Multimodal Analgesia in Inguinal Hernia Repair Using a Cyclooxygenase-2-Specific Inhibitor: A Randomised Controlled Trial
Mostafa Somri1,2*, Christopher Hadjittofi3, Sarel Halachmi2,4, Hoash Naser1, Majed Kabaha2,5, Dan Shteinberg2,6, Constantinos A Parisinos7, Ibrahim Matter2,8
Affiliation
- 1Department of Anesthesia, Bnai Zion Medical Center, 47 Golomb St. Haifa, Israel
- 2The Ruth and Bruce Rappaport Faculty of Medicine, Technion - Israel Institute of Technology, Efron St. Haifa, Israel
- 3HPB & Liver Transplant Surgery Department, Royal Free London NHS Foundation Trust, Pond Street, London, NW3 2QG, United Kingdom
- 4Department of Urology, Bnai Zion Medical Center, 47 Golomb St. Haifa, Israel
- 5Department of Cardiothoracic Surgery, Carmel Medical Center, 7 Michal St. Haifa, Israel
- 6Department of General Surgery, Bnai Zion Medical Center, 47 Golomb St. Haifa, Israel
- 7Department of Gastrointestinal Medicine, Barts Health NHS Trust, W Smithfield, London, EC1A 7BE, United Kingdom
- 8Department of General Surgery, Bnai Zion Medical Center, 47 Golomb St. Haifa, Israel
Corresponding Author
Mostafa Somri, MD, Bnai Zion Medical Center, 47 Golomb St. Haifa, Israel 31048, Tel: +97248359230; E-mail: somri_m@yahoo.com
Citation
Somri, M., et al. Multimodal Analgesia in Inguinal Hernia Repair Using a Cyclooxygenase-2-Specific Inhibitor: A randomised controlled trial. (2016) J Anesth Surg 2(2): 61-65.
Copy rights
©2016 Somri, M. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Rofecoxib; Spinal; Anesthesia; Multimodal; Analgesia; Hernia
Abstract
Aim: To determine whether preoperative use of the highly cyclooxygenase-2-specific inhibitor rofecoxib combined with spinal anesthesia offers superior postoperative analgesia compared to spinal anesthesia alone.
Methods: Single-centre, randomised, double-blinded, placebo-controlled trial, including 40 adult patients undergoing elective open inguinal hernia repair. Both the control (n = 20) and treatment (n = 20) groups received 10-12 mg of bupivacaine as local anesthesia and 25 μg of fentanyl as spinal anesthesia. Additionally, control patients received oral placebo, whereas the treatment group received 50 mg of oral rofecoxib 2 hours preoperatively.
Results: Resting pain scores were significantly lower in the treatment group at 4, 16 and 24 hours postoperatively, as well as on discharge (p < 0.05). Pain scores on active straight-leg raise were significantly lower at 16 hours, 24 hours, and on discharge (p < 0.01, p < 0.05 and p < 0.05 respectively). Time to first analgesic dose was longer (p < 0.001) and average analgesic doses were lower (p < 0.001) in the treatment group. Finally, side-effect profiles were similar between groups.
Conclusions: Preoperative rofecoxib administration in combination with spinal anesthesia is superior to spinal anesthesia alone in controlling pain after inguinal hernia repair.
Introduction
Open inguinal hernia repair (IHR) is one of the commonest surgical procedures and one which may provoke pain of variable intensity and duration. Acute untreated pain can occasionally persist as a chronic problem due to increased prostaglandin synthesis[1,2]. Maximal prostaglandin concentration is reached at 3-4 hours after injury and this inflammatory process may last for 12 to 48 hours, which correlates with the peak intensity of postoperative pain[3]. In many cases, IHR is performed in an ambulatory fashion, therefore effective pain management allows early discharge and a comfortable post-operative period for the patient at home.
Surgical trauma causes immediate changes in both peripheral and central nervous systems, leading to augmentation of pain perception in the final destination – the cerebral cortex[1,3]. The surgical incision induces secretion of cyclooxygenase-2 (COX-2), which increases the production of prostaglandins (PGs), and which in turn excite peripheral nociceptors. These special pain fibres not only transmit the pain signal to the central nervous system but also amplify tissue sensitivity to the surgical trauma, giving rise to the phenomenon known as “local hyperalgesia”[4]. Similar hyper-reaction mechanisms in spinal neurons cause pain fibre hyper excitability, leading again to amplification of tissue sensitivity to a given pain stimulus (secondary hyperalgesia)[5]. Administration of preoperative analgesia is thought to inhibit the excess secondary excitability of the peripheral and spinal neurons, thus blockading the development of secondary hyperalgesia and blunting pain[5,6].
In one of the early studies describing the merits of preoperative or preemptive analgesia by Crile[7], local anesthesia was used in addition to general anesthesia in order to reduce scar pain due to chronic nociceptor inflammation and irritation. The ideal preoperative or preemptive agents were not completely defined, with several studies investigating various agents and modes of administration such as non-steroidal anti-inflammatory drugs (NSAIDs), opioids, local infiltration, and combined epidural and general anesthesia[7-13].
NSAIDs inhibit the COX group of isoenzymes, which are responsible for converting arachidonic acid to PGs[14-16]. The COX-2 isoenzyme in particular is secreted by immune cells and is directly associated with inflammation, pain and fever[17,18].
Rofecoxib (Vioxx®) is a NSAID with anti-inflammatory and analgesic properties. Its anti-inflammatory action is mainly achieved via selective peripheral COX-2 inhibition, although it may also cross the blood-brain barrier[15,16,19]. As rofecoxib is COX-2 specific, it confers a significantly lower risk of gastric irritation and peptic ulceration than other NSAIDs, and can thus be administered to a preoperatively fasted patient[20]. Spinal anesthesia using local anesthetics combined with opioids affects the transmission, modulation and modification stages of nociceptive afferent impulses and its analgesic qualities are superior to local anesthesia alone[21,22].
To our knowledge, no studies have investigated the combined use of anti-inflammatory analgesics with spinal anesthesia/analgesia for pre, intra and postoperative multimodal pain protection in patients undergoing day-case IHR. The aim of our study was therefore to assess the efficacy of preoperative combined administration of rofecoxib and standard spinal anesthesia in the reduction of postoperative pain following IHR.
Materials and Methods
Study population
A single-centre, randomised, double-blind, placebo-controlled study was conducted following the approval and according to the regulations of the Helsinki Committee 2003. The study was conducted between January and September 2003 at Bnai Zion Medical Center, Haifa, Israel. Inclusion criteria consisted of patient age between 18 and 70 years, a maximal American Society of Anesthesiologists (ASA) Score of II, and a negative pregnancy test result in the case of female patients undergoing elective open IHR. Exclusion criteria consisted of current or previous chronic NSAID or opioid treatment, peptic ulcer disease, liver or renal insufficiency, asthma, cardiovascular disease, allergy to rofecoxib or other NSAIDs. Patients in whom spinal anesthesia failed were also excluded from the study. All operations were performed by the same surgeon at the same hospital, and all patient participants provided written informed consent.
Study protocol
Patients were blindly and randomly allocated to 2 groups consisting of 20 patients each by random number table. The treatment group received 50mg of oral rofecoxib under the brand name Vioxx® (Merck Sharp & Dohme) two hours prior to spinal anesthesia induction. The control group received placebo medication identical in size and shape to the treatment drug, two hours prior to induction of spinal anesthesia.
All patients received spinal anesthesia using 10-12mg of bupivacaine for local anesthesia and 25 μg of fentanyl. The agents were administered by a senior anesthetist who was not involved in data collection. In cases of postoperative breakthrough pain, intravenous tramadol was administered at a dose of 1mg/kg intravenously over 20 minutes.
Data collection
In addition to patient age, gender, weight and operated side, the following data were collected:
- Pain level at rest by Visual Analog Scale (VAS) measurement (0 or “No pain” to 10 or “Worst possible pain”) at 4, 8, 16, and 24 hours postoperatively, as well as on discharge.
- Pain level on active straight-leg raise (ASLR) on the operated side to 20-30cm by VAS measurement at 8, 16, and 24 hours postoperatively.
- Postoperative time to administration of first analgesic dose (Time-to-analgesia; TTA).
- Number of analgesic doses administered in the first 24 hours after surgery.
- Laboratory studies, including full blood count, urea, electrolytes, and liver biochemistry both pre and postoperatively.
Power and statistical analysis
An a-priori power analysis was performed to determine the minimum group size (n = 16) for a power of 80% and a = 0.05, with the following assumptions:
- A 30% difference in postoperative analgesic doses
- A 40 minute difference for TTA
- A 30 % difference in the postoperative VAS score.
p < 0.05 was considered statistically significant. Comparison of numerical data such as age, gender distribution, weight and TTA was performed by the non-parametric Mann-Whitney test. Comparison of the VAS data was performed using the Friedman non-parametric paired test. Statistical analysis was performed using SPSS version 14 (SPSS Inc. Chicago, IL, USA.)
Results
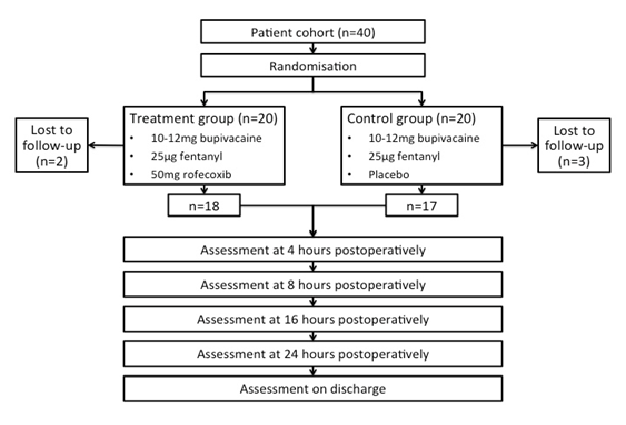
Following the exclusion of 5 patients who were lost to follow-up, 18 and 17 patients comprised the treatment and control groups respectively (Figure 1). Age, weight, height, gender distribution, operative time and anesthesia time were similar in both groups with no statistically significant difference (Table 1). On admission and during the immediate preoperative phase, all patients were pain-free. In all cases, an elective open primary inguinal hernia repair (herniorrhaphy) was successfully performed.
Figure 1: Study flow diagram
Table 1: Patient characteristics
| Parameter | Treatment group (n = 18; mean ± SD) | Control group(n = 17; mean ± SD) | p value |
|---|---|---|---|
| Age (years) | 44.33 ± 18.54 | 53.5 ± 20.96 | 0.95 |
| Weight (kg) | 75.88 ± 8.27 | 78.32 ± 5.14 | 0.13 |
| Height (cm) | 173.42 ± 16.23 | 175.12 ± 18.18 | 0.22 |
| %Male/%Female | 72.2/27.8 | 64.7/35.3 | 0.58 |
| Operative time (min) | 116.76 ± 19.78 | 114.71 ± 19.96 | 0.88 |
| Anaesthesia duration (min) | 137.50 ± 19.19 | 136.18 ± 19.40 | 0.56 |
Average pain levels were significantly lower in the treatment group at 4, 16, and 24 hours postoperatively, as well as on discharge (p < 0.05). Pain score was also lower in the treatment group at 8 hours postoperatively, without however reaching statistical significance (Table 2). Pain scores on ASLR were significantly lower in the treatment group at 16 and 24 hours postoperatively, as well as on discharge (p < 0.01, p < 0.05, and p < 0.05 respectively). Similarly, ASLR pain scores at 8 hours were lower in the treatment group, without statistical significance (Table 3).
Table 2: Pain scores at rest according to the Visual Analog Scale
| Time after operation | Treatment group (n = 18; mean ± SD) | Control group (n = 17; mean ± SD) | p value |
|---|---|---|---|
| 4 hours | 3.89 ± 1.32 | 5.88 ± 1.16 | < 0.05 |
| 8 hours | 4.00 ± 1.68 | 4.65 ± 1.41 | 0.207 |
| 16 hours | 3.00 ± 0.84 | 4.35 ± 1.17 | < 0.05 |
| 24 hours | 1.67 ± 0.59 | 3.82 ± 1.18 | < 0.05 |
| Discharge | 1.39 ± 0.50 | 3.76 ± 0.83 | < 0.05 |
Table 3: Pain scores on active straight-leg raise according to the Visual Analog Scale
| Time after operation | Treatment group (n = 18; mean ± SD) | Control group (n = 17; mean ± SD) | p value |
|---|---|---|---|
| 8 hours | 5.17 ± 0.8 | 6.24 ± 0.7 | 0.32 |
| 16 hours | 3.95 ± 0.8 | 6.59 ± 0.65 | <0.01 |
| 24 hours | 2.84 ± 1 | 5.12 ± 1.2 | < 0.05 |
| Discharge | 3.15 ± 0.9 | 5.32 ± 0.88 | < 0.05 |
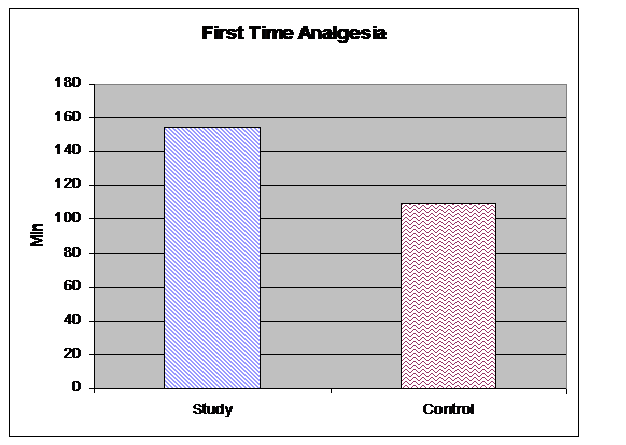
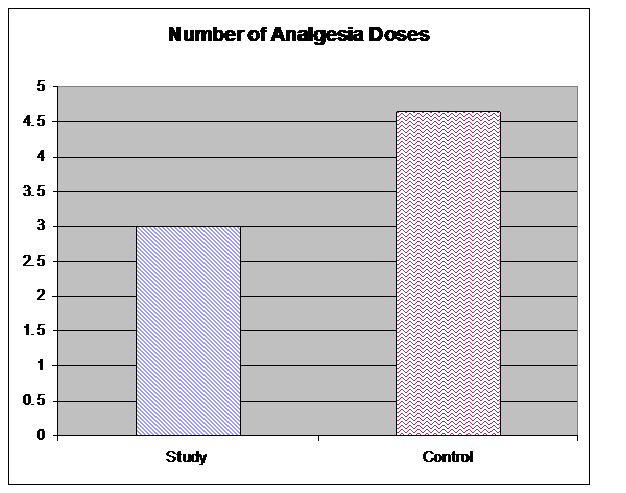
TTA was significantly longer in the treatment group following surgery than in the control group (157 ± 45 min vs. 110 ± 90 min; p = 0.001) (Figure 2). The number of analgesic doses was also significantly lower in the treatment group, with an average of 3.01 doses vs. 4.65 doses in the control group (p = 0.001) (Figure 3). Laboratory study results for all patients fell within normal reference ranges both pre and postoperatively.
Figure 2: Postoperative time (minutes) to first dose of analgesia on demand (p < 0.001)
Figure 3: Number of postoperative analgesic doses administered until discharge (p < 0.001)
Finally, the two groups experienced a similar rate of side-effects. With respect to the treatment vs. control group, 5 vs. 6 patients experienced nausea, 3 vs. 4 patients experienced headache, 2 vs. 2 patients experienced dizziness, and 4 vs. 5 patients experienced constipation. All side-effects were grade 1 (mild) and were managed conservatively with rest and intravenous fluid administration.
Discussion
This study clearly demonstrated the effectiveness of selective COX-2 inhibitor administration prior to surgical trauma in IHR for the reduction of postoperative pain as compared to spinal anesthesia alone.
Pain scores at rest and on ASLR, as well as TTA and number of analgesic doses favoured the treatment group overall, reaching statistical significance in all instances except at 8 hours postoperatively. The absence of statistical significance in pain reduction in the treatment group at 8 hours postoperatively may in part be explained by the additive effects of postoperative intravenous tramadol, which was first requested and administered at 157 ± 45 min and at 110 ± 90 min in the treatment and control groups respectively. Alternatively, the similarity in pain scores at 8 hours postoperatively may be influenced by the relatively small study sample. In any case, this remains to be confirmed with further studies into the effects of newer selective COX-2 inhibitors on larger patient cohorts.
The most practically important parameter, is the longterm effect of balanced protective analgesia. Pain scores on discharge were significantly lower in the treatment group, affording patients with a more comfortable discharge home, where they were able to independently manage mild pain using over-thecounter non-opioid analgesia.
Reuben et al.[23] produced similar results in a study investigating the use of intra-articular rofecoxib to prevent post-arthroscopy pain. A meta-analysis of 13 randomised clinical trials by Desjardins et al.[24] studying the long-term effect of a single rofecoxib dose revealed that a dose of 50mg is highly effective for more than 24 hours due to its longer half-life as compared to other NSAIDs such as ibuprofen and naproxen.
Selective and non-selective COX-2 inhibitors are effective in pain relief. Studies on pain following dental or maxillofacial operations demonstrated analgesic effectiveness from a single 50mg oral dose of rofecoxib[25]. Although several studies found rofecoxib to have similar analgesic effects to other NSAIDS such as ibuprofen and naproxen, its long-term analgesic effects were not examined as in the current study[26-28].
Spinal anesthesia is widely used in surgical procedures, including IHR as in the current case. In addition to a rapid analgesic effect, spinal anesthesia provides efficient sensorimotor blockade[17,29]. Randomised trials on the effectiveness of adding spinal anesthesia to general anesthesia in order to reduce postoperative analgesic requirements following abdominal surgery have produced conflicting results[18,20].
Tverskoy et al. showed that postoperative pain can be reduced in patients undergoing inguinal herniorrhaphy by substituting general with spinal anesthesia, and moreover if local and general anesthesia are used in combination[31]. In the current study, spinal anesthesia alone was compared to spinal anesthesia combined with rofecoxib, a combination which exerted more effective analgesic effects for up to 24 postoperative hours. This combination suppresses nerve excitation through a dual pathway; central pain is blocked by the spinal anesthetic and peripheral pain is blocked by the NSAID, which decreases nociceptor sensitivity.
Preemptive analgesia has been defined as treatment which starts before surgery, prevents the establishment of central sensitisation caused by incisional injury (covering the intraoperative period) and prevents the establishment of central sensitisation caused by incisional and inflammatory injuries (covering both the intraoperative and initial postoperative periods).
A comprehensive systematic review of preemptive analgesia showed that preemptive analgesia using spinal anesthesia alone or preoperative NSAIDs is not significantly effective in pain control following open IHR[32]. The authors found that only preoperative epidural anesthesia which continues throughout the operation is sufficiently effective. This review supported our decision to examine the combination of regional anesthesia and NSAIDs for combined balanced protective analgesia rather than pre-emptive analgesia.
Limitations to the current study include the fact that although results are statistically significant, patient groups are relatively small (n = 18 and n = 17). Furthermore, on 30 September, 2004, Merck withdrew rofecoxib from the market due to evidence of increased myocardial infarction and thrombotic stroke risks associated with its long-term use[35]. The withdrawal came into force after the completion of this study, and no perioperative cardiovascular events were observed.
In this study, the technique of combined balanced analgesia rather than preemptive analgesia was used, achieving effective suppression of the afferent input during the intraoperative and immediate postoperative period by spinal anesthesia, as well as prevention of peripheral and central sensitisation by the anti-inflammatory effect of rofecoxib due to its long duration of effect.
In light of the discontinuation of rofecoxib, these findings remain to be confirmed by further studies in larger patient groups using newer long-acting NSAIDs such as etoricoxib.
Conclusion
In conclusion, the administration of oral rofecoxib prior to elective IHR combined with spinal anesthesia is significantly more effective than spinal anesthesia alone in post-operative pain control.
References
- 1. Callesen, T., Bech, K., Nielsen, R., et al. Pain after groin hernia repair. (1998) Br J Surg 85(10): 1412-1414.
- 2. Johansson, B., Hallerbäck, B., Stubberöd, A., et al. Preoperative local infiltration with ropivacaine for postoperative pain relief after inguinal hernia repair. A randomised controlled trial. (1997) Eur J Surg 163(5): 371-378.
- 3. Kissin, I. Preemptive analgesia. (2000) Anesthesiology 93(4): 1138-1143.
- 4. Samad, T.A., Moore, K.A., Sapirstein, A., et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. (2001) Nature 410(6827): 471-475.
- 5. Woolf, C.J., Chong, M.S. Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. (1993) Anesth Analg 77(2): 362-379.
- 6. Cousins, M.J., Power, I., Smith, G. 1996 Labat lecture: pain--a persistent problem. (2000) Reg Anesth Pain Med 25(1): 6-21.
- 7. Crile, G.W. The kinetic theory of shock and its prevention through anoci-association (shockless operation). (1913) Lancet 2(4688): 7-16.
- 8. Fletcher, D., Kayser, V., Guilbaud, G. Influence of timing of administration on the analgesic effect of bupivacaine infiltration in carrageenin-injected rats. (1996) Anesthesiology 84(5): 1129-1137.
- 9. Pedersen, J.L., Crawford, M.E., Dahl, J.B., et al. Effect of preemptive nerve block on inflammation and hyperalgesia after human thermal injury. (1996) Anesthesiology 84(5): 1020-1026.
- 10. Rockemann, M.G., Seeling, W., Bischof, C., et al. Prophylactic use of epidural mepivacaine/morphine, systemic diclofenac, and metamizole reduces postoperative morphine consumption after major abdominal surgery. (1996) Anesthesiology 84(5): 1027-1034.
- 11. Wall, P.D. The prevention of postoperative pain. (1988) Pain 33(3): 289-290.
- 12. Woolf, C.J. Evidence for a central component of post-injury pain hypersensitivity. (1983) Nature 306(5944): 686-688.
- 13. Yashpal, K., Katz, J., Coderre, T.J. Effects of preemptive or postinjury intrathecal local anesthesia on persistent nociceptive responses in rats. Confounding influences of peripheral inflammation and the general anesthetic regimen. (1996) Anesthesiology 84(5): 1119-1128.
- 14. Mizuno, K., Yamamoto, S., Lands, W.E. Effects of non-steroidal anti-inflammatory drugs on fatty acid cyclooxygenase and prostaglandin hydroperoxidase activities. (1982) Prostaglandins 23(5): 743-757.
- 15. Needleman, P., Turk, J., Jakschik, B.A., et al. Arachidonic acid metabolism. (1986) Annu Rev Biochem 55: 69-102.
- 16. Vane, J.R., Bakhle, Y.S., Botting, R.M. Cyclooxygenases 1 and 2. (1998) Annu Rev Pharmacol Toxicol 38: 97-120.
- 17. Liu, S.S., Ware, P.D., Allen, H.W., et al. Dose-response characteristics of spinal bupivacaine in volunteers. Clinical implications for ambulatory anesthesia. (1996) Anesthesiology 85(4): 729-736.
- 18. Dakin, M.J., Osinubi, O.Y., Carli, F. Preoperative spinal bupivacaine does not reduce postoperative morphine requirement in women undergoing total abdominal hysterectomy. (1996) Reg Anesth 21(2): 99-102.
- 19. Malmberg, A.B., Yaksh, T.L. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. (1992) Science 257(5074): 1276-1279.
- 20. Meade, E.A., Smith, W.L., DeWitt, D.L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. (1993) J Biol Chem 268(9): 6610-6614.
- 21. Naulty, J.S., Datta, S., Ostheimer, G.W., et al. Epidural fentanyl for postcesarean delivery pain management. (1985) Anesthesiology 63(6): 694-698.
- 22. Naulty, J.S. Continuous infusions of local anesthetics and narcotics for epidural analgesia in the management of labor. (1990) Int Anesthesiol Clin 28(1): 17-24.
- 23. Reuben, S.S., Bhopatkar, S., Maciolek, H., et al. The preemptive analgesic effect of rofecoxib after ambulatory arthroscopic knee surgery. (2002) Anesth Analg 94(1): 55-59.
- 24. Desjardins, P.J., Mehlisch, D.R., Chang, D.J., et al. The time to onset and overall analgesic efficacy of rofecoxib 50 mg: a meta-analysis of 13 randomized clinical trials. (2005) Clin J Pain 21(3): 241-250.
- 25. Roszkowski, M.T., Swift, J.Q., Hargreaves, K.M. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. (1997) Pain; 73(3): 339-345.
- 26. Ehrich, E.W., Dallob, A., De Lepeleire, I., et al. Characterization of rofecoxib as a cyclooxygenase-2 isoform inhibitor and demonstration of analgesia in the dental pain model. (1999) Clin Pharmacol Ther 65(3): 336-347.
- 27. Morrison, B.W., Christensen, S., Yuan, W., et al. Analgesic efficacy of the cyclooxygenase-2-specific inhibitor rofecoxib in post-dental surgery pain: a randomized, controlled trial. (1999) Clin Ther 21(6): 943-953.
- 28. Morrison, B.W., Fricke, J., Brown, J., et al. The optimal analgesic dose of rofecoxib: Overview of six randomized controlled trials. (2000) J Am Dent Assoc 131(12): 1729-1737.
- 29. Casati, A., Vinciguerra, F. Intrathecal anesthesia. (2002) Curr Opin Anaesthesiol 15(5): 543-551.
- 30. Vaida, S.J., David, B.B., Somri, M., et al. The influence of preemptive spinal anesthesia on postoperative pain. (2000) J Clin Anesth 12(5): 374-377.
- 31. Tverskoy, M., Cozacov, C., Ayache, M., et al. Postoperative pain after inguinal herniorrhaphy with different types of anesthesia. (1990) Anesth Analg; 70(1): 29-35.
- 32. Møiniche, S., Kehlet, H., Dahl, J.B. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. (2002) Anesthesiology 96(3): 725-741.
- 33. Bombardier, C., Laine, L., Reicin, A., et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. (2000) N Engl J Med 343(21): 1520-1528.
- 34. Weaver, A.L. Rofecoxib: clinical pharmacology and clinical experience. (2001) Clin Ther 23(9): 1323-1338.
- 35. Fitzgerald, G.A. Coxibs and cardiovascular disease. (2004) N Engl J Med 351(17): 1709-1711.