Modified Posterior Exenteration in Ovarian Cancer, Frequency and Morbidity of the Procedure
Affiliation
Department of Obstetrics and Pediatrics, Buenos Aires University, Fellow ACOG Buenos Aires Argentine Section, Chief of Obstetrics Service, Hospital Alemán.
Corresponding Author
Dr. Ricardo Illia, MD, Department of Obstetrics and Pediatrics, Buenos Aires University, Fellow ACOG Buenos Aires Argentine Section, Chief of Obstetrics Service, Hospital Alemán, Avenida Pueyrredón - 1640, Buenos Aires - 1118, Argentina, Tel: +54 11 4827-7000/Fax: +54 11 4827-7000; E-mail: rhillia@gmail.com
Citation
Illia, R., et al. Modified posterior exenteration in ovarian cancer, Frequency and morbidity of the procedure. (2018) J Gynecol Neonatal Biol 4(1): 4- 9.
Copy rights
© 2018 Illia, R. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Introduction
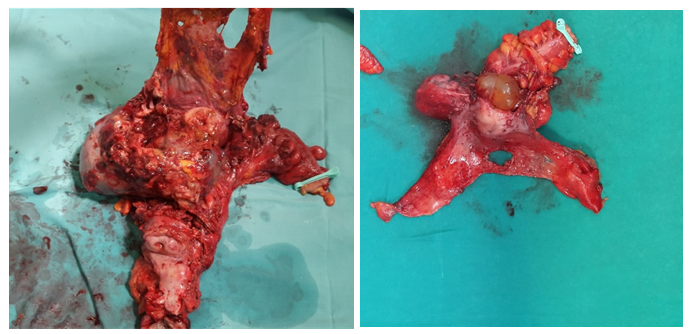
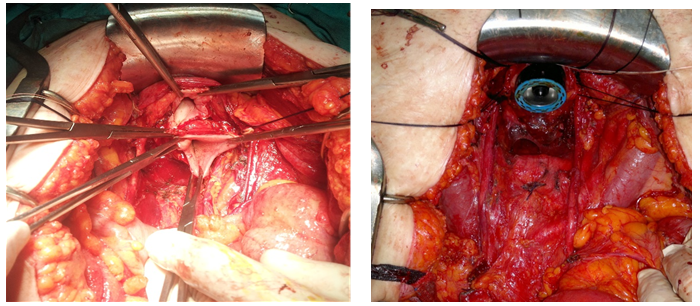
Ovarian cancer as a world wide incidence of 6.1 /100,000 representing a total of 238719 new cases per year of which 63% will die of their disease[1]. One of the most significative features is the diagnosis in advanced stages. Three quarters (77%) of these patients have metastatic disease (regional or disseminated) at the time of manifestation[2]. The standard treatment is surgery followed by chemotherapy with platinum and taxane[3-4]. Multiple retrospective analysis showed that debulking surgery acts as an independent factor in survival in ovarian cancer, but despite this knowledge, debulking rates vary widely between different institutions and published works ranging between 15 - 85%. Multiple procedures should be performed to achieve complete cytoreduction[5-12], one of them is the Modified Posterior Exenteration (MPE), (Figure 1) defined as a block resection of uterus, adnexa, rectosigmoid and pelvic peritoneum above the elevator muscles of anus[13]. The aim of this study is to determine the need for this procedure in the context of primary and interval cytoreductive surgery in ovarian cancer and report its complications.
Figure 1: Modified Posterior Exenteration. Ultra-low resection Modified posterior exenteration with pelvic Peritoneum resection.
Materials and Methods
It has been analyzed retrospectively the patients undergoing primary and interval debulking for ovarian cancer between January 2004 and December 2014, at the Service of Gynecology of the German Hospital of Buenos Aires. The study included all the patients who underwent modified posterior exenteration during primary or interval surgery in stages IIb, III, IV, evaluating frequency and postoperative complications within the next 30 days. All the patients were operated exclusively by Gynecological Oncologists of the of Gynecology Service. The data was obtained from computerized record of clinical history of the institution.
Results
It has been analyzed 168 patients diagnosed with ovarian carcinoma with an average age of 60 years (28 - 90). In 128 cases was performed primary cytoreduction and in 40 interval cytoreductive surgery. MPE was performed in 65 patients (38.7%), 44 (34.3%) at primary surgery and 21 (52.2%) at interval surgery. In the 44 cases of primary surgery 36 were stage III-IV and 8 stage IIb (Table1), (Figure 4) Optimal cytoreduction (residual tumor less than 1 cm) was achieved in 61 patients (93.8 %) and complete cytoreduction (no large disease) in 49 (75 %) patients. As part of the debulking procedure all patients also underwent multiorgan resections to achieve it. In all the patients mechanical bowel preparation with hyperosmotic saline laxative was indicated. During and after surgery prophylaxis with low molecular weight heparin was performed.
Table 1:
| Surgery | Modified Posterior Exenteration (%) | Total |
|---|---|---|
| Primary Debulking (IIb, III-IV) | 44 (34,3%) | 128 |
| Interval Debulking | 21 (52%) | 40 |
| Total | 65 (38,7%) | 168 |
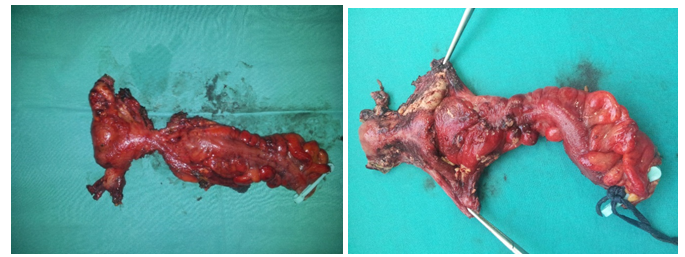
In all cases end-to-end anasthomosys with mechanical circular staplerwas performed. Only in 2 cases (3%) a defunctioning loop ileostomy was indicated to protect an very low (less than 6 cm from the anal margin) colorectal resection. We performed the closure of the ileostomy after 30 days of the primary surgery. One of them required read mission for dehydration due to a large enterectomy (short bowel syndrome). No fistulas or anastomotic dehiscence occurred. In any patient was necessary colostomy. All patients received platinum-based chemotherapy and, since 2006, those who had complete or optimal debulking during primary surgery received intraperitoneal chemotherapy. (Figure 2)
Figure 2: MPE after neoadyuvant chemotherapy, MPE after neoadyuvant chemotherapy.
Table 2:
| Features | Proportion (%) |
|---|---|
| Age (years) | 60 (28-90) |
| Ca 125 | 932 (10-10752) |
| Procedure time (minutes) | 211 (99-690) |
| Hospital stay (days) | 9 (4-31) |
| Histological type | |
| Seorus | 53 (81,5%) |
| Endometrioid | 6 (9,2%) |
| Clear cells | 5 (7,7%) |
| Mucinous | 1 (1,6%) |
| Tumor Grade | |
| I | - |
| II | 7(10,8%) |
| III | 58 (89,2%) |
| Residual disease | |
| Complete | 49 (75,5%) |
| Optimal | 12 (18,4%) |
| Suboptimal | 4 (6,1%) |
| Upper abdominal surgery required | |
| Si | 16 (24,6%) |
| No | 49 (75,4%) |
The most common histological type was serous papillary in 53 cases (81.5%), the rest were endometrioid, clear cells and mucinous adenocarcinoma. Other study variables are described at table 2. In those patients in which MPE was made during primary vs. Interval surgery had higher stay (3 days vs. 1 day) in intensive care unit during the postoperative due to a higher surgical effort to achieve complete cytoreduction. Among the postoperative complications we observed a 20% associated specifically to the MPE (prolonged ileus, feeling of incomplete evacuation, rectal incontinence, bleeding, bladder dysfunction and retroanastomosis abscess) and 20% associated to a common pelvic surgery (infections, thromboembolism and/or other cardiopulmonary events). Two patients died at the postoperative period, both of them having been subjected to multi-organ resections (table 3), (Figure 5, 6, 7, 8)
Table 3:
| Complications | Proportion | |
|---|---|---|
| Especifically related to MPE | Prolongued ileus | 4 (6,1%) |
| Tenesmus / incomplete evacuation sensation | 4 (6,1%) | |
| Hemorrhage (anemia) | 2 (3%) | |
| Retro anastomosis abscess | 1 (1,5%) | |
| Partial rectal incontinence | 1 (1,5%) | |
| Bladder dysfunction | 1 (1,5%) | |
| Common to an any pelvic surgery | Abdominal Wall infections | 8 (12,3%) |
| Deep venous thrombosis | 2 (3%) | |
| Lung thromboembolism | 1 (1,5%) | |
| Death | 2 (3%) | |
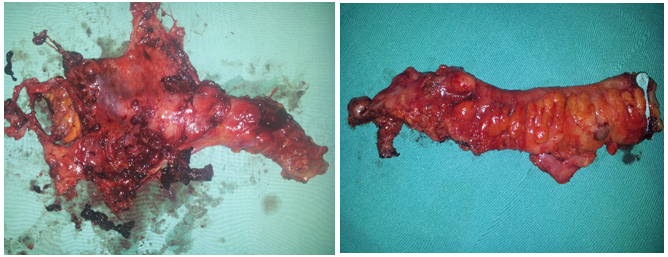
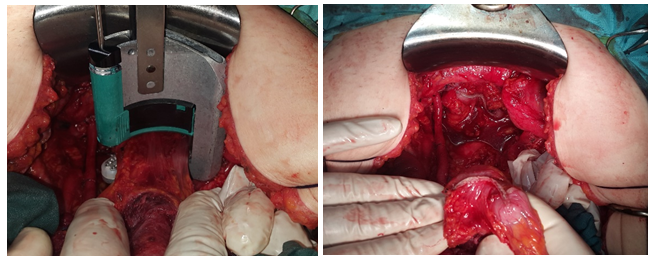
Figure 3: MPE in hysterectomized patient, MPE in hysterectomized patient.
Figure 4: MPE in stage IIb and MPE in extensive rectal disease.
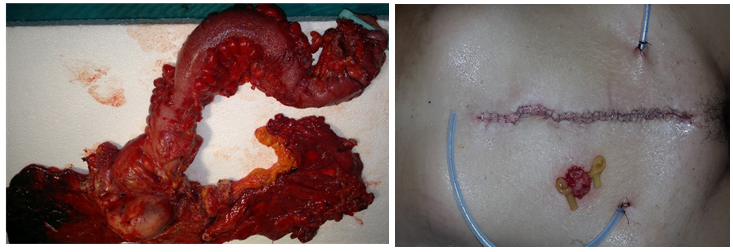
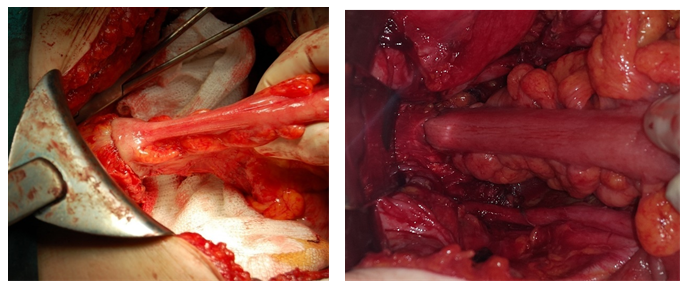
Figure 5: MPE and left hemicolectomy Defunctioning loop ileostomy in an ultra-low resection.
Figure 6: Upper view of vagina and distal rectum after MPE Circular mechanical stapler coming up from anus.
Figure 7: Rectum resection with curve cutter stapler Rectum resection with linear stapler.
Figure 8: End to end anastomosis with circular stapler End to end anastomosis with linear stapler.
Discussion
Cytoreductive surgery was proposed in 1935 by Meigs[14] arguing that the improvement of postoperative effect of radiotherapy was directly related with the amount of tumor mass removed. In 1968 Munnell reports that the maximum surgical efforts influenced survival[15] while in 1975 Griffiths showed, in 102 patients with ovarian carcinoma stage II - III, an inverse relationship between residual tumor mass and survival, being this worse if the residual tumor size was greater than 1.5 cm[16].
In 1992 - 1994 Hoskins in two trials of the GOG, 52 and 97, compare adjuvant chemotherapy with cisplatin and cyclophosphamide in patients with stage III and less than 1 cm residual disease (GOG 52) or greater than 1 cm (GOG 97) after primary debulking. Survival was superior in patients without visible disease compared with those with lower and higher than 2 cm residual tumor. Also, benefit in survival was found if less than 2 cm residual disease were compared with those larger than 2 cm (3.7). At present the best results in terms of survival are achieved in those patients where cytoreduction is complete associated to adjuvant intraperitoneal chemotherapy[12]. It’s well known that to achieve complete cytoreduction several surgical procedures must be performed, one of them is the block rectosigmoid anterior resection with uterus and pelvic peritoneum above the elevator muscle of anus. (Figure 3)
In 1950 Appebly highlights the need to remove the adjacent organs that were closely attached to prostate cancer[17]. Huddson and Chair in 1973 called “radical oophorectomy “to the retroperitoneal approach for block resection in ovarian cancer[18]. Years after, in 1984, Berek reported the need for anterior resection of the rectosigmoid with an end to end anastomosis to facilitate remotion of gynecologic malignancies. In this trial 72 patients underwent radical surgery; 48.6 % had ovarian cancer and colostomy with posterior end to end anastomosis were required in 25 % of these[19]. In 1989, Sonnendecker publishes the results of rectosigmoid resection in 20 patients without protective colostomy resulting in no wound dehiscence and only one rectovaginal fistula[20]. Einsenkop in 1991 published the block resection of rectosigmoid with uterus, pelvic peritoneum or not, over the elevator muscles of anus promoting the modified posterior exenteration denomination. Subsequently published several experiences reflecting the frequency, safety, efficacy and the low rate of complications of the procedure[21-29].
Revaux in 2012 compared morbidity and survival of the modified posterior exenteration during primary surgery or interval, showing a survival improvement when MPE was performed during the first surgery (49.4 vs 27.1 months) with no differences in digestive complications or no digestive[30]. Chang and Bristow recently published surgical details for block resection rectosigmoid, pelvic peritoneum, uterus and adnexa[31]. One of the most important features of the posterior exenteration in ovarian cancer, unlike the cervix cancer, is that it is performed on non irradiated tissues, which may explain the low rate of complications and the feasibility of the procedure.
Conclusion
In our Institution, the MPE was performed as part of debulking surgery for ovarian carcinoma in 38.7 % of the patients included in the study. This procedure allowed a 93.8% of optimal or complete cytoreduction with a low rate (20%) of complications associated with MPE, suggesting that it should not be an impediment for achieving cytoreduction but a routine procedure.
References
1) GLOBOCAN 2012 (IARC) Section of Cancer Surveillance.
Pubmed||Crossref||Others
2) Siegel, R., Ma, J., Zou, Z., et al. Cancer Statistics. (2014) CA cancer J Clin 64(1): 9–29.
Pubmed ||Crossref|| Others
3) Hoskins, W.J., McGuire, W.P., Brady, M.F., et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. (1994) Am J Obstet Gynecol 170: 974 – 979.
Pubmed ||Crossref|| Others
4) Ozols, R.F., Bundy, B.N., Greer, B.E., et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. (2003) J Clin Oncol 21(17): 3194–3200.
Pubmed ||Crossref|| Others
5) Bristow, R.E., Tomacruz, R.S., Armstrong, D.K., et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. (2002) J Clin Oncol 20: (5) 1248–1259.
Pubmed ||Crossref|| Others
6) Omura, G.A., Bundy, B.N., Berek, J.S., et al. Randomized trial of cyclophosphamide plus cisplatin with or without doxirubicin in ovarian carcinoma: a gynecologic oncology group study. (1989) J Clin Oncol 7(4): 457–465.
Pubmed ||Crossref|| Others
7) Hoskins, W.J., Bundy, B.N., Thigpen, J.T., et al. The influence of cytoreductive surgery on recurrence-free interval and survival in small volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. (1992) Gynecol Oncol 47(2): 167–171.
Pubmed ||Crossref|| Others
8) Alberts, D.S., Liu, P.Y., Hannigan, E.V., et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med (1996) 335(26): 1950–1955.
Pubmed ||Crossref|| Others
9) Ozols, R.F., Bundy, B.N., Greer, B.E., et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecology Oncology Group study. (2003) J Clin Oncol 21(17): 3194–3200.
Pubmed ||Crossref|| Others
10) Aletti, G., Dowdy, S.C., Gostout, B.S., et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. (2006) Obstet Gynecol 107(1): 77–85.
Pubmed ||Crossref|| Others
11) Chi, D.S., Eisenhauer, E.L., Lang, L., et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)?. (2006) Gynecol Oncol 103(3): 559–564.
Pubmed ||Crossref|| Others
12) Tewari, D., Java, J.J., Salani, R., et al. Long-Term Survival Advantage and Prognostic Factors Associated With Intraperitoneal Chemotherapy Treatment in Advanced Ovarian Cancer: A Gynecologic Oncology Group Study.(2015) J clin oncol 33(13): 1460-1466.
Pubmed ||Crossref|| Others
13) Eisenkop, S.M., Nalick, R.H., Teng, N.N. Modified posterior exenteration for ovarian cancer. (1991) Obstet Gynecol 78(5 Pt 1): 879-885.
Pubmed ||Crossref|| Others
14) Meigs, J.V., Macmillan, Co., Tumors of the Female Pelvic Organs. (1934) Am J Surg 26(3): 605.
Pubmed|| Crossref|| Others
15) Munnell, E.W. The changing prognosis and treatment in cancer of the ovary. A report of 235 patients with primary ovarían carcinoma 1952-1961. (1968) Am J Obstet Gynecol 100(6): 790-805.
Pubmed ||Crossref|| Others
16) Griffiths, C.T. Surgical resection of tumor bulk in the primary treatment of ovarian cancer. Natl Cancer Inst Monogr (1975) 42: 101–104.
Pubmed ||Crossref|| Others
17) Appleby, L.H. Proctocystectomy; the management of colostomy with ureteral transplants. Am J Surg (1950) 79(1): 57-60.
Pubmed ||Crossref|| Others
18) Hudson, C.N., Chir, M. Surgical treatment of ovarian cancer. (1973) Gynecol oncol 1: 370-378.
Pubmed||Crossref||Others
19) Berek, J.S., Hacker, N.F., Lagasse, L.D. Rectosigmoid colectomy and reanastomosis to facilitate resection of primary and recurrent gynecologic cancer. (1984) Obstet Gynecol 64(5): 715-720.
Pubmed ||Crossref||Others
20) Sonnendecker, E.W., Beale, P.G. Rectosigmoid resection without colostomy during primary cytoreductive surgery for ovarian carcinoma. (1989) Int Surg 74(1): 10-12.
Pubmed ||Crossref|| Others
21) Bridges, J.E., Leung, Y., Hammond, I.G., et al. En bloc resection of epithelial ovarian tumors with concomitant rectosigmoid colectomy: the KEMH experience. (1993) Int J Gynecol Cancer 3(4): 199-202.
Pubmed ||Crossref|| Others
22) Scarabelli, C., Gallo, A., Franceschi, S., et al. Primary cytoreductive surgery with rectosigmoid colon resection for patients with advanced epithelial ovarian carcinoma. (2000) Cancer 88(2): 389-397.
Pubmed || Crossref|| Others
23) Obermair, A., Hagenauer, S., Tamandl, D., et al. Safety and efficacy of low anterior en bloc resection as part of cytoreductive surgery for patients with ovarian cancer. (2001) Gynecol Oncol 83(1): 115-120.
Pubmed ||Crossref|| Others
24) Clayton, R.D., Obermair, A., Hammond, I.G., et al. The Western Australian experience of the use of en bloc resection of ovarian cancer with concomitant rectosigmoid colectomy. (2002) Gynecol Oncol 84(1): 53-57.
Pubmed ||Crossref|| Others
25) Bristow, R.E., delCarmen, M.G., Kaufman, H.S., et al. Radical oophorectomy with primary stapled colorectal anastomosis for resection of locally advanced epithelial ovarian cancer. (2003) J Am Coll Surg 197(4): 565-574.
Pubmed ||Crossref|| Others
26) Bedirli, A., Mentes, B.B., Onan, A., et al. Colorectal intervention as part of surgery for patients with gynaecological malignancy. (2005) Colorectal Dis 7(3): 228-231.
Pubmed ||Crossref|| Others
27) Mourton, S.M., Temple, L.K., Abu-Rustum, N.R., et al. Morbidity of rectosigmoid resection and primary anastomosis in patients undergoing primary cytoreductive surgery for advanced epithelial ovarian cancer. (2005) Gynecol Oncol 99(3): 608-614.
Pubmed ||Crossref|| Others
28) Houvenaeghel, G., Gutowski, M., Buttarelli, M., et al. Modified posterior pelvic exenteration for ovarian cancer. (2009) Int J Gynecol Cancer 19(5): 968-973.
Pubmed||Crossref|| Others
29) Peiretti, M., Bristow, R.E., Zapardiel, I., et al. Rectosigmoid resection at the time of primary cytoreduction for advanced ovarian cancer. A multi-center analysis of surgical and oncological outcomes. (2012) Gynecol Oncol 126(2): 220-223.
Pubmed ||Crossref|| Others
30) Revaux, A., Rouzier, R., Ballester, M., et al. Comparison of morbidity and survival between primary and interval cytoreductive surgery in patients after modified posterior pelvic exenteration for advanced ovarian cancer. (2012) Int J Gynecol Cancer 22(8): 1349-1354.
Pubmed||Crossref|| Others
31) Chang, S.J., Bristow, R.E. Surgical technique of en bloc pelvic resection for advanced ovarian cancer. (2015) J Gynecol Oncol 26(2): 155.
Pubmed ||Crossref|| Others