legal framework challenges. ADSCs therapies are hampered by long development duration and undergo lengthy clinical phases execution to reach the market because of current regulatory framework[181,182]. ADSCs clinical translation can be speeded up by adopting translational modalities and basic quality framework from bone marrow and blood transfusion methodologies[183]. Further, risk-based classification of regenerative therapeutics of Japanese regulatory model which heralded new reforms, enabling new outlook to regulatory translations ensuring the patient safety and clinical research can also speed up the translation[184]. The need of the hour is for tailor made regulations based on product classification, with minimal regulatory intervention in initial stages of clinical development to ensure proper development of product, procedures and its real-time cold chain logistics.
Conclusion
ADSCs as a therapeutic agent has several advantages over drugs in many clinical indications. Recent progress of ADSCs towards various clinical trials suggest its usability as core active therapeutic by tapping its natural abilities of anti-inflammation, repair, regeneration and immunomodulation properties. ADSCs have been shown to slowdown the disease progression and can be tested as a potential therapy for incurable diseases. This review focusses on the scientific evidence of using ADSCs for some clinical indications. Current regulations delay the clinical translation of ADSCs. The Japanese risk-based regulatory approaches[184] on stem cells can provide a path to develop regulatory framework to speed up the release of ADSCs based product to market with uncompromised safety and quality.
Acknowledgement
SRTE is fully supported by Sri Sringeri Sharada Peetam
Authors’ Contributions
Dr. Sanjay Gottipamula: The first author made a significant contribution to conception and design, administrative support, provision of study materials, search, navigation, data collection and assembly of data, data analysis and interpretation, drafting and revising the manuscript.
Dr. Kumar Chokalingam: The second author made significantly contributed in research navigation, data collection, interpretation and drafting of the manuscript.
Dr. K.N Sridhar: Full corresponding author of the article and made a significant contribution in revising, provision of study materials and final approval of the manuscript.
Conflict of Interest
The author discloses no conflict of Interest.
References
- 1. Spear, B.B., Heath-Chiozzi, M., Huff, J. Clinical application of pharmacogenetics. (2001) Trends mol med 7(5): 201-204.
- 2. Onakpoya, I.J., Heneghan, C.J., Aronson, J.K. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. (2016) BMC med 14: 10.
- 3. Light, D.W., Lexchin, J., Darrow, J.J. Institutional corruption of pharmaceuticals and the myth of safe and effective drugs. (2013) J law med ethics 41(3): 590-600.
- 4. Onakpoya, I.J., Heneghan, C.J., Aronson, J.K. Worldwide withdrawal of medicinal products because of adverse drug reactions: a systematic review and analysis. (2016) Crit rev toxicol 46(6): 477-489.
- 5. Aziz, Aly, L.A., Menoufy, H.E., Ragae, A., et al. Adipose stem cells as alternatives for bone marrow mesenchymal stem cells in oral ulcer healing. (2012) Int j stem cells 5(2): 104-114.
- 6. Riazifar, M., Pone, E.J., Lotvall, J., et al. Stem Cell Extracellular Vesicles: Extended Messages of Regeneration. (2017) Annu rev pharmacoltoxicol 57: 125-154.
- 7. An, H.Y., Shin, H.S., Choi, J.S., et al. Adipose Mesenchymal Stem Cell Secretome Modulated in Hypoxia for Remodeling of Radiation-Induced Salivary Gland Damage. (2015) PloS one 10(11): e0141862.
- 8. Hu, L., Wang, J., Zhou, X., et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. (2016) Sci rep 6: 32993.
- 9. Strem, B.M., Hicok, K.C., Zhu, M., et al. Multipotential differentiation of adipose tissue-derived stem cells. (2005) Keio j med 54(3): 132-141.
- 10. Shen, F.H., Werner, B.C., Liang, H., et al. Implications of adipose-derived stromal cells in a 3D culture system for osteogenic differentiation: an in vitro and in vivo investigation. (2013) spine j 13(1): 32-43.
- 11. Rezanejad, H., Soheili, Z.S., Haddad, F., et al. In vitro differentiation of adipose-tissue-derived mesenchymal stem cells into neural retinal cells through expression of human PAX6 (5a) gene. (2014) Cell tissue res 356(1): 65-75.
- 12. Montespan, F., Deschaseaux, F., Sensebe, L., et al. Osteodifferentiatedmesenchymal stem cells from bone marrow and adipose tissue express HLA-G and display immunomodulatory properties in HLA-mismatched settings: implications in bone repair therapy. (2014) J immunol res (2014): 230346.
- 13. Bajek, A., Gurtowska, N., Olkowska, J., et al. Does the Harvesting Technique Affect the Properties of Adipose-Derived Stem Cells?-The Comparative Biological Characterization. (2017) J cell biochem 118(5): 1097-1107.
- 14. Harats, M., Millet, E., Jaeger, M., et al. Adipocytes Viability After Suction-Assisted Lipoplasty: Does the Technique Matter? (2016) Aesthetic plastsurg 40(4): 578-583.
- 15. Duscher, D., Atashroo, D., Maan, Z.N., et al. Ultrasound-Assisted Liposuction Does Not Compromise the Regenerative Potential of Adipose-Derived Stem Cells. (2016) Stem cell transl med 5(2): 248-257.
- 16. Bourin, P., Bunnell, B.A., Casteilla, L., et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). (2013) Cytotherapy 15(6): 641-648.
- 17. Oberbauer, E., Steffenhagen, C., Wurzer, C., et al. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: current state of the art. (2015) Cell regen 4: 7.
- 18. Aronowitz, J.A., Lockhart, R.A., Hakakian C.S., et al. Adipose Stromal Vascular Fraction Isolation: A Head-to-Head Comparison of 4 Cell Separation Systems #2. (2016) Ann plastsurg 77(3): 354-362.
- 19. Aronowitz, J.A., Ellenhorn, J.D. Adipose stromal vascular fraction isolation: a head-to-head comparison of four commercial cell separation systems. (2013) Plastreconstrsurg 132(6): 932e-939e.
- 20. Fernandes-Platzgummer, A., Carmelo, J.G., da, Silva, C.L., et al. Clinical-Grade Manufacturing of Therapeutic Human Mesenchymal Stem/Stromal Cells in Microcarrier-Based Culture Systems. (2016) Methods molbiol 1416: 375-388.
- 21. Jossen, V., Schirmer, C., MostafaSindi, D., et al. Theoretical and Practical Issues That Are Relevant When Scaling Up hMSCMicrocarrier Production Processes.( 2016) Stem cells int 2016: 4760414.
- 22. Dos, Santos, F., Campbell, A., Fernandes-Platzgummer, A., et al. A xenogeneic-free bioreactor system for the clinical-scale expansion of human mesenchymal stem/stromal cells. (2014) Biotechnolbioeng 111(6): 1116-1127.
- 23. Portron, S.,Merceron, C., Gauthier, O., et al. Effects of in vitro low oxygen tension preconditioning of adipose stromal cells on their in vivo chondrogenic potential: application in cartilage tissue repair. (2013) PLoS One 8(4): e62368.
- 24. Mei, L., Shen, B., Ling, P., et al. Culture-expanded allogenic adipose tissue-derived stem cells attenuate cartilage degeneration in an experimental rat osteoarthritis model. (2017) PLoS One 12(4): e0176107.
- 25. Lafosse, A., Desmet, C., Aouassar, N., et al. Autologous Adipose Stromal Cells Seeded onto a Human Collagen Matrix for Dermal Regeneration in Chronic Wounds: Clinical Proof of Concept. (2015) PlastReconstrSurg 136(2): 279-295.
- 26. Lai,T., Yang,Y., Ng, S.K. Advances in Mammalian cell line development technologies for recombinant protein production. (2013) Pharmaceuticals (Basel) 6(5): 579-603.
- 27. Dumont, J.,Euwart. D., Mei, B., et al. Human cell lines for biopharmaceutical manufacturing: history, status, and future perspectives. (2016) Crit Rev Biotechnol 36(6): 1110-1122.
- 28. Al, Battah, F., De, Kock, J., Vanhaecke, T., et al. Current status of human adipose-derived stem cells: differentiation into hepatocyte-like cells. (2011) ScientificWorldJournal 11: 1568-1581.
- 29. Syed, B.A., Evans, J.B. Stem cell therapy market. (2013) Nat Rev Drug Discov 12(3): 185-186.
- 30. M’Koma, A.E. Inflammatory bowel disease: an expanding global health problem. (2013) Clin med insights Gastroenterol 6: 33-47.
- 31. Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. (2015) Nat rev Gastroenterolhepatol 12(4): 205-217.
- 32. Burisch, J., Munkholm, P. The epidemiology of inflammatory bowel disease. (2015) Scandinavian journal of gastroenterology 50(8): 942-951.
- 33. Doster, D.L., Jensen, A.R., Khaneki, S., et al. Mesenchymal stromal cell therapy for the treatment of intestinal ischemia: Defining the optimal cell isolate for maximum therapeutic benefit. (2016) Cytotherapy 18(12): 1457-1470.
- 34. Crafts, T.D., Hunsberger, E.B., Jensen, A.R., et al. Direct peritoneal resuscitation improves survival and decreases inflammation after intestinal ischemia and reperfusion injury. (2015) J Surg Res 199(2): 428-434.
- 35. Roussel, A., Castier, Y., Nuzzo, A., et al. Revascularization of acute mesenteric ischemia after creation of a dedicated multidisciplinary center. (2015) J VascSurg 62(5): 1251-1256.
- 36. Paladino, N.C., Inviati, A., Di, Paola, V., et al., Predictive factors of mortality in patients with acute mesenteric ischemia. A retrospective study(2014) Ann ItalChir 85(3): 265-270.
- 37. Sandborn, W.J. The Present and Future of Inflammatory Bowel Disease Treatment. (2016) GastroenterolHepatol (N Y) 12(7): 438-441.
- 38. Anderson, P., Souza-Moreira, L., Morell, M., et al. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. (2013) Gut 62(8): 1131-1141.
- 39. Spees, J.L., Lee, R.H., Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. (2016) Stem cell res ther 7(1): 125.
- 40. Wang, X., Liu, C., Li, S., et al . Hypoxia precondition promotes adipose-derived mesenchymal stem cells based repair of diabetic erectile dysfunction via augmenting angiogenesis and neuroprotection. (2015) PloS one 10(3): e0118951.
- 41. Jensen, A.R., Doster, D.L., Hunsberger, E.B., et al . Human Adipose Stromal Cells Increase Survival and Mesenteric Perfusion Following Intestinal Ischemia and Reperfusion Injury. (2016) Shock 46(1): 75-82.
- 42. Lippi, G., Plebani, M. Biomarker research and leading causes of death worldwide: a rather feeble relationship. (2013) Clinchem lab med 51(9): 1691-1693.
- 43. Chin, J.H. Stroke in sub-Saharan Africa: an urgent call for prevention. (2012) Neurology 78(13): 1007-1008.
- 44. Barone, F.C., Feuerstein, G.Z. Inflammatory mediators and stroke: new opportunities for novel therapeutics. (1999) J Cereb Blood Flow Metab 19(8): 819-834.
- 45. Ali, M.R., Salim Hossain, M., Islam, M.A., et al. Aspect of thrombolytic therapy: a review. (2014) ScientificWorldJournal 2014: 586510.
- 46. Chen, J., Tang, Y.X., Liu, Y.M., et al. Transplantation of adipose-derived stem cells is associated with neural differentiation and functional improvement in a rat model of intracerebral hemorrhage. (2012) CNS NeurosciTher 18(10): 847-854.
- 47. Chan, T.M., Harn, H.J., Lin, H.P., et al. The use of ADSCs as a treatment for chronic stroke. (2014) Cell transplant 23(4-5): 541-547.
- 48. Lee, R.H., Pulin, A.A., Seo, M.J., et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. (2009) Cell stem cell 5(1): 54-63.
- 49 Zangi, L., Margalit, R., Reich-Zeliger, S., et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. (2009) Stem cells 27(11): 2865-2874.
- 50. Koul, P.A. Chronic obstructive pulmonary disease: Indian guidelines and the road ahead. (2013) Lung India 30(3): 175-177.
- 51. Di, Petta, A. Pathogenesis of pulmonary emphysema - cellular and molecular events. (2010) Einstein 8(2): 248-251.
- 52. Xu, B., Chen, H., Xu, W., et al. Molecular mechanisms of MMP9 overexpression and its role in emphysema pathogenesis of Smad3-deficient mice. (2012) Am j physiol Lung cell molphysiol 303(2): L89-96.
- 53. Sestini, P., Renzoni, E., Robinson, S., et al. Short-acting beta 2 agonists for stable chronic obstructive pulmonary disease. (2002) Cochrane database syst rev (4): CD001495.
- 54. Salpeter, S.R., Ormiston, T.M., Salpeter, E.E. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. (2004) Chest 125(6): 2309-2321.
- 55. Cho, R.J., Kim, Y.S., Kim, J.Y., et al. Human adipose-derived mesenchymal stem cell spheroids improve recovery in a mouse model of elastase-induced emphysema. (2017) BMB reports 50(2): 79-84.
- 56. Parekkadan, B., Milwid, J.M. Mesenchymal stem cells as therapeutics. (2010) Annu rev biomed eng 12: 87-117.
- 57. Nejad-Moghaddam, A., Ajdari, S., Tahmasbpour, E., et al. Adipose-Derived Mesenchymal Stem Cells for Treatment of Airway Injuries in A Patient after Long-Term Exposure to Sulfur Mustard. (2017) Cell j 19(1): 117-126.
- 58. Hong ,Y., Kim, Y.S., Hong, S.H., et al. Therapeutic effects of adipose-derived stem cells pretreated with pioglitazone in an emphysema mouse model. (2016) Expmol med 48(10): e266.
- 59. Bianco, A., Nigro, E., Monaco, M.L., et al. The burden of obesity in asthma and COPD: Role of adiponectin. (2017) Pulmpharmacolther 43: 20-25.
- 60. Cheng, S.L., Lin, C.H., Yao, C.L. Mesenchymal Stem Cell Administration in Patients with Chronic Obstructive Pulmonary Disease: State of the Science. (2017) Stem cells int 2017: 8916570.
- 61. Arthur, K.C., Calvo, A., Price, T.R., et al. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. (2016) Nat commun 7: 12408.
- 62. Chew, J., Gendron, T.F., Prudencio, M., et al. Neurodegeneration. C9ORF72 repeat expansions in mice cause TDP-43 pathology, neuronal loss, and behavioral deficits. (2015) Science 348(6239): 1151-1154.
- 63. Webster, C.P., Smith, E.F., Bauer, C.S., et al. The C9orf72 protein interacts with Rab1a and the ULK1 complex to regulate initiation of autophagy. (2016) The EMBO journal 35(15): 1656-1676.
- 64. Chen, S., Sayana, P., Zhang, X., et al. Genetics of amyotrophic lateral sclerosis: an update. (2013) Molecular neurodegeneration 8: 28.
- 65. Rowland, L.P. Amyotrophic lateral sclerosis. (1994) Curropinneurol 7(4): 310-315.
- 66. Chio, A., Logroscino, G., Traynor, B.J., et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. (2013) Neuroepidemiology 41(2): 118-130.
- 67. Morrison, B., Hensley, K., Pioro, E.P., et al. Amyotrophic lateral sclerosis and novel therapeutic strategies. (2012) Neurol res int 2012: 798028.
- 68. Lewis, C.M., Suzuki, M. Therapeutic applications of mesenchymal stem cells for amyotrophic lateral sclerosis. (2014) Stem cell res ther 5(2): 32.
- 69. Marconi, S., Bonaconsa, M., Scambi, I., et al. Systemic treatment with adipose-derived mesenchymal stem cells ameliorates clinical and pathological features in the amyotrophic lateral sclerosis murine model. (2013) Neuroscience 248: 333-343.
- 70. Kim, K.S., Lee, H.J., An, J., et al. Transplantation of human adipose tissue-derived stem cells delays clinical onset and prolongs life span in ALS mouse model. (2014) Cell transplant 23(12): 1585-1597.
- 71. Staff, N.P., Madigan, N.N., Morris, J., et al. Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. (2016) Neurology 87(21): 2230-2234.
- 72. Haidet-Phillips, A.M., Hester, M.E., Miranda, C.J., et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. (2011) Nature biotechnology 29(9): 824-828.
- 73. Papadeas, S.T., Kraig, S.E., O’Banion, C., et al. Astrocytes carrying the superoxide dismutase 1 (SOD1G93A) mutation induce wild-type motor neuron degeneration in vivo. (2011) ProcNatlAcadSci U S A 108(43): 17803-17808.
- 74. Koh, S.H., Baik, W., Noh, M.Y., et al. The functional deficiency of bone marrow mesenchymal stromal cells in ALS patients is proportional to disease progression rate. (2012) Expneurol 233(1): 472-480.
- 75. Cho, G.W., Noh, M.Y., Kim, H.Y., et al. Bone marrow-derived stromal cells from amyotrophic lateral sclerosis patients have diminished stem cell capacity. (2010) Stem cells and development 19(7): 1035-1042.
- 76. Bonafede, R., Scambi, I., Peroni, D., et al. Exosome derived from murine adipose-derived stromal cells: Neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. (2016) Exp cell res 340(1): 150-158.
- 77. Bonafede, R., Mariotti, R. ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles. (2017) Front cell neurosci 11: 80.
- 78. Fontanilla, C.V., Gu, H., Liu, Q., et al. Adipose-derived Stem Cell Conditioned Media Extends Survival time of a mouse model of Amyotrophic Lateral Sclerosis. (2015) Sci rep 5: 16953.
- 79. Tripodo, G., Chlapanidas, T., Perteghella, S., et al. Mesenchymal stromal cells loading curcumin-INVITE-micelles: a drug delivery system for neurodegenerative diseases. (2015) Colloids surf B Biointerfaces 125: 300-308.
- 80. Karussis,D., Karageorgiou, C., Vaknin-Dembinsky, A., et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. (2010) Arch neurol 67(10): 1187-1194.
- 81. Leray, E., Moreau, T., Fromont, A., et al. Epidemiology of multiple sclerosis. (2016) Rev neurol 172(1): 3-13.
- 82. Goldenberg, M.M. Multiple sclerosis review. (2012) P T 37(3): 175-184.
- 83. Steinman, L. Multiple sclerosis: a two-stage disease. (2001) Nat immunol 2(9): 762-764.
- 84. Siatskas, C., Bernard, C.C. Stem cell and gene therapeutic strategies for the treatment of multiple sclerosis. (2009) Currmol med 9(8): 992-1016.
- 85. Weber, M.S., Menge, T., Lehmann-Horn, K., et al. Current treatment strategies for multiple sclerosis - efficacy versus neurological adverse effects. (2012) Curr pharm des 18(2): 209-219.
- 86. Giacoppo, S., Bramanti, P., Mazzon, E. The transplantation of mesenchymal stem cells derived from unconventional sources: an innovative approach to multiple sclerosis therapy. (2017) Arch immunoltherexp65(5): 363-379.
- 87. Li, J., Chen, Y., Chen, Z., et al. Therapeutic effects of human adipose tissue-derived stem cell (hADSC) transplantation on experimental autoimmune encephalomyelitis (EAE) mice. (2017) Sci rep 7: 42695.
- 88. Marin-Banasco, C., Benabdellah, K., Melero-Jerez, C., et al. Oliver Gene therapy with mesenchymal stem cells expressing IFN-ss ameliorates neuroinflammation in experimental models of multiple sclerosis. (2017) Br j pharmacol 174(3): 238-253.
- 89. Payne, N.L., Dantanarayana, A., Sun, G., et al. Early intervention with gene-modified mesenchymal stem cells overexpressing interleukin-4 enhances anti-inflammatory responses and functional recovery in experimental autoimmune demyelination. (2012) Cell adhmigr 6(3): 179-189.
- 90. Strong, A.L., Bowles, A.C., Wise, R.M., et al. Human Adipose Stromal/Stem Cells from Obese Donors Show Reduced Efficacy in Halting Disease Progression in the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. (2016) Stem cells 34(3): 614-626.
- 91. Barnes, J., Mayes, M.D. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. (2012) Curropinrheumatol 24(2): 165-170.
- 92. Medsger, T.A. Epidemiology of systemic sclerosis. (1994) Clindermatol 12(2): 207-216.
- 93. Buchman, A.L. Side effects of corticosteroid therapy. (2001) J clingastroenterol 33(4): 289-294.
- 94. Khanna,D., Denton, C.P. Evidence-based management of rapidly progressing systemic sclerosis. . (2010) Best pract res Clinrheumatol 24(3): 387-400.
- 95. Mineda, K., Kuno, S., Kato, H., et al. Chronic inflammation and progressive calcification as a result of fat necrosis: the worst outcome in fat grafting. (2014) Plastreconstrsurg 133(5): 1064-1072.
- 96. Glasgold, R.A., Glasgold, M.J., Lam, S.M. Complications following fat transfer. (2009) Oral maxillofacsurgclin North Am 21(1): 53-58.
- 97. Lam, S.M., Glasgold, R.A., Glasgold, M.J. Limitations, complications, and long-term sequelae of fat transfer. (2008) Facial plastsurgclin North Am 16(4): 391-399.
- 98. Maria, A.T., Toupet, K., Maumus, M., et al. Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. (2016) J autoimmun 70: 31-39.
- 99. Granel, B., Daumas, A., Jouve, E., et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. (2015) Ann rheum dis 74(12): 2175-2182.
- 100. Detiger, S.E., Helder, M.N., Smit, T.H., et al. Adverse effects of stromal vascular fraction during regenerative treatment of the intervertebral disc: observations in a goat model. (2015) Euro spine j 24(9): 1992-2000.
- 101. Zanotti, C., Martinez-Puente, C., Pascual, I., et al. An assessment of the incidence of fistula-in-ano in four countries of the European Union. (2007) Int j colorectal dis 22(12): 1459-1462.
- 102. Ommer, A., Herold, A., Berg, E., et al. Cryptoglandular anal fistulas. (2011) DeutschesArzteblatt international 108(42): 707-713.
- 103. Sainio, P. Fistula-in-ano in a defined population. Incidence and epidemiological aspects. (1984) Ann chirgynaecol 73(4): 219-224.
- 104. Chauhan, N.S., Sood, D., Shukla, A. Magnetic Resonance Imaging (MRI) Characterization of Perianal Fistulous Disease in a Rural Based Tertiary Hospital of North India. (2016) Polish journal of radiology 81: 611-617.
- 105. Marzo, M., Felice, C., Pugliese, D., et al. Management of perianal fistulas in Crohn’s disease: an up-to-date review. (2015) World j gastroenterol 21(5): 1394-1403.
- 106. Dignass, A.U. Mechanisms and modulation of intestinal epithelial repair. (2001) Inflamm bowel dis 7(1): 68-77.
- 107. Scharl, M., Rogler, G. Pathophysiology of fistula formation in Crohn’s disease. (2014) World j gastrointestpathophysiol 5(3): 205-212.
- 108. Al-Maawali, A.K., Nguyen, P., Phang, P.T. Modern Treatments and Stem Cell Therapies for Perianal Crohn’s Fistulas. (2016) Can j gastroenterolhepatol (2016): 1651570.
- 109. D’Haens, G.R., Panaccione, R., Higgins, P.D., et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? (2011) Am j gastroenterol 106(2): 199-212.
- 110. Bouguen, G., Siproudhis, L., Gizard, E., et al. Long-term outcome of perianal fistulizingCrohn’s disease treated with infliximab. (2013) Clingastroenterolhepatol 11(8): 975-981.
- 111. Ciccocioppo, R., Corazza, G.R. Mesenchymal stem cells for fistulisingCrohn’s disease. (2016) Lancet 388(10051): 1251-1252.
- 112. Poritz, L.S., Rowe, W.A., Koltun, W.A. Remicade® does not abolish the need for surgery in fistulizingCrohn’s disease. (2002) Diseases of the colon &rectum 45(6): 771-775.
- 113. Topstad, D., Panaccione, R., Heine, J., et al. Combined seton placement, infliximab infusion, and maintenance immunosuppressives improve healing rate in fistulizinganorectalCrohn’s disease. (2003) Dis colon rectum 46(5): 577-583.
- 114. Parks, A.G., Gordon, P.H., Hardcastle, J.D. A classification of fistula-in-ano. (1976) Br j surg 63(1): 1-12.
- 115. Whiteford, M.H., Kilkenny, J., Hyman, N., et al. Practice parameters for the treatment of perianal abscess and fistula-in-ano (revised). (2005) Dis colon rectum 48(7): 1337-1342.
- 116. Dudukgian, H., Abcarian, H. Why do we have so much trouble treating anal fistula? (2011) World journal of gastroenterology 17(28): 3292-3296.
- 117. Sheikh, P. Controversies in fistula in ano. (2012) Indian j surg 74(3): 217-220.
- 118. Garcia-Aguilar, J., Davey, C.S., Le, C.T., et al. Patient satisfaction after surgical treatment for fistula-in-ano. (2000) Diseases of the colon and rectum 43(9): 1206-1212.
- 119. Abou-Zeid, A.A. Anal fistula: intraoperative difficulties and unexpected findings. (2011) World j gastroenterol 17(28): 3272-3276.
- 120. Grimaud, J.C., Munoz–Bongrand, N., Siproudhis, L., et al. Fibrin glue is effective healing perianal fistulas in patients with Crohn’s disease. (2010) Gastroenterology 138(7): 2275-2281.
- 121. O’riordan, J., Datta, I., Johnston, C., et al. A systematic review of the anal fistula plug for patients with Crohn’s and non-Crohn’s related fistula-in-ano. (2012) Dis Colon Rectum 55(3): 351-358.
- 122. Garcia-Olmo, D., Garcia-Arranz, M., Garcia, L.G., et al. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn’s disease: a new cell-based therapy. (2003) Int j colorectal dis 18(5): 451-454.
- 123. Garcia-Olmo, D., Herreros, D., Pascual, M., et al. Treatment of enterocutaneous fistula in Crohn’s Disease with adipose-derived stem cells: a comparison of protocols with and without cell expansion. (2009) Int j colorectal dis 24(1): 27-30.
- 124. García-Olmo, D., García-Arranz, M., Herreros, D., et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. (2005) Dis colon rectum 48(7): 1416-1423.
- 125. Garcia-Olmo, D., Herreros, D., Pascual, I., et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. (2009) Dis colon rectum 52(1): 79-86.
- 126. Lee, W.Y., Park, K.J., Cho, Y.B., et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. (2013) Stem cells 31(11): 2575-2581.
- 127. Guadalajara, H., Herreros, D., De-La-Quintana, P., et al. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. (2012) Int j colorectal dis 27(5): 595-600.
- 128. Molendijk, I., Bonsing, B.A., Roelofs, H., et al. Allogeneic bone marrow–derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. (2015) Gastroenterology 149(4): 918-927.
- 129. Garcia-Arranz, M., Herreros, M.D., Gonzalez-Gomez, C., et al. Treatment of Crohn’s-Related Rectovaginal Fistula With Allogeneic Expanded-Adipose Derived Stem Cells: A Phase I-IIa Clinical Trial. (2016) Stem cells transl medicine 5(11): 1441-1446.
- 130. Panes, J., Garcia-Olmo, D., Van, Assche, G., et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. (2016) Lancet 388(10051): 1281-1290.
- 131. Bender, E. Cell-based therapy: Cells on trial. (2016) Nature 540(7634): S106-S108.
- 132. Cao, Y., Ding, Z., Han, C., et al. Efficacy of Mesenchymal Stromal Cells for Fistula Treatment of Crohn’s Disease: A Systematic Review and Meta-Analysis. (2017) Dig dis sci 62(4): 851-860.
- 133. Ryska, O., Serclova, Z., Mestak, O., et al. Local application of adipose-derived mesenchymal stem cells supports the healing of fistula: prospective randomised study on rat model of fistulisingCrohn’s disease. (2017) Scand j gastroenterol 52(5): 543-550.
- 134. Johnson, V.L., Hunter, D.J. The epidemiology of osteoarthritis. (2014) Best pract res Clinrheumatol 28(1): 5-15.
- 135. Peat, G., Mc, Carney, R., Croft, P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. (2001) Ann rheum dis 60(2): 91-97.
- 136. Cross, M., Smith, E., Hoy, D., et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. (2014) Ann rheum dis 73(7): 1323-1330.
- 137. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. (2016) Lancet 388(10053): 1545-1602.
- 138. Buckwalter, J.A., Saltzman, C., Brown, T. The impact of osteoarthritis: implications for research. (2004) Clinorthop related res S6-S15.
- 139. Parrilli, A., Giavaresi, G., Ferrari, A., et al. Subchondral bone response to injected adipose-derived stromal cells for treating osteoarthritis using an experimental rabbit model. (2017) Biotech histochem 92(3): 201-211.
- 140. Glyn-Jones, S., Palmer, A.J., Agricola, R., et al. Osteoarthritis. (2015) Lancet 386(9991): 376-387.
- 141. Goldring, S.R. Alterations in periarticular bone and cross talk between subchondral bone and articular cartilage in osteoarthritis. (2012) TherAdvMusculoskelet Dis 4(4): 249-258.
- 142. Loeser, R.F., Goldring, S.R., Scanzello, C.R., et al. Osteoarthritis: a disease of the joint as an organ. (2012) Arthritis rheum 64(6): 1697-1707.
- 143. Sellam, J., Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. (2010) Nat rev Rheumatol 6(11): 625-635.
- 144. Yu, S.P., Hunter, D.J. Managing osteoarthritis. (2015) Australian Prescriber 38(4): 115-119.
- 145. Clouet, J., Vinatier, C., Merceron, C., et al. From osteoarthritis treatments to future regenerative therapies for cartilage. (2009) Drug discov today 14(19-20): 913-925.
- 146. Liu, S.H., Driban, J.B., Eaton, C.B., et al. Objectively Measured Physical Activity and Symptoms Change in Knee Osteoarthritis. (2016) Am j med 129(5): 497-505.
- 147. Burke, J., Hunter, M., Kolhe, R., et al. Therapeutic potential of mesenchymal stem cell based therapy for osteoarthritis. (2016) Clintransl med 5(1): 27.
- 148. Bosomworth, N.J. Exercise and knee osteoarthritis: benefit or hazard? (2009) Canfam physician 55(9): 871-878.
- 149. Fransen, M., Mc, Connell, S. Exercise for osteoarthritis of the knee. (2008) Cochrane database syst rev (4): Cd004376.
- 150. Fransen, M., Mc, Connell, S. Land-based exercise for osteoarthritis of the knee: a metaanalysis of randomized controlled trials. (2009) J Rheumatol 36(6): 1109-1117.
- 1541. Burke, J., Hunter, M., Kolhe, R., et al. Therapeutic potential of mesenchymal stem cell based therapy for osteoarthritis. (2016) Clinical and translational medicine 5(1): 27.
- 152. Nuesch, E., Rutjes, A.W., Husni, E., et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. (2009) Cochrane database syst rev (4): Cd003115.
- 153. Wright, E.A., Katz, J.N., Abrams, S., et al. Trends in prescription of opioids from 2003-2009 in persons with knee osteoarthritis. (2014) Arthritis care res 66(10): 1489-1495.
- 154. Lanas, A. Nonsteroidalantiinflammatory drugs and cyclooxygenase inhibition in the gastrointestinal tract: a trip from peptic ulcer to colon cancer. (2009) Am J Med Sci 338(2): 96-106.
- 155. Crofford, L.J. Use of NSAIDs in treating patients with arthritis. (2013) Arthritis Res Ther 15(3): S2.
- 156. Brater, D.C., Harris, C., Redfern, J.S., et al. Renal effects of COX-2-selective inhibitors. (2001) Am J Nephrol 21(1): 1-15.
- 157. Dockerty, T., Latham, S.K., Smith, T.O. Why don’t patients take their analgesics? A meta-ethnography assessing the perceptions of medication adherence in patients with osteoarthritis. (2016) Rheumatolint 36(5): 731-739.
- 158. Kongtharvonskul, J., Anothaisintawee, T., McEvoy, M., et al. Efficacy and safety of glucosamine, diacerein, and NSAIDs in osteoarthritis knee: a systematic review and network meta-analysis. (2015) Eur j med res 20: 24.
- 159. Kovic, S.V., Vujovic, K.S., Srebro, D., et al. Prevention of Renal Complications Induced by Non- Steroidal Anti-Inflammatory Drugs. (2016) Current medicinal chemistry 23(19): 1953-1964.
- 160. Laev, S.S., Salakhutdinov, N.F. Anti-arthritic agents: progress and potential. (2015) Bioorg med chem 23(13): 3059-3080.
- 161. Lee, T., Lu, N., Felson, D.T., et al. Use of non-steroidal anti-inflammatory drugs correlates with the risk of venous thromboembolism in knee osteoarthritis patients: a UK population-based case-control study. (2016) Rheumatology (Oxford) 55(6): 1099-1105.
- 162. Hunter, D.J., Schofield, D., Callander, E. The individual and socioeconomic impact of osteoarthritis. (2014) Nat rev Rheumatol 10(7): 437-441.
- 163. Desando, G., Cavallo, C., Sartoni, F., et al. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. (2013) Arthritis Res Ther 15(1): R22.
- 164. Kuroda, K., Kabata, T., Hayashi, K., et al. The paracrine effect of adipose-derived stem cells inhibits osteoarthritis progression. (2015) BMC MusculoskeletDisord 16: 236.
- 165. Jin, R., Shen, M., Yu, L., et al. Adipose-Derived Stem Cells Suppress Inflammation Induced by IL-1β through Down-Regulation of P2X7R Mediated by miR-373 in Chondrocytes of Osteoarthritis. (2017) Mol Cells 40(3): 222-229.
- 166. Hildner, F., Concaro, S., Peterbauer, A., et al. Human adipose-derived stem cells contribute to chondrogenesis in coculture with human articular chondrocytes. (2009) Tissue eng Part A 15(12): 3961-3969.
- 167. Shi, J., Liang, J., Guo, B., et al. Adipose-Derived Stem Cells Cocultured with Chondrocytes Promote the Proliferation of Chondrocytes. (2017) Stem cells international 2017: 1709582.
- 168. Maumus, M., Manferdini, C., Toupet, K., et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. (2013) Stem cell res 11(2): 834-844.
- 169. Toghraie, F., Razmkhah, M., Gholipour, MA., et al. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. (2012) Arch Iran med 15(8): 495-499.
- 170. Kriston-Pal, E., Czibula, A., Gyuris, Z., et al. Characterization and therapeutic application of canine adipose mesenchymal stem cells to treat elbow osteoarthritis. (2017) Can J Vet Res 81(1): 73-78.
- 171. Black, L.L., Gaynor, J., Gahring, D., et al. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. (2007) Vet Ther 8(4): 272-284.
- 172. Black, L.L., Gaynor. J., Adams. C., et al. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. (2008) Vet Ther 9(3): 192-200.
- 173. Guercio, A., Di, Marco, P., Casella, S., et al. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. (2012) Cell BiolInt 36(2): 189-194.
- 174. Ko, J-Y., Lee, J., Lee, J., et al. Intra-articular Xenotransplantation of Adipose-Derived Stromal Cells to Treat Osteoarthritis in a Goat Model. (2017) Tissue Engineering Regenerative Med 14(1): 65-71.
- 175. ter, Huurne, M., Schelbergen, R., Blattes, R., et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. (2012) Arthritis Rheum 64(11): 3604-3613.
- 176. Frisbie, D.D., Kisiday, J.D., Kawcak, C.E., et al. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. (2009) J orthop res 27(12): 1675-1680.
- 177. Mei, L., Shen, B., Ling, P., et al. Culture-expanded allogenic adipose tissue-derived stem cells attenuate cartilage degeneration in an experimental rat osteoarthritis model. (2017) PLoS One 12(4): e0176107.
- 178. Jo, C.H., Lee, Y.G., Shin, W.H., et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. (2014) Stem cells 32(5): 1254-1266.
- 179. Koh, Y.G., Jo, S.B., Kwon, O.R., et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. (2013) Arthroscopy 29(4): 748-755.
- 180. Pers, Y.M., Rackwitz, L., Ferreira, R., et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. (2016) Stem cells transl med 5(7): 847-856.
- 181. Kolkundkar, U., Gottipamula, S., Majumdar, A. Cell therapy manufacturing and quality control: current process and regulatory challenges. (2014) J Stem Cell Res Ther 4(9): 1-10.
- 182. Ghooi, R.B., Bhosale, N., Wadhwani, R., et al. Assessment and classification of protocol deviations. (2016) PerspectClin Res 7(3): 132-136.
- 183. Quinley, E.D. Quality and Regulatory Issues in Cellular Therapy. (2013) Transfusion Med Hemostasis 577-584.
- 184. Tobita, Morikuni., Kenji, Konomi., Yasuhiro, \Torashima., et al. “Japan’s challenges of translational regenerative medicine: act on the safety of regenerative medicine.” (2016) Regenerative Therapy 4: 78-81.