Management of the Airway in Transoral Robotic Surgery for Head and Neck Cancer
Belen San Antonio2, Manuel Perez-Marquez3, Jose L Ayala2, Raimundo Gutierrez-Fonseca1
Affiliation
- 1Department of Otorhinolaryngology, Rey Juan Carlos University Hospital, Madrida
- 2Department of Anesthesiology, Rey Juan Carlos University Hospital, Madrid
- 3Intensive Care Unit, Rey Juan Carlos University Hospital, Madrid
Corresponding Author
Jose Granell, Rey Juan Carlos University Hospital,c/ Gladiolo s/n, 28933 Mostoles, Madrid, E-mail: jose.granell@hospitalreyjuancarlos.es
Citation
Granell, J., et al. Management of the Airway in Transoral Robotic Surgery for Head and Neck Cancer. (2017) J Anesth Surg 4(1): 9- 14.
Copy rights
© 2017 Granell, J. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Postoperative intubation; Transoral robotic surgery; Oncological indications
Abstract
Aim of the study: To evaluate the safety and effectiveness of a conservative management of the airway without tracheotomy in a new transoral robotic surgery program for head and neck cancer.
Materials and Method: Observational prospective study on a cohort without a control group. We included patients diagnosed of oropharyngeal, hypopharyngeal or laryngeal cancer who underwent transoral robotic surgery between July 2013 and July 2016.
Results: Thirty-six patients met the inclusion criteria; 72% were oropharyngeal tumors (most frequently, 13 cases, tumors of the base of the tongue). The most frequent local extension was T2 (18 cases) but almost two-thirds (64%) were classified as advanced tumors (stages III and IV) due to the N stage. Lymph node surgery and transoral primary tumor surgery were staged when required. The intervention was successful in all cases. After robotic surgery, the patients in risk remained intubated for 24 hours. All were managed without a tracheostomy except for a patient with a synchronous diagnosis of sleep apnea (who received a temporary prophylactic tracheostomy) and a case of combined transoral-transcervical surgery (who received a non-programmed tracheotomy). There were no relevant perioperative incidences related to the airway except for a case of delayed bleeding.
Conclusions: In our early experience, with a conservative management protocol with two-stage surgery and programmed postoperative intubation, transoral robotic surgery for oncological indications has been feasible and safe without a tracheotomy.
Introduction
The introduction of technological innovations has become a constant of current clinical practice. Robotic assisted surgery is one of the most important innovations of recent years within the scope of Minimally Invasive approach Surgery (MIS). The first widely used robotic surgery system, the da Vinci (Intuitive Surgical Inc. Sunnyvale, California, USA), was marketed at the end of 1999. The current model, the Xi, released in year 2014, is the fourth generation. It is a remote surgery system designed to optimize MIS. Although not originally conceived for transoral application, the experimental development has shown that it is safe and effective for the treatment of diseases of the upper aerodigestive tract[1]. Clinical experience has made it particularly useful for the surgical treatment of head and neck cancer[2]. Virtually it allows the transoral resection of any lesion of the oropharynx[3,4], hypopharynx or larynx[5], provided that it is limited to soft tissue. Indications are still being defined today[6].
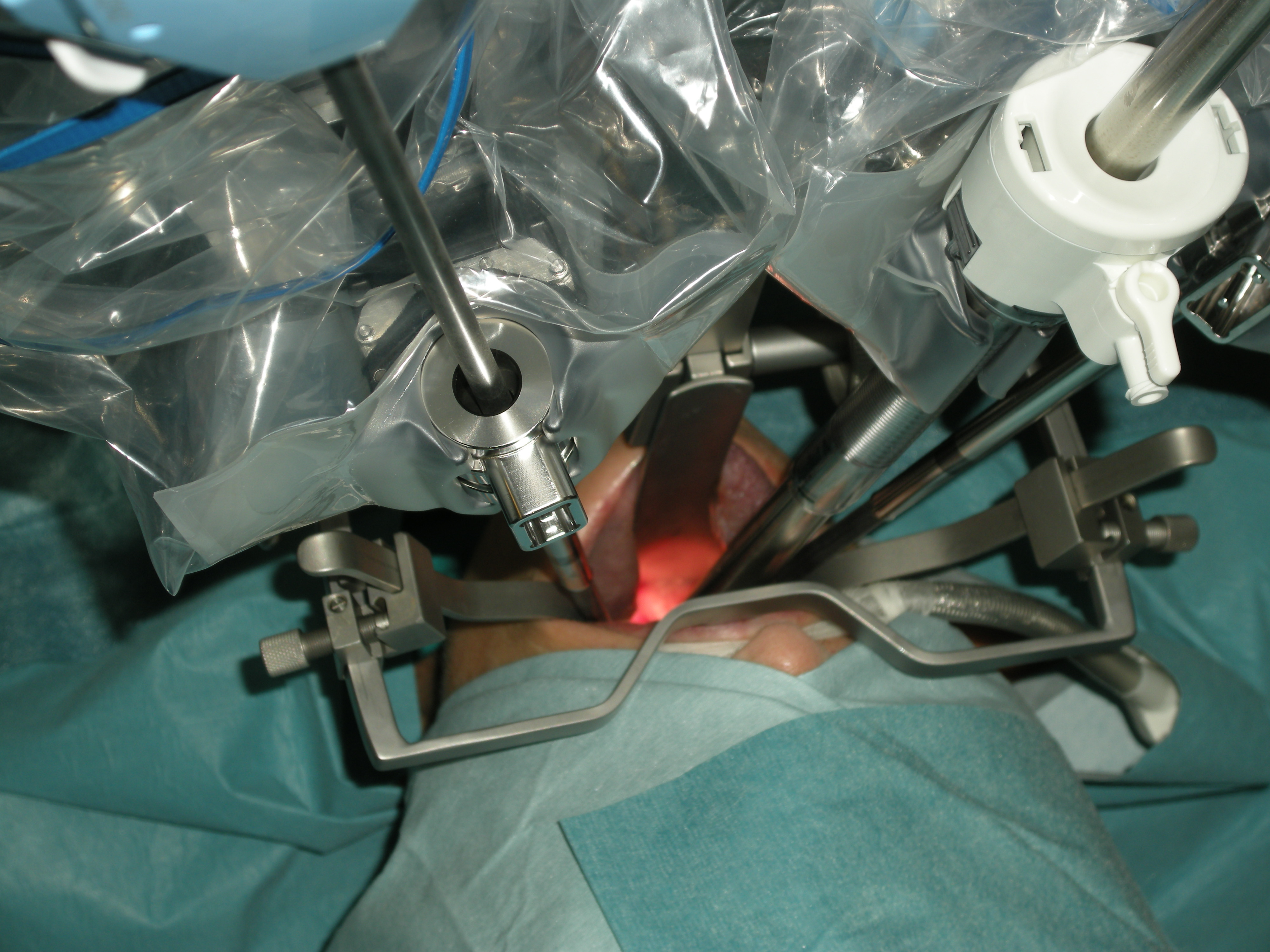
TransOral Robotic Surgery (TORS) is a new challenge for the anaesthesiologist. Any transoral surgical approach implies a conflict of space between the working space of the surgeon and that of the anaesthetist, who must control the airway in the same anatomical territory of the surgical approach. In TORS, the placement of voluminous auto-static device to gain exposure and the introduction of three robotic arms through the mouth makes the situation even more complex (Figure 1). In oncologic surgery, frequently with extensive resections and with the risk of postoperative airway compromise, or potentially serious intra-or postoperative haemorrhagic complications, the situation can become a real challenge if we are to perform the procedures without a safety tracheostomy.
Figure 1: Transoral robotic approach. The exposure is obtained with the FKWO pharyngolaryngoscope. The camera arm enters in a central position and the other two robotic arms (instrument arms) converge from the sides.
TORS is in fair expansion[7]. Although it is true that they are not particularly complex interventions from the anaesthetic point of view, it is appropriate to know some peculiarities of the perioperative care. At the start of the TORS program at our centre we did plan to prospectively evaluate safety in the application of this new technology, and thus specifically control the potential level of damage. This is particularly important in those aspects that involve significant differences with standard treatment protocols. Since the standard surgical approaches of the oropharynx and many lesions of the hypopharynx and supraglottis associate temporal tracheotomy, this was considered a point of particular interest. Therefore, it was proposed to describe the perioperative management of the airway in patients with head and neck cancer who underwent TORS.
The primary objective of this study was to evaluate the feasibility and safety of conservative management of the airway without tracheotomy in patients undergoing TORS for head and neck cancer. As a secondary objective, we searched for possible risk factors that could condition airway management problems, defined as variations on the established protocol.
Materials and Methods
We designed a prospective observational study. A time horizon was proposed from the beginning of the program in July 2013, until July 2016. We included patients with a diagnosis of carcinoma of the oropharynx, hypopharynx or larynx undergoing TORS. Data were collected prospectively from diagnosis to completion of treatment.
The TORS-oncology program protocol is supervised by the institutional quality control system[8]. Patients with tumors of the upper aerodigestive tract that can be surgically treated with functional preservation and are resectable by TORS are included, subject to agreement in the Multidisciplinary Committee on Head and Neck Cancer.
The anaesthetic management protocol includes a standard pre-anaesthetic assessment, with particular attention to the prediction of the difficult airway)[9,10]. Unless there is a reasonable contraindication, interventions are scheduled without a tracheotomy. The surgical position is supine, with atlo-occipital extension for transoral access from the patient’s head. Patient monitoring is performed and the usual aesthetic protocol is used: pre-oxygenation for 2 minutes with oropharyngeal cannula, intravenous induction with propofol, fentanyl and rocuronium, inhaled anaesthesia with sevoflorane with dose adjusted according to BIS monitor (Covidien Mansfield, USA). For the most frequent indication of TORS, on the oropharynx, a nasotracheal intubation facilitates surgical work. If possible, the nostril contralateral to the tumor is chosen. In anticipation of a difficult airway, a Froba tracheal tube introducer (Cook Inc. Bloomington, IN. USA) and alternative intubation device such as the Airtrack (Ajl Prodol meditec Ltd. Guangdong, China) are prepared if conventional intubation fails. In all cases of pharyngolaryngeal surgery, the material for an emergency tracheotomy is ready for use, and the surgeon is present in the operating room during the intubation manoeuvre. For the orotracheal intubation we use a reinforced tube (Teleflex medical. Malasya) number 7 and for nasotracheal intubation a Portex nasal tube (Smith Medical Int. Kent. UK) also of number 7 or 6.5. Once the airway has been secured the patient receives eye protection goggles, a dental protector, and two venous accesses are warranted. Placement of a central venous access is not considered necessary, since it is a surgery in which discrete hematic loss and minimal hemodynamic instability is foreseen, being exceptional that vasoactive drugs are required. However, a cannula is put into the radial artery to facilitate gasometry and laboratory monitoring during surgery and in the immediate postoperative period, since patients will be intubated for at least 24 hours in the Intensive Care Unit (ICU). High doses of intraoperative analgesia are required: fentanyl up to a total of 8 - 10 mcg/kg and/or remifentanil infusion. It is also usual to require some occasional urapidil bolus for the control of blood pressure and sympathetic stimulation that occurs at specific times of surgery, such as lingual traction in the introduction of retractor FKWO (Olympus Corp. Tokyo, Japan). Although the surgical time of excision may be relatively short, intraoperative biopsies are often required, and might lengthen the anaesthetic time. At the end of the procedure, an orotracheal tube replaces the nasal tube if the last was used during surgery. The patient is transferred to the ICU where is kept under orotracheal intubation for the next 24 hours, being extubated the next day.
The management protocol contemplates the accomplishment of surgery in two times in case a neck dissection is required. Neck surgery is scheduled between one and two weeks prior to transoral surgery. Times given in the current study refer only to TORS itself.
For the present study, variables related to airway characteristics and management, and potential risk factors for complications were collected[11]. Preoperatively, in addition to the oncological diagnosis (TNM staging), age, BMI and ASA classification, Mallampatti, difficulty in cervical extension, oral opening and teeth status were documented. Also Cormack-Lehane grade, type of intubation and eventually the existence of a difficult intubation, the quality of the exposure (insufficient, bad, moderate, good or excellent), type of surgery, surgical time, and presence of intraoperative complications. During the postoperative period, intubation time with mechanical ventilation was recorded and total time at the ICU (hours), as well as any complications. The series is described according to the indicated variables. We analyse the compliance with the airway management protocol and the implication of the potential risk factors inthe non-compliance. For all of the cases we did use a da Vinci S HD system, except for the last supraglottic laryngectomy case, which was done with the da Vinci Xi system.
Results
July 2013 to July 2016 were first 3 years of the TORS - oncology program at our centre. The study group consisted of 32 men and 3 women (one patient was operated twice because of two metachronous tumors). The mean age was 63.4 years (SD = 8.9 years). All tumors were epidermoid carcinomas except for one liposarcoma. Most tumors (72.2%) were located in the oropharynx (13 at the base of the tongue, 6 at the palatine tonsil, 1 at the tonsillar pillar, 3 at the lateral wall and 2 at the posterior wall); The rest were supraglottic tumors (9 cases), and a pyriform sinus tumor (hypopharynx). Two tumors belong to the same patient in whom a robotic supraglottic laryngectomy was performed for a pT2pN0, and 6 months later the robotic transoral excision of a second primary (pT1) of the posterior wall of the oropharynx. Half of the cases (18) were classified as T2. In order of frequency the rest were T1 (4), T4a (3) and T3 (1). Therefore, 11.1% were locally advanced tumors (T3 and T4), but due to the presence of lymph node metastases, the percentage of cases classified as advanced stages (III and IV) rose to 64%. As corresponds to a series with primarily surgical treatment, all cases were M0. Also, as per staging, 86% of the patients received a neck dissection. In half of the cases it was bilateral (all of then functional neck dissections, except for two necks with radical dissections), an in 77.8 it was staged (before TORS). Simultaneous surgery was more frequent in the last year for patients with radical tonsillectomy and unilateral functional neck dissection.
All patients in whom TORS was indicated received anaesthetic approval with an ASA II (66.7%) or III (33.3%). In two cases a difficult airway was predicted in patients with a Mallampatti III and IV respectively. The mean BMI was 24.3 kg / m² (SD = 2.3 kg / m²). 22.2% of patients had normal dentition, 33.3% had partial dentition, and 16.7% had edentulous teeth. The remaining 27.8% were considered to have a septic mouth; in 3 cases in which the possibility of adjuvant radiotherapy was likely, a complete extraction of the remaining teeth was performed, so that, for the purposes of the approach, they became edentulous. In no case was it considered that there were relevant limitations in oral opening or cervical extension.
Elective tracheotomy was scheduled in a patient with a tongue-base tumor diagnosed with sleep apnea-hypopnea syndrome (OSAS) who was using a CPAP device. Although our TORS-SAHS protocol does not include routine tracheotomy, in this case it was considered an unnecessary risk not to do so. Another patient with a combined transoral-transcervical surgery got a non-programmed tracheostomy. In the rest of the cases, natural airway management was planned, including one patient with previous supracricoid hemipharyngolaryngectomy (10 years earlier).
The mean time for the setup of the patient and the operating room was 55 minutes (SD = 11 minutes). The mean transoral surgery time was 59 minutes (SD = 29 minutes), with a minimum of 24 minutes and a maximum of 142 minutes.
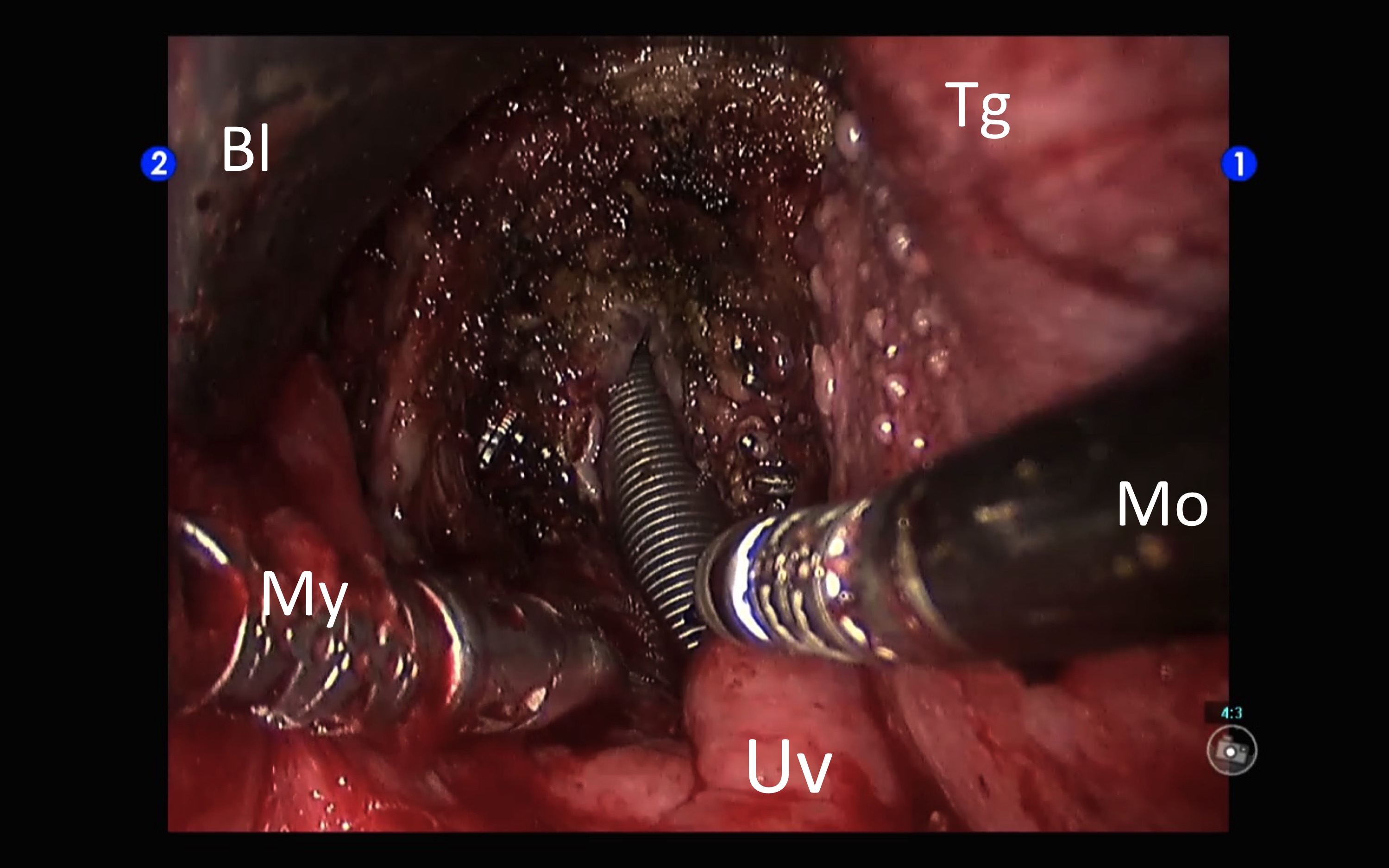
Only one of the predicted difficult airways cases met difficult intubation criteria. However, in 5 other patients there was an unplanned difficult intubation, two of them with Cormack-Lehane III and another IV. In all, 6 cases met difficult intubation criteria, although all situations were resolved with a standard management and the expected surgical procedures could be completed. In spite of the incidences in intubation, the surgeon rated the quality of the transoral exposure as good or excellent in all but one of the cases (it was considered to be poor in a patient with a T1 pyriform sinus tumor). In no case was necessary to convert to open or discontinue the procedure because of exposure problems. All of the procedures were successfully completed. In most cases (72%) nasotracheal intubation was used. It was performed with a nasal tube except in two cases where a reinforced tube was used. In the oral route the most frequent tube size was number 7, and in the nasal one equally number 7 and 6.5. The change from nasal to orotracheal at the end of the interventions was performed without difficulty (Figure 2).
Figure 2: Surgical bed at the end of robotic transoral resection of the base of the tongue and supraglottis (pT3pN2aM0 squamous cell carcinoma of the left valecula). It was a Mallampatti IV that was handled with nasotracheal intubation. At the moment of changing the tube we take advantage of the excellent surgical exposure of the glottis after the excision. Bl: short TORS blade of the FKWO. Tg: tongue. Mo: 5 mm monopolar cautery. Uv: uvula. My: 5 mm Maryland dissector.
All of the patients were submitted to the Intensive Care Unit for postoperative surveillance, most off them under mechanical ventilation. The risk assessment at ICU admission had an average of 7.35 (SD = 4.12) on the Apache II scale, and 20.29 (SD = 6.16) on SAPS II. The mean length of stay in ICU was 58 hours (SD = 25 hours). In patients with orotracheal intubation, mean intubation time was 30 hours (SD = 13 hours). Four patients were not extubated “the next day”. A multivariate logistic regression analysis was performed to identify variables associated with prolonged intubation between the previous risk (age, locally advanced tumor, VAD prediction), intraoperative (difficult intubation, transoral surgery time, extent of resection) and operating room (risk scales Apache II and SAPS II at ICU admission). No statistical significance was found for any of them.
Complications were considered as prolongations in the time of intubation and a case of lingual oedema that motivated one of the prolonged intubations, besides pneumonia during the postoperative period in one of the laryngeal tumors. There were no intraoperative haemorrhagic complications, but two postoperative complications: diffuse haemorrhage of the tongue base surgical site the same afternoon of the intervention (with the patient intubated) and a bleeding after a week of a tongue base resection. Both required surgical revision and were resolved without further incidents. The patient with a programmed tracheostomy for simultaneous SAHS, a valecula tumor that required resection of the base of the tongue and supraglottis, was extubated after 14 days.
27% of patients received adjuvant radiotherapy and 33% received radio chemotherapy. At no time of treatment was there any incidence with the airway. At the end of the treatment, all the patients but three had a complete oral diet. Two patients with laryngeal tumors had a temporarily gastrostomy during radiochemotherapy because of unsafe swallowing in the early postoperative period. Three patients have a gastrostomy at the end of the follow-up: the patient with the previous supracricoid surgery, the one with the delayed bleeding, who suffered neurological complications, and the one with a combined resection (also still with a tracheostomy). No patient experienced delayed respiratory complications attributable to aspiration.
Discussion
Temporary elective tracheotomy is a safety measure in major head and neck surgical procedures; is a constant for certain approaches, such as the transmandibular approach used for the lateral wall of the oropharynx and the base of the tongue[12]. The risk is related to the potential compromise of the airway in the postoperative period due to oedema and haemorrhage, which may be associated with an important morbidity and mortality due to the eventual impossibility for reintubation and bronchial aspiration.
Although transoral surgery (be it robotic or with other instrumentation) by definition implies a minimization of the damage associated with the approach, this risk of complications continues to exist. Even so, the expansion of transoral approaches favoured by Transoral Laser Microsurgery (TLM) was associated with a decrease in the number of temporary tracheotomies[13], while allowing more ambitious transoral resections, particularly in the larynx[14]. TLM techniques on the larynx are reproducible by TORS, but TORS also allows more ambitious resections in the oropharynx. Of course, this potentially increases the risk of complications.
In the published series, the management of the airway in oncological patients operated by TORS is diverse. In one of the first ones, published by the authors who originally developed the technique, out of 27 patients, most of them remained intubated for 24 to 72 hours[15]. In another series of 54 patients, 12 remained intubated 48 hours and 2 patients had tracheotomy[16]. In a third early series of 45 cases, which mostly included tonguebase cancer, 14 patients left the operating theatre with a tracheotomy[17]. In more recent series, the percentage of tracheotomies is lower (4 of 26)[18] or even null[19], although for the same locations (e.g. supraglottis) there are still large variations that include “always”[20] or “never”[21]. This probably reflects a certain degree of arbitrariness. The original protocol of the University of Pennsylvania indicates tracheotomy in resections combining tongue base and supraglottis, if a difficult reintubation is expected (e.g. by previous radiotherapy) or by other medical indications (e.g. morbid obesity)[22]. In our case, two of these indications (combined resection and OSAS) have been managed with prolonged intubation. The same protocol indicates prolonged intubation for most supraglottic laryngectomies or when further oedema is expected from resections adjacent to the valecula or epiglottis, or prolonged surgeries with increased risk of tongue oedema. Our initial protocol routinely includes programmed intubation for 24 hours. Eventually sedation to maintain intubation also improves analgesic control in the first hours and may also reduce the risk of bleeding due to the absence of voluntary mobility of the intervened tissues, as well as the non-existence of Valsalva manoeuvres. It also minimizes the risk of an eventual need for emergency reintubation. In more recent series non-staged surgery and extubation in the operating room have been found to be safe[23]. Therefore a less conservative protocol than ours is probably also safe, although certainly each team has to adapt toits own experience and the circumstances of each hospital.
On a case-by-case basis, patients in whom the tube was maintained longer than initially scheduled, the first was a T2N2c tumor of the tongue base, the only case in which bilateral cervical lymph node dissection was performed simultaneously, in addition to bilateral resection with exposition of both lingual arteries, which, independently of the robotic instrumentation, placed us, in the absence of a tracheotomy, in an unprecedented postoperative situation that was handled with extreme caution. The second was a vertical hemilaryngectomy for a glottic-supraglottic T2, an intervention that also places a particular risk for the airway. The third was a patient with resection of the tongue and supraglottis, a situation also particularly compromised for eventual haemorrhagic complications. These types of combined resections are common in the most usual indication of TORS that is for lesions of the base of the tongue. The last case is a resection by a T1 of base of tongue that developed a marked oedema of tongue in the postoperative that forced to delay extubation. Lingual oedema is a problem intrinsic to the transoral approach, possibly related not only to surgical time, but also to other factors such as the type of retractor (and the particular pressure in each case) or the quality of exposure.
Neck surgery at a separate time is another option. In addition to decreasing postoperative oedema, it allows us to control the most relevant vascular structures of the primary site (particularly the lingual artery). On the other hand, the risk of intraoperative communication of transoral and cervical resections decreases, neutralizing one of the possible indications for reconstruction[24]. Complex reconstruction conditions the need for a tracheotomy. Secondly, to separate the transoral time optimizes robotic operating time.
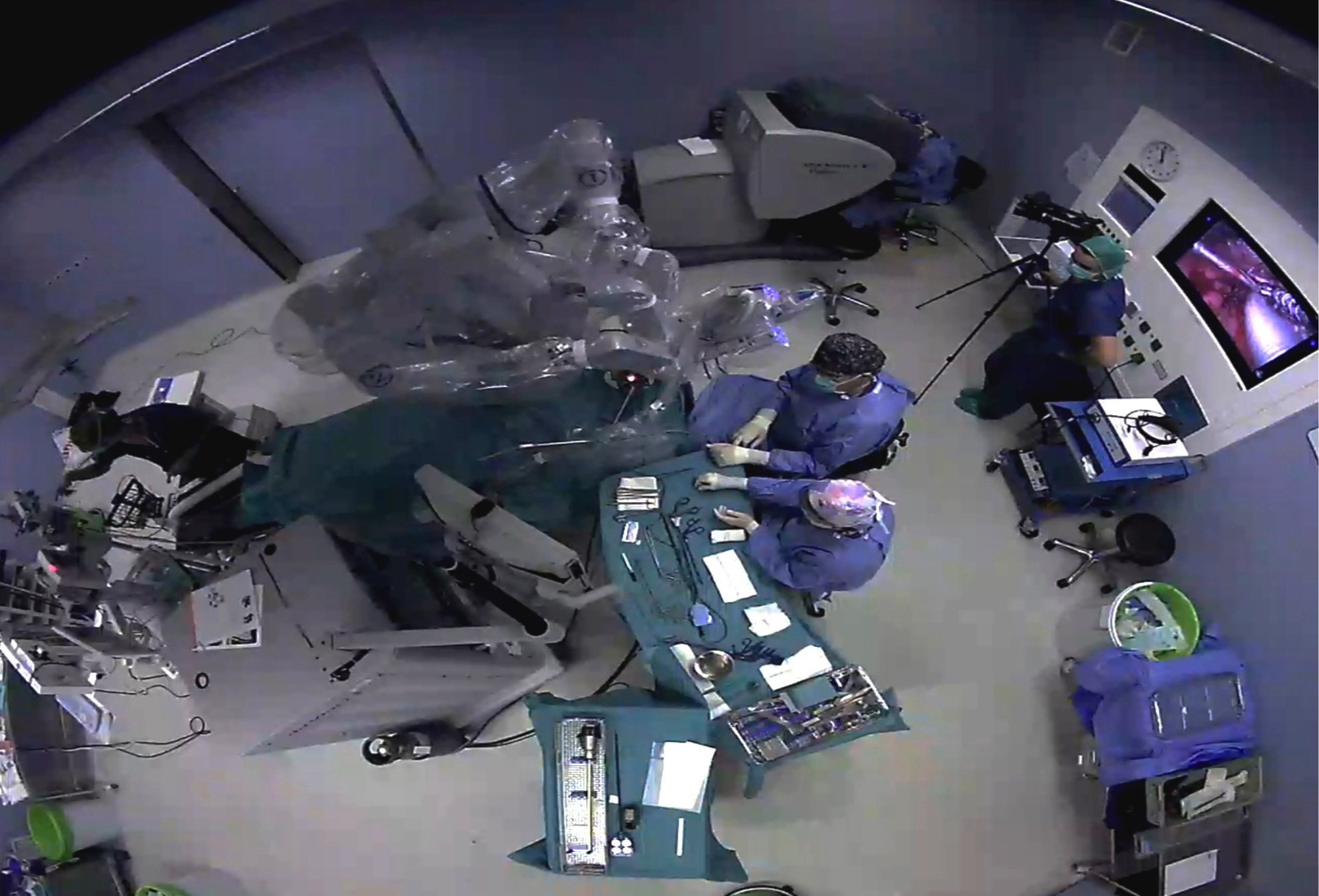
It is clear that the management of these patients involves care that goes beyond the robotic instrumentation itself, and that justifies the design of the transoral robotic surgery program. Regarding the intraoperative situation there are some important considerations from the anaesthetic point of view. The patient, once intubated, will be rotated 180º from the anaesthesia machine. The robot pedestal (patient-side cart), instrument tables, assistant surgeon and nurse will occupy the patient’s head and chest area (Figures 3). A rigid retractor, the FKWO, and the arms of the robot occupy the surgical field, so that the head and thorax are difficult to access, which must be foreseen in relation to the fixation of tube and eventual vascular accesses. Obviously a large enough operating room is needed, in addition to a good coordination of the entire surgical team. Specific training is required for all staff, including nursing staff. Even so, we assume an increase in the time of response to an emergency as a result of this difficult access to the patient. We have not had any such situation that is unlikely if we check the published series.
Figure 3: Operating theatre setup for TORS. As usual in head and neck procedures, the anaesthesia carriage is placed at the patient’s feet. In the case of robotic surgery, the head is also occupied by bulky items of equipment, and there is a complex cabling between them. In addition the patient’s car is in a blocking situation while the trocars are placed in the robotic arms. This should be taken into account for potential emergency situations. The surgeon is at the console, away from the surgical field. An assistant-surgeon and the assistant are placed inside the sterile field at the head of the patient.
Regarding the type of tube although the initial recommendations included that of using a laser-protected tube because of the risk of damaging it with the monopolar coagulation spatula commonly used in TORS. In clinical practice this is very unlikely. It is also a problem for the nasal intubations because the laser tubes are short. We use conventional tubes. Nasal intubation moves the tube away from the resection area, particularly in the oropharynx. The short intervention time also allows us to use smaller diameter tubes.
We have to highlight some limitations of the study. It is an observational study on consecutive patients selected based on a surgical indication. The selection of patients who will undergo TORS might be different between centres. See again the description of patient´s features. Also the management of the postoperative period and the airway itself might be variable depending on factors like the availably of ICU or ENT surgeon on-call. As a general rule, putting patient safety first should be the motto.
The concept of MIS is intimately linked to the very development of robotic surgery. From our point of view, the objective of minimizing morbidity includes in head and neck surgery the avoidance of tracheotomy if we consider that this attitude is safe. In our series, a conservative protocol with two-stage surgery and prolonged programmed intubation has allowed us to do a non-tracheotomy surgery in almost all of the cases. Thus, in our experience, the realization of TORS in patients with pharyngolaryngeal tumors has been safe without a tracheotomy.
References
- 1. Weinstein, G.S., O'Malley, B.W., Magnuson, J.S., et al. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. (2012) Laryngoscope 122(8): 1701-1707.
- 2. Kelly, K., Johnson-Obaseki, S., Lumingu, J., et al. Oncologic, functional and surgical outcomes of primary Transoral Robotic Surgeryfor early squamous cell cancer of the oropharynx: A systematic review. (2014) Oral Oncol 50(8): 696-703.
- 3. Granell, J., Mendez-Benegassi, I., Millas, T., et al. Transoral robotic surgery: step-by-step radical tonsillectomy. (2014) Case Reports in Otolaryngology.
- 4. O'Malley, B.W., Weinstein, G.S., Snyder, W., et al. Transoral robotic surgery (TORS) for base of tongue neoplasms. (2006) Laryngoscope 116(8): 1465-1472.
- 5. Weinstein, G.S., O'Malley, B.W., Snyder, W., et al. Transoral robotic surgery: supraglottic partial laryngectomy. (2007) Ann Otol Rhinol Laryngol 116(1): 19-23.
- 6. Granell, J., Weinstein, G.S., Gutierrez-Fonseca, R. Adjusting the focus of transoral robotic surgery. (2015) Acta Otorrinolaringol Esp 66(3): 181-182.
- 7. Mydlarz, W.K., Chan, J.Y., Richmon, J.D. The role of surgery for HPV-associated head and neck cancer. (2015) Oral Oncol 51(4): 305-313.
- 8. Transoral Robotic Surgery Program in Otorhinolaryngology. Otorrinolaringología.
- 9. Apfelbaum, J.L., Hagberg, C.A., Caplan, R.A., et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. (2013) Anesthesiology 118(2): 251-270.
- 10. Law, J.A., Broemling, N., Cooper, R.M., et al. The difficult airway with recommendations for management--part 2--the anticipated difficult airway. (2013) Can J Anaesth 60(11): 1119-1138.
- 11. Espinosa Domínguez, E., Reverón Gómez, M.A., Pérez Méndez, L., et al. Risk factors for postoperative complications in major head and neck surgery. (2011) Rev Esp Anestesiol Reanim 58(4): 218-222.
- 12. Cameron, M., Corner, A., Diba, A., et al. Development of a tracheostomy scoring system to guide airway management after major head and neck surgery. (2009) Int J Oral Maxillofac Surg 38(8): 846-849.
- 13. Cabanillas, R., Rodrigo, J.P., Llorente, J.L., et al. Head Neck. Functional outcomes of transoral laser surgery of supraglottic carcinoma compared with a transcervical approach. (2004) Head Neck 26(8): 653-659.
- 14. Vilaseca, I., Bernal-Sprekelsen, M. Transoral laser microsurgery for locally advanced laryngeal cancer. (2013) Acta Otorrinolaringol Esp 64(2): 140-149.
- 15. Weinstein, G.S., O'Malley, B.W., Snyder, W., et al. Transoral robotic surgery: radical tonsillectomy. (2007) Arch Otolaryngol Head Neck Surg 133(12): 1220-1226
- 16. Iseli, T.A., Kulbersh, B.D., Iseli, C.E., et al. Functional outcomes after transoral robotic surgery for head and neck cancer. (2009) Otolaryngol Head Neck Surg 141(2): 166-171.
- 17. Moore, E.J., Olsen, K.D., Kasperbauer, J.L. Transoral robotic surgery for oropharyngeal squamous cell carcinoma: a prospective study of feasibility and functional outcomes. (2009) Laryngoscope 119(11): 2156-2164.
- 18. Hammoudi, K., Pinlong, E., Kim, S., et al. Transoral robotic surgery versus conventional surgery in treatment for squamous cell carcinoma of the upper aerodigestive tract. (2014) Head Neck 37(9): 1304-1309.
- 19. Dabas, S., Dewan, A., Ranjan, R., et al. Transoral robotic surgery in management of oropharyngeal cancers: a preliminary experience at a tertiary cancer centre in India. (2014) Int J Clin Oncol 20(4): 693-700.
- 20. Park, Y.M., Kim, W.S., Byeon, H.K., et al. Surgical techniques and treatment outcomes of transoral robotic supraglottic partial laryngectomy. (2013) Laryngoscope 123(3): 670-677.
- 21. Mendelsohn, A.H., Remacle, M., Van Der Vorst, S., et al. Outcomes following transoral robotic surgery: supraglottic laryngectomy. (2013) Laryngoscope 123(1): 208-214.
- 22. Chi, J.J., Mandel, J.E., Weinstein, G.S., et al. Anesthetic considerations for transoral robotic surgery. (2010) Anesthesiol Clin 28(3): 411-422.
- 23. Kucur, C., Durmus, K., Gun, R., et al. The safety and efficacy of concurrent neck dissection and transoral robotic surgery. (2016) Head Neck 38 Suppl 1: E519-23.
- 24. de Almeida, J.R., Genden, E.M. Robotic assisted reconstruction of the oropharynx. (2012) Curr Opin Otolaryngol Head Neck Surg 20(4): 237-245.