Neutrophil to Lymphocyte Ratio as a Prognostic Biomarker for Morbidity and Mortality in Patients Undergoing Benign Surgery: A Systematic Review
Camilla Godthaab
Affiliation
Center for Surgical Science, Department of Surgery, Zealand University Hospital, Lykkebækvej 1, Køge
Corresponding Author
Camilla Godthaab, Medical Student and Scholar, Center for Surgical Science, Zealand University Hospital, Lykkebækvej 1, 4600 Køge, Tel: +4530709356; E-mail: Camilla_godthaab@hotmail.com
Citation
Godthaab, C., et al. Neutrophil to Lymphocyte Ratio as a Prognostic Biomarker for Morbidity and Mortality in Patients Undergoing Benign Surgery: A Systematic Review. (2018) J Anesth Surg 5(2): 106-122.
Copy rights
© 2018 Godthaab, C. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Abstract
Background: Neutrophil to lymphocyte ratio (NLR) reflects the degree of systemic inflammation. Several clinical trials have shown that a high preoperative NLR predicts morbidity and mortality after surgery for malignant disease. Whether preoperative NLR predicts morbidity and mortality after benign surgery is uncertain. The aim of this systematic review was to investigate whether preoperative NLR predicts postoperative morbidity and mortality after benign surgery.
Method: A systematic review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-analyses. The population was limited to adults undergoing benign surgery. The search was performed in PubMed, Scopus, Embase and the Cochrane Library. Risk of bias was evaluated with ACROBAT-NRSI.
Results: 25 clinical observational studies with a total of 10,015 patients were included. Types of surgery were cardiac surgery (N = 11), vascular surgery (N = 6) and non-cardiac non-vascular surgery (N = 8). The studies in cardiac and vascular surgery showed associations between preoperative NLR and postoperative mortality. 11 out of 13 studies in cardiac and vascular surgery showed a significant association between NLR and postoperative morbidity. In orthopaedic surgery, NLR predicted troponin elevation after hip surgery and postoperative infections after knee surgery, while no association was shown between preoperative NLR and postoperative complications in patients amputated due to diabetic foot ulcers. NLR predicted postoperative complications after prosthesis implantation and bowel resection. NLR did not predict the risk of postoperative complications after abdominal and miscellaneous non-cardiac surgery.
Conclusion: In cardiac and vascular surgery, a high preoperative NLR was significantly associated with postoperative morbidity and mortality. The predictive value of NLR in non-cardiac non-vascular surgery was unclear and should be investigated in larger clinical studies.
Introduction
In recent years, the role of the neutrophil to lymphocyte ratio (NLR) has gained significant attention in a variety of malignant diseases including colorectal cancer, hepatocellular cancer, advanced pancreatic cancer, ovarian cancer and oesophageal cancer, as an independent predictor of morbidity and mortality[1-5]. A systematic review including 40,559 patients showed that a high pre-operative NLR was associated with overall survival and disease-free survival in solid tumours[6]. Likewise, a clinical study including 404 patients with gastric cancer undergoing curative gastrectomy showed that a high pre-operative NLR (NLR > 3) independently predicted the development of postoperative infectious complications and NLR > 3 was independently associated with overall- and cancer-specific survival[7]. A cohort study including 418 patients reported that NLR was the most efficient biomarker to predict recurrence-free survival, cancer-specific survival and overall survival in patients undergoing radical cystectomy for bladder cancer compared to platelet-lymphocyte ratio and absolute platelet counts[8]. Moreover, NLR has been studied in a range of clinical non-surgical settings and has e.g. been shown to be a better indicator than total white blood cell count in the staging of acute pancreatitis[9]. Whether preoperative NLR predicts morbidity and mortality after benign surgery is unclear. The purpose of this systematic review was to investigate whether a pre-operative NLR is a predictor of postoperative morbidity and mortality after benign surgery.
Methods
A systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (PRISMA)[10]. A version of the review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42016050756).
The population was limited to patients ≥18 years who underwent benign surgery. Studies including patients who underwent transplantation or patients with cancer were excluded. Only studies with a pre-operative NLR were included. Outcomes were postoperative morbidity and mortality. We excluded non-English publications and unpublished studies including proceeding abstracts. Date of publication was not restricted. A literature search was conducted in November 2017 in PubMed, Scopus, Embase and the Cochrane Library. In PubMed the following search terms were used: Humans, adults, surgical specialties, surgery, operation, surgical procedures - operative, resection, incision, neutrophils, lymphocytes, neutrophil, lymphocyte, neutrophil/lymphocyte, neutrophil-to-lymphocyte ratio, NLR, survival, mortality, mortalities, death, morbidity, mortalities, morbidities. The PubMed MesH terms were modified to corresponding terms in the other databases. Reference lists from included articles were manually searched for additional publications of relevance. Two reviewers independently screened all articles on title and abstract and resolved disagreement through discussion.
Two reviewers extracted data on; author, study design, number of participants, characteristics of the population, type of surgery and priority, lengths of follow-up, pre-operative NLR and clinical outcomes. Disagreements were resolved through discussion.
Methodological quality assessment
A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI) was used to assess the methodological quality of the individual studies[11]. The quality assessment evaluated the potential risk of introducing bias or limit applicability of the studies. ACROBAT-NRSI assesses the studies across seven specified domains of bias distributed in two types of pre-intervention bias, one kind of intervention bias and four types of post-intervention bias. All studies were rated on the risk of pre-intervention bias, intervention bias and post-intervention bias. The rating was divided into ‘low’, ‘moderate’, ‘serious’, ‘high’ or ‘non relevant’. For each study, the overall risk of bias was determined by the highest rated risk of bias in any of the domains.
Results
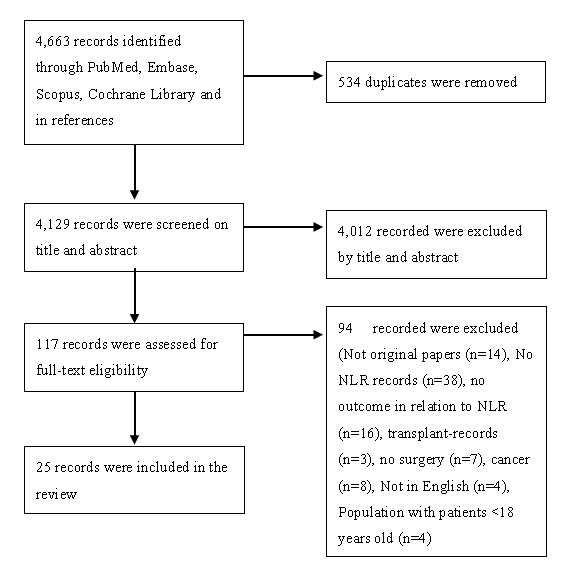
A total of 4,663 articles were identified in the PubMed database, Scopus, EMBASE and Cochrane clinical trials. After manual removal of 534 duplicates, 4,129 articles were left for screening on title and abstract. 4,012 articles were excluded after screening on title and abstract. After full-text review, 94 articles were excluded and 23 articles were included in the systematic review. Furthermore, a manual search of reference lists led to inclusion of 2 additional articles, figure 1.
Figure 1: Flowchart of included articles and the screening process. The violation of any inclusion criterion resulted in the exclusion of the article.
Study characteristics
25 clinical studies with a total of 10,015 patients were included[12-36]. The studies consisted of 10 prospective cohort studies[12-14,18,19,22,25-28] retrospective cohort studies[15-17,20,21,23,29-36] and two case-control study[24,31]. Lengths of follow-up ranged from one day to108 months after surgery.
The mean age ranged from 37.6 ± 13.1 years[36] to 82 ± 8years[28], table1.
The studies were divided into three categories according to the type of surgery: cardiac surgery (N = 11)[12-17,28,30-32,37], vascular surgery (N = 6)[1-23,33] and non-cardiac non-vascular surgery (N = 8)[24-27,29,34-36] including four studies in orthopaedic surgery[24,25,29,35], two studies in abdominal surgery[26,36], one study in urology[34] and one study in miscellaneous types of non-cardiac non-vascular surgery[27].
Table 1: Clinical studies assessing Neutrophile to Lymphocyte Ratio prior to benign surgery.
| Author | N | Design | Priority | Type of surgery | Age, years | Population NLR | Population Morbidity | Population Mortality | Follow up after surgery |
|---|---|---|---|---|---|---|---|---|---|
| Cardiac surgery | |||||||||
| Azab B et al (2013)[12] | 1,126 | Prospective cohort study | Elective | CABG | Tertile 1: Sternotomy 62.5 ± 10.2 MICS: 60.7 ± 10.9 Tertile 2: Sternotomy 64.3 ± 11.0 MICS: 62.6 ± 10.7 Tertile 3: Sternotomy 67.5 ± 11.0 MICS: 65.6 ± 10.7 | - | - | - | 49 ± 15.2 months |
| Gibson P et al (2007)[13] | 1,938 | Prospective cohort study | - | CABG | 65±9 | 2.43 (1.86-3.36) | - | 177/1,938 (9.1%) | 3.6 (1.4-4.7) year (median, q1-q3) |
| Gibson P et al (2010)[14] | 275 | Prospective cohort study | Elective | CABG | 65 (58-70) (median (q1-q3)) | - | Atrial fibrillation 107/275 patients (38.9%) | - | 7 days or until discharge |
| Tasoglu I et al (2013)[15] | 444 | Retrospective cohort study | Elective | CABG | 61.9±10.6 | - | SVGF: 258/444 patients (58.1%) | - | Tertile 1: 41.7 ± 4.2 months Tertile 2: 37.5 ± 6.2 months Tertile 3:43.5 ± 5.4 months |
| Aydınlı Bet al (2016)[29] | 1500 | Retrospective cohort study | Elective | CABG, isolated single cardiac valve surgery, combined surgery | 60±11.7 | 3.2 ± 2.3 | CAE 296/1500 patients (19.8%) | - | - |
| Sevuk U et al (2016)[31] | 172 | Retrospective case-control study | Elective | CABG | Post-pericardiotomy syndrome 60.5 (52.25-66) No post-pericardiotomy syndrome 61 (54-67)(median (q1-q3)) | - | Post-pericardiotomy syndrome 72/172 patients (41.8%) | - | 15 ± 1 day |
| Saskin H et al (2015)[32] | 916 | Retrospective cohort study | Elective | CABG | 60 ± 8.3 | - | - | - | 30 days |
| Kim W et al (2015)[16] | 590 | Retrospective cohort study | - | Open cardiac or thoracic aorta surgery with cardiopulmonary bypass | Quartile 1: 66 (55-73) Quartile 2: 64 (56-74) Quartile 3: 68 (55-74) Quartile 4: 66 (56-73) (median (q1-q3)) | - | Acute kidney injury 166/590 patients (28.1%) | - | 1 year |
| Yost GL et al (2015)[17] | 273 | Retrospective cohort study | Acute | Implantation of left ventricular assist devices | 59.85 ± 12.95 | - | Right ventricular failure: 84/273 patients (30.8%) | 30-days mortality 9/273 patients (3.3%) 1-year mortality 117/273 patients (42.9%) 2-years mortality 174/273 patients (63.7%) | 2 years |
| Lafci G et al (2014)[21] | 104 | Retrospective cohort study | Acute | Type I aorta dissection | 55.2 ± 14 | - | - | In-hospital mortality 33/104 patients (31.7%) | - |
| Kalkan ME et al (2017)[30] | 184 | Retrospective cohort study | Acute | Type I aorta dissection | 53.1 ± 11.4 | - | Re-operative surgery 9/184 patients (4.8%) Multi-organ dysfunction 23/184 patients (12.5%) Major bleeding 22/184 patients (11.9%) AKI 26/184 patients (14.1%) Stroke 12/184 patients (6.5%) Extremity embolism 1/184 patients (0.5%) Hospital-related infection 36/184 patients (19.5%) | In-hospital cardiovascular mortality 38/184 patients (20.7%) | - |
| Vascular surgery | |||||||||
| Appleton ND et al (2014)[18] | 350 | Prospective cohort study | Elective, acute | Repair of abdominal aortic aneurysms | 72.9 ± 7.9 | - | - | 30 days mortality 32/350 patients (9.4%) 1-year mortality: 136/350 (38.8%) | |
| Halazun H et al (2014)[19] | 432 | Prospective cohort study | Elective | CEA for high-grade carotid artery stenosis | - | 3.4 ± 2.9 | Postoperative cognitive dysfunction 70/432 patients (16.4%) | - | 1 day |
| Kordzadeh A et al (2015)[20] | 80 | Retrospective cohort study | Acute | Ruptured abdominal aortic aneurysms | 75 (51-92) (median (q1-q3)) | 9.40 (4.14-13.69) (median (q1-q3)) | 30 days morbidity (Clavien-Dindo ≥ 3)41/80 patients (51.2%) | 30-days mortality 11/80 patients (13.8%) | 30 days |
| Kullar P et al (2012)[22] | 126 | Prospective cohort study | Elective, acute | Lower limb revascularisation | Patients with graft patency 73 (64-78)Patients witn no graft patency 77 (71-81) (median (q1-q3)) | - | Graft patency 79/126 patients (62.7%) | - | 1 year |
| Tasoglu I et al (2014)[23] | 245 | Retrospective cohort study | Acute | Embolectomy (open) for acute limb ischemia | 66.0 ± 13.3 | - | - | 30-days mortality 25/245 patients (10%) Long-term mortality 49/245 patients (20.0%) | 26 months (mean) |
| Wang Q et al (2017)[33] | 270 | Retrospective cohort study | Acute, elective | Major amputation (above and below knee) or minor amputation (toe and foot) due to ischaemia | 71 ± 6 | 7.9 ± 8.0 | Myocardial infarction or stroke: 9/270 patients (3.3%) | 30-days mortality 22/270 patients (8.1) | 30 days |
| Non-cardiac non-vascular surgery | |||||||||
| Gölge UH et al (2016)[24] | 133 | Retrospective case-control study | Elective | Total knee arthroplasty | Patients with prosthetic joint infection: 64.3 ± 9.3 (48-82) Control group: 66.2 ± 7.4 (45-85) | - | - | - | 30.5±4.01 weeks |
| Fisher A et al (2016)[28] | 294 | Prospective cohort study | Acute | Hip fracture surgery | 82.1 ± 8.0 | - | Troponin rise: 75/294 patients (18.1%) | In-hospital mortality 10/294 patients (2.4%) | - |
| Sedlár M et al (2015)[25] | 104 | Prospective cohort study | Acute | Hip fracture surgery | 80 ± 9 | 10.0 ± 7.7 | - | 5-years mortality 64/104 patients (61.5%) | 60 months (48-84) (median (q1-q3)) |
| Forget P et al (2015)[26] | 82 | Prospective cohort study | - | Major abdominal surgery | 62 (27-80) (median (q1-q3)) | 4.0±4.91 | Postoperative complications: 45/82 patients (54.9) | - | 30 days |
| Alkhamis T et al (2014)[27] | 60 | Prospective cohort study | Elective | Surgery due to intestinal organ diseases, expansive process of central nervous system, or degenerative hip disease | 62.5 (56-72.5) (median (q1-q3)) | - | - | - | 5 days |
| Bolat D et al (2017)[34] | 153 | Retrospective kohort study | Elective | Penile prosthesis implantation | 56.4 ± 8.0 | - | Postoperative infectious complications: 18/153 patients (11.8%) | - | 56 ± 30.4 months |
| Metineren H et al (2017)[35] | 56 | Retrospective cohort study | - | Limb amputation as a result of diabetic foot ulcer | 72.73 ± 10.53 | 8.25 (1.3-70) (median (min-max)) | - | 2-weeks mortality due to sepsis 24/56 patients (42%) | 2 weeks |
| Kang W-M et al (2017)[36] | 108 | Retrospective cohort study | - | Abdominal surgery (bowel resection) due to Crohn’s disease | 37.6 ± 13.1 | 5.9 ±12.1 | Postoperative complications: 30/108 patients (27.8%) | 1/108 (0.9%) | 17 days |
*clavien-dindo ≥ 3
Continuous data were expressed as mean +/- standard deviation unless indicated otherwise
95% CI: 95% confidence interval; q1: 1st quartile; q3: 3th quartile
AF: Atrial Fibrillation; AKI: Acute Kidney Injury; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; AUC: Area Under the Curve (i.e., ROC curve); CABG: Coronary Artery Bypass Grafting; CAD: Coronary Artery Disease; CAE: Combined Adverse Events (myocardial infarction, cardiac reoperation, prolonged mechanical ventilation ( > 48h), prolonged hospital stay, rehospitalization or mortality); CCS: Canadian Cardiovascular Society; CEA: Carotid Endarterectomy; DBP: Diastolic Blood Pressure; HB: Hemoglobin; HTN: Hypertensive; HR: Hazard Ratio; LOS: Length of hospital stay; LVEF: Left Ventricular Ejection Fraction; MCV: Mean Corpuscular Volume; MI: Myocardial infarction; MICS: Minimally Invasive Cardiac Surgery; MPV: Mean Platelet Volume; NLR: Neutrophil-Lymphocyte Ratio; OR: Odds Ratio; PCI: Percutaneous Coronary Intervention; PLR: Platelet:Lymphocyte Ratio; PPS: Post –Pericadiotomy Syndrome; PTH: Parathyroid Hormone; rAAA: ruptured Abdominal Aortic Aneurysm; RDW: Red Cell Distribution Width; ROC: Receiver Operating Characteristic; RV: Right Ventricular; SBP: Systolic Blood Pressure; SVGF: Saphenous Vein Graft Patency
Cardiac surgery
Six studies included patients undergoing coronary artery bypass grafting (CABG)(n = 4,771)[12-15,31,32] and one study included patients undergoing CABG, isolated single cardiac valve surgery and combined surgery (n = 1500)[29]. An eighth study included patients undergoing different types of open cardiac or thoracic aortic surgery with cardio-pulmonary bypass (n = 590)[19]. A ninth study included patients with advanced heart failure who received left ventricular assist devices (n = 273)[17]. Two studies included patients who underwent surgery for acute type I aorta dissection (n = 288)[21,30] table 1.
In one study patients who underwent CABG with a high preoperative NLR (NLR > 3.00 (2.30-3.85)) had a significantly higher risk of developing atrial fibrillation compared with patients with a low NLR[14]. Another study found no association between high NLR and atrial fibrillation[38], table 2.
NLR was independently associated with the risk of saphenous vein graft failure[15], adjusted OR = 1,39 (1,19-1,61), and was an independent predictor for combined adverse events[29], OR = 0.21 (0.2-0.3), table 2. NLR was independently associated with sternum revision[38], adjusted OR = 2.95 (1.32-6.63), but no association was found between NLR and post-pericardiotomy syndrome[31] or neurologic events[38] in patients who underwent CABG, table 2.
NLR was also independently associated with right ventricular failure[17] in patients with advanced heart failure implanted with left ventricular assist devices OR = 1.12, CI = 1.04-1.20, p = 0.003), table 2.
Likewise, patients with an NLR ≥ 2 had a significantly higher risk of developing acute kidney injury[16], table 2.
NLR was independently associated with multi-organ dysfunction, major bleeding and hospital-related infection[30], in patients with aorta dissection, table 2.
Six studies evaluated the association between preoperative NLR and postoperative mortality[12,13,30,37,39,40]. All but one study[39] reported a high pre-operative NLR to be associated with overall postoperative mortality[12,13,32,36,40], table 2.
Table 2:
|
Cardiac Surgery |
||||||
|---|---|---|---|---|---|---|
|
Author |
NLR and Morbidity |
NLR and Mortality |
ROC |
Variables in the adjusted analysis |
||
|
Categorical |
Continuous |
Categorical |
Continuous |
|||
|
Azab B et al (2013)[12] |
- |
- |
30-days, 6-months, 1- and 5-years mortality rates: NLR < 2.3: 0.5%, 1.6%, 2.4% and 8% 2.3 ≤ NLR ≤ 3.4: 1.3%, 4.3%, 5.1% and 13% NLR > 3.4: 2.7% 7.7%, 9.9% and 20%) p < 0.0001 |
uHR 1.09 per unit NLR (1.06-1.13), p < 0.001 aHR 1.06 per unit NLR (1.01-1.10), p = 0.008 |
- |
Mortality, continuous: Age, gender, family history of coronary artery disease, smoking, dialysis, COPD, ACE-I, statin, aspirin, post-operative sepsis, preoperative glucose |
|
Gibson P et al (2007)[13] |
- |
- |
All-cause mortality: uHR NLR quartile 1 (low): 1 NLR quartile 2: 0.89 (0.56-1.42) NLR quartile 3: 0.89 (0.56-1.43) NLR quartile 4 (high): 2.06 (1.39-3.06), p < 0.001 aHR NLR quartile 1 (low): 1 NLR quartile 2: 0.81 (0.51-1.30) NLR quartile 3: 0.77 (0.48-1.23) NLR quartile 4 (high): 1.42 (0.95-2.15), p = 0.09 |
All-cause mortality: Survived: NLR=2.39 (1.84-3.27) Deceased: NLR=2.79 (2.00-4.22) uHR: 1.13 per unit NLR (95% CI 1.08-1.18) p < 0.001 aHR: 1.08 per unit NLR (95% CI 1.02-1.15), p = 0.008 Cardiovascular mortality: uHR: 1.12 per unit NLR (95% CI 1.06-1.18) p < 0.001 aHR: 1.08 per unit NLR (95% CI 1.00-1.16) p = 0.046 |
- |
All-cause mortality, categorical: Euroscore All-cause mortality, continuous: Preoperative total WCC, preoperative monocyte count, Euroscore. Cardiovascular mortality, continuous: Preoperative total WCC, preoperative monocyte count, Euroscore. |
|
Gibson P et al (2010)[14] |
Risk of atrial fibrillation NLR quartile 1 (low): 20/68 patients (29%) NLR quartile 2: 20/69 patients (29%) NLR quartile 3: 31/69 patients (45%) NLR quartile 4 (high): 36/69 patients (52%) P = 0.001 NLR > 2.63: uOR 2.23 (1.36-3.67) p = 0.002 aOR 1.76 (1.04-2.97), p = 0.03 |
No atrial fibrillation: NLR = 2.42 (1.94-3.23) Atrial fibrillation: NLR = 3.00 (2.30-3.85), p = 0.001 Risk of atrial fibrillation uOR 1.29 per unit NLR, p = 0.007 aOR 1.29 (1.07-1.55), p = 0.007 |
- |
- |
Atrial fibrillation NLR cut-off 2.63 sensitivity 63% specificity 68% AUC 0.61 (0.55-0.68), P = 0.001. |
Morbidity, categorical: age, sex, BMI, previous myocardial infarction, ejection fraction, diabetes mellitus, current smoker, hypertension, Euroscore, parsonnet score, New York Heart Association functional class III/IV, Canadian Cardiovascular Society angina class III/IV, estimated glomerular filtration rate, aspirin/clopidogrel, Beta-blocker, statin, ACE inhibitor/angiotensin receptor blocker, preoperative haemoglobin, preoperative total white blood cell count, high sensitivity C-reactive protein, off-pump procedure, preoperative intra-aortic balloon pump, no. of bypass grafts, internal mammary artery used, bypass time, cross-clamp time, perioperative inotropes, postoperative total WBC count, postoperative C-reactive protein, postoperative troponin I at 48 hours, postoperative AF Morbidity, continuous: Euroscore |
|
Tasoglu I et al (2013)[15] |
Risk of SVGF: uOR1.42 per unit NLR (1.22-1.74) P < 0.001 aOR1.39 per unit NLR (1.19-1.61) P < 0.001 |
Morbidity, continuous: Creatinine > 1.2, target artery diameter < 1.5 mm, smoking, diabetes, interval between operation and angiogram |
||||
|
Aydınlı Bet al (2016)[29] |
Combined adverse events NLR = 5.1 ± 3.7 No CAE: NLR = 2.7 ± 1.4 P = < 0.001 Risk of CAE aOR 4.76, (-3.39-6.67), p < 0.001 |
Morbidity, continuous: Euroscore, hemoglobin, red cell distribution width, mean platelet volume, platelet:lymphocyte ratio |
||||
|
Sevuk U et al (2016)[31] |
- |
PPS: NLR = 2.39 (1.8-3.15) No PPS: NLR = 2.6 (1.9-3.3), p = 0.35 Median (q1-q3) |
||||
|
Saskin H et al (2015)[32] |
Sternum revision: uOR 3.38 per unit NLR (95% CI 1.93-5.91), p = 0.0001 aOR 2.95 per unit NLR (95% CI 1.32-6-63), p = 0.009 Neurologic events: uOR 1.52 per unit NLR (95% CI 0.88-2.63), p = 0.14 Atrial fibrillation: uOR 1.81 per unit NLR (95% CI 1.49-2.19), p = 0.0001 aOR 1.17 per unit NLR (95% CI 0.92-1.50), p = 0.20 |
Morbidity, continuous: sex, age, ejection fraction, diabetes mellitus, hypertension, hyperlipidemia, smoking, fasting blood glucose, preoperative LDL, preoperative platelet, preoperative lymphocyte, PLR, preoperative neutrophil, preoperative CRP, preoperative hematocrit, preoperative hemoglobin, aortic cross clamp time, use of blood products, use of inotropic support, amount of drainage |
||||
|
Kim W et al (2015)[16] |
NLR<1.5: AKI: 29/166 patients (17.6%) uOR = 1 1.5 ≤ NLR < 2: AKI: 34 (20.6%) uOR= 1.40 (95% CI 0.80-2.46) P = 0.237 2 ≤ NLR < 3: AKI: 50 (30.3%) uOR: 2.08 (95% CI 1.23-3.53) p = 0.006 NLR ≥ 3: AKI: 52 (31.5%) uOR: 2.19 (95% CI 1.29-3.70) p = 0.004 |
No AKI: NLR =1.93 (1.43-2.83) AKI: NLR = 2.30 (1.67-3.29) uOR: 1.07 (95% CI 0.99-1.12Al) P = 0.086 |
Unadjusted 1-year survival stratified by NLR-quartiles, Log-rank test, P = 0.314 |
|||
|
Yost GL et al (2015)[17] |
RV failure: Tertile 1 (NLR = 2.21 ± 0.66): 19/91 patients (20.9%) Tertile 2 (NLR = 4.01 ± 0.59): 25/91 patients (27.5%) Tertile 3 (NLR = 9.15 ± 6.05): 40/91 patients (44.0%), p < 0.001 |
Risk of RV failure: uOR 1.117 per unit NLR (95% CI 1.039-1.201), P = 0.003 |
30-days survival: NLR tertile 1 (low): 91/91 patients (100%) NLR tertile 2: 89/91 patients (97.8%) NLR tertile 3 (high): 84/91 patients (92.3%), p = 0.011 1-year survival: NLR tertile 1 (low): 58/91 patients (87.9%) NLR tertile 2: 53/91 patients (82.8%) NLR tertile 3: 45/91 patients (69.2%), p = 0.022 |
2-years all-cause mortality: aOR 1.159 per unit NLR (95% CI 1.022-1.314), p = 0.021 |
Mortality, continuous: Age, serum sodium, BUN, creatinine, BNP, AST, bilirubin, WBC counts, blood pressure, mitral regurgitation, previous stroke, chronic kidney disease |
|
|
Lafci G et al (2014)[21] |
- |
- |
- |
Deceased in-hospital: NLR = 12.3 ± 7.4 Survived: NLR 9.0 ± 6.3 p = 0.025 uHR 1.07 per unit NLR (95% CI 1.02-1.13), p = 0.03 aHR 1.05 per unit NLR (95% CI 1.01-1.10), p = 0.03 |
Mortality: NLR cut-off 8.0, sensitivity 70% specificity 53% AUC: 0.634 (95% CI 0.516-0.753) |
Mortality, continuous: cross-clamp time, cardiopulmonary bypass time, intensive care unit duration, ventilation time, hemorrhage amount, aspartate aminotransferase level, platelet count |
|
Kalkan ME et al (2017)[30] |
Reoperation NLR > 6: 7/91 patients (8%) NLR ≤ 6: 2/93 patients (2%) p = 0.079 Multi-organ dysfunction: NLR > 6: 16/91 patients (17%) NLR ≤ 6: 7/93 patients (8%) p = 0.032 Major bleeding: NLR > 6: 17/91 patients (19%) NLR ≤ 6: 5/93 patients (5%) p = 0.005 Acute renal failure: NLR > 6: 15/91 patients (16%) NLR ≤ 6: 11/93 patients (12%) p = 0.112 Stroke: NLR > 6: 9/91 patients (10%) NLR ≤ 6: 3/93 patients (3%) p = 0.06 Extremity emboli: NLR > 6: 0/91 patients (0%) NLR ≤ 6: 1/93 patients (1%) p = 0.505 Hospital-related infection: NLR > 6: 23/91 patients (25%) NLR ≤ 6: 13/93 patients (14%) p = 0.041 |
In-hospital mortality NLR > 6: 28/91 patients (30%) NLR ≤ 6 10/93 patients (10%) p = 0.001 |
In-hospital cardiovascular mortality: uOR 1.182 per unit NLR (95% CI 1.077-1.298), p < 0.001 aOR 1.147 per unit NLR (95% CI 1.030-1.276), p = 0.012 |
In-hospital cardiovascular mortality cut-off: NLR > 6.5 Sensitivity 71% Sensibility 63% |
AUC: 0.71 (95% CI 0.631-0.789), p < 0.001 Mortality, continuous: WBC, Operation duration, NLR |
|
aOR: Adjusted odds ratio
COPD: Chronic obstructive pulmonary disease
uOR: unadjusted odds ratio
WCC: White cell count
Vascular surgery
Two studies included patients undergoing surgery for aortic aneurysms (n = 782)[18,20]. A high preoperative NLR was shown to be a predictor of postoperative short- and long-term mortality[18,20] and postoperative morbidity[20], table 3. Morbidity was defined according to the Clavien-Dindo classification ≥ 3[20].
In patients who underwent elective carotid endarterectomy[19] or embolectomy for acute limb ischemia[23], a high pre-operative NLR (NLR ≥ 5[19] and NLR ≥ 5.2[23]) predicted postoperative cognitive dysfunction[19] and poor limb survival[23], table 3.
NLR was an independent predictor of myocardial infarction, stroke and death, in patients with critical limb ischemia, who underwent major amputation[33], table 3.
In patients who underwent lower limb revascularization, no association was shown between preoperative NLR and postoperative graft patency in the multivariate analysis[22].
Table 3:
|
Vascular surgery |
||||||
|---|---|---|---|---|---|---|
|
Author |
NLR and Morbidity |
NLR and Mortality |
ROC |
Variables in the adjusted analysis |
||
|
Categorical |
Continuous |
Categorical |
Continuous |
|||
|
Appleton ND et al (2014)[18] |
30 days mortality: NLR > 5: 12/ 52 patients (23%)NLR < 5 20/298 patients (6.7%), uOR 4.17 (95% CI 1.90-9.18), p = 0.0007 1-year mortality NLR < 5: 102/298 patients (34.3%) NLR > 5: 26/52 patients (50%), uOR 1.92 (1.06-3.48), p = 0.043 |
Deceased within 30 days: NLR = 4.2 (2.6-7.5) Survived: NLR = 2.8 (2.1-3.8), p = 0.0001 Deceased within 1 year: NLR = 3.2 (2.5-4.6) Survived: NLR = 2.6 (2.0-3.6), p = 0.00004 median (q1-q3) |
||||
|
Halazun H et al (2014)[19] |
Cognetive dysfunction: NLR<5: 46/360 patients (12.8%) NLR ≥ 5: 25/72 patients (34.7%) p < 0.001 Risk of cognetive dysfunction: NLR ≥ 5: aOR 3.38 (95% CI 1.81-6.27), p < 0.001 |
“Cognetive dysfunction: |
Morbidity, categorical: sex, education, statin use, diabetes mellitus |
|||
|
Kordzadeh A et al (2015)[20] |
“30-days morbidity (Clavien-Dindo ≥ 3): NLR < 5: 6/25 patients (24%) |
“30-days mortality: NLR < 5: 2/25 patients (8%) NLR > 5: 9/55 patients (16.4%) |
Morbidity, categorical: hypertension |
|||
|
Kullar P et al (2012)[22] |
“Risk of graft patency: |
Morbidity, continuous: postoperative NLR, smoking, vein graft, age |
||||
|
Tasoglu I et al (2014)[23] |
“Amputation within 30 days: |
“Non-amputated: |
“30 days mortality: |
“Amputation within 30 days cut-off: NLR ≥ 5.2 |
Morbidity, categorical and continuous: COPD, diabetes mellitus, no arterial back bleeding |
|
|
Wang Q et al (2017)[33] |
Clinical complications (myocardial infarction, stroke, death): NLR ≥ 8.08 aOR 26.23 (95% CI 5.80-118.58), p < 0.001 |
“Clinical complications (myocardial infarction, stroke, death): NLR = 20.12 ± 16.29 |
- |
- |
“Clinical complications cut-off: NLR ≥ 8.08 |
Morbidity, categorical: gender, age, progress classification, smoking history, hypertension, diabetes mellitus, coronary heart disease, hyperlipidemia, cerebral apoplexy, PLR, MCV, RDW |
aOR: Adjusted odds ratio
PLR: Platelet Lymphocyte Count
RDW: Red Cell Distribution Witdt
uOR: Unadjusted Odds Ratio
Non-cardiac non-vascular surgery
A study including 1,087 patients examined whether preoperative NLR predicted the risk of infection in patients undergoing total knee arthroplasty[24]. Non-infected patients had a significantly lower NLR than infected patients after surgery[24], table 4. A second study included 294 patients aged 60 years and over undergoing hip-fracture surgery[28]. NLR predicted both postoperative myocardial injury (NLR > 5.1)[28] and in-hospital mortality (NLR > 8.5)[28], table 4. A third study included 56 patients who underwent limb amputation as a result of diabetic foot ulcer[35] and a fourth study included 104 patients with hip fractures undergoing surgery[25]. NLR did not predict mortality after surgery[25,35].
A fifth study on 108 patients examined the risk of postoperative complications in patients with Crohn’s disease who underwent abdominal surgery[36]. NLR predicted postoperative complications, a OR = 2.78 (1.04-7.43)[36], table 4. Another study on 82 patients examined the risk of postoperative complications within 30 days of major abdominal surgery[26]. There was no significant difference in preoperative NLR for patients with or without medical- or surgical complications[26], table 4.
A study examined NLR as a predictor of early penile prosthesis implant infection[34]. A preoperative NLR ≥ 6.2 was associated with postoperative infectious events[34], table 4.
Finally, a study included 60 patients who underwent elective surgery due to intestinal disease, expansive process of the central nervous system or degenerated hip disease[27]. Preoperative NLR did not predict the risk of postoperative sepsis within day 5 of surgery[27].
Table 4:
|
Non-cardiac non-vascular surgery |
||||||
|---|---|---|---|---|---|---|
|
Author |
NLR and Morbidity |
NLR and Mortality |
ROC |
Variables in the adjusted analysis |
||
|
Categorical |
Continuous |
Categorical |
Continuous |
|||
|
Gölge U et al (2016)[24] |
“No infection NLR = 2.1 ± 0.7 |
Infection NLR cut-off: 2.45 Sensitivity 90% Specificity 72% |
||||
|
Fisher A et al (2016)[28] |
“Risk of troponin elevation |
“No troponin elevation |
“In-hospital mortality NLR > 8.5 |
“Deceased in-hospital death: |
“In-hospital mortality NLR cut-off: |
“Morbidity and mortality, continuous: |
|
Sedlár M et al (2015)[25] |
“Deceased, |
|||||
|
Forget P et al (2015)[26] |
“No postoperative complications: NLR = 4.13 ± 4.43 |
|||||
|
Alkhamis T et al (2014)[27] |
“Postoperative sepsis NLR cut-off: |
|||||
|
Bolat D et al (2017)[34] |
“No postoperative complication (infection) |
“Postoperative infection NLR cut-off: |
||||
|
Metineren H et al (2017)[35] |
“Deceased within 2 weeks: |
|||||
|
Kang W-M et al (2017)[36] |
“Risk of postoperative complications, |
“Postoperative complications, |
“Postoperative complications NLR cut-off: |
Morbidity, continuous: gender, age, smoking history, history of appendectomy, emergency operation, blood type, extraintestinal manifestations, perianal lesions, preoperative BMI, Onodera prognostic nutrition index, primary lesions, disease type, preoperative duration, preoperative neutrophil count, preoperative lymphocyte count, preoperative haemoglobin, preoperative albumin, preoperative enteral nutrition |
||
Risk of bias within the studies
The methodological assessment is summarized in table 5.
In 23 out of 25 studies, risk of bias was scored to moderate. Two studies were scored to serious risk of bias, since there was no adjustment for confounding[18,24]. The populations examined were generally representative with patients of all ages in both genders. The quality of definitions according to diagnosis was specific in general. Objectives and cut-offs were in the majority of studies decided in advance and definitions of postoperative outcomes were clear. The studies differed in clinical diagnostic methods but followed clinical standards.
Table 5: Risk of bias within studies.
|
Study |
Bias due to confounding |
Bias in selection of participants into the study |
Bias in measurement of interventions |
Bias due to departures from intended interventions |
Bias due to missing data |
Bias in measurement of outcomes |
Bias in selection of the reported results |
Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
|
Azab B et al (2013)[12] |
Moderate |
Low |
Low |
Non relevant |
Low |
Low |
Moderate |
Moderate |
|
Gibson P et al (2007) [13] |
Moderate |
Low |
Low |
Non relevant |
Moderate |
Low |
Moderate |
Moderate |
|
Gibson P et al (2010) [14] |
Moderate |
Moderate |
Low |
Non relevant |
Moderate |
Low |
Moderate |
Moderate |
|
Tasoglu I et al (2013) [15] |
Moderate |
Low |
Low |
Non relevant |
Low |
Low |
Low |
Moderate |
|
Kim W et al (2015)[16] |
Moderate |
Moderate |
Low |
Non relevant |
Low |
Low |
Low |
Moderate |
|
Yost GL et al (2015) [17] |
Moderate |
Low |
Low |
Non relevant |
Low |
Moderate |
Moderate |
Moderate |
|
Lafci G et al (2014)[21] |
Moderate |
Low |
Moderate |
Non relevant |
Low |
Low |
Moderate |
Moderate |
|
Kalkan ME et al (2017) |
Low |
Moderate |
Moderate |
Non relevant |
Low |
Low |
Moderate |
Moderate |
|
Sevuk U et al (2016) |
Moderate |
Moderate |
Low |
Non relevant |
Low |
Moderate |
Moderate |
Moderate |
|
Saskın H MD et al (2015) |
Low |
Moderate |
Low |
Non relevant |
Low |
Low |
Low |
Moderate |
|
Appleton ND et al (2014) [1] |
Moderate |
Low |
Moderate |
Non relevant |
Moderate |
Low |
Serious |
Serious |
|
Halazun H et al (2014) [19] |
Low |
Low |
Low |
Non relevant |
Moderate |
Low |
Low |
Moderate |
|
Kordzadeh A et al (2015) [20] |
Moderate |
Low |
Moderate |
Non relevant |
Low |
Low |
Moderate |
Moderate |
|
Kullar P et al (2012) [22] |
Moderate |
Low |
Low |
Non relevant |
Low |
Moderate |
Moderate |
Moderate |
|
Tasoglu I et al (2014) [23] |
Moderate |
Low |
Low |
Non relevant |
Low |
Moderate |
Moderate |
Moderate |
|
Wang Q et al (2017) |
Moderate |
Moderate |
Moderate |
Non relevant |
Low |
Low |
Moderate |
Moderate |
|
Gölge G et al (2016) [24] |
Serious |
Moderate |
Moderate |
Non relevant |
Moderate |
Moderate |
Moderate |
Serious |
|
Sedlár M et al (2015) [25] |
Moderate |
Low |
Low |
Non relevant |
Moderate |
Low |
Moderate |
Moderate |
|
Forget P et al (2015) [26] |
Moderate |
Moderate |
Moderate |
Non relevant |
Moderate |
Moderate |
Moderate |
Moderate |
|
Alkhamis T et al (2014) [30] |
Moderate |
Low |
Moderate |
Non relevant |
Moderate |
Moderate |
Moderate |
Moderate |
|
Fisher A et al (2016) [2] |
Moderate |
Moderate |
Low |
Non relevant |
Moderate |
Moderate |
Moderate |
Moderate |
|
Aydınlı B et al (2016) [29] |
Moderate |
Low |
Low |
Non relevant |
Low |
Moderate |
Low |
Moderate |
|
Bolat D et al (2017) |
Moderate |
Moderate |
Moderate |
Non relevant |
Moderate |
Moderate |
Moderate |
Moderate |
|
Metineren H et al (2017) |
Moderate |
Moderate |
Moderate |
Non relevant |
Moderate |
Low |
Moderate |
Moderate |
|
Kang W-M et al (2017) |
Low |
Low |
Moderate |
Non relevant |
Moderate |
Low |
Low |
Moderate |
Discussion
A total of 25 clinical observational studies with 10,015 surgical patients were included. All but two studies in cardiac- and vascular surgery showed a significant association between preoperative NLR and postoperative mortality while 11out of 13 studies reported that high NLR predicted postoperative cardiovascular morbidity. In orthopaedic surgery, NLR predicted troponin elevation after hip surgery and postoperative infections after knee surgery, while no association was shown between preoperative NLR and postoperative complications in patients amputated due to diabetic foot ulcers. NLR predicted postoperative complications after prosthesis implantation[34] and bowel resection[36].
NLR did not predict the risk of postoperative complications after abdominal and miscellaneous non-cardiac surgery.
NLR reflects a systemic inflammatory state[24]. This association between the inflammatory response and the clinical outcome after surgery is complex. In studies investigating NLR in relation to cancer surgery, it has been reported that neutrophils secrete factors favourable for growth of malignant solid tumours e.g. tumour growth promoting factors[6,41,42]. However, the pathophysiological link between preoperative NLR and clinical outcomes after benign surgery is unclear. Neutrophils release inflammatory mediators, proteolytic enzymes, arachidonic acid derivatives, and superoxide radicals that may promote rupture of coronary plaques and cause further tissue injury[13]. NLR could reflect an imbalance in the acute immunological response. The antibacterial response of natural killer cells and activated T-cells may be supressed by an increased number of neutrophils[47]. The high NLR may then reflect an increased neutrophil-dependent inflammatory response and a decreased lymphocyte-mediated antibacterial immune response[47]. This may weaken the lymphocyte-mediated antibacterial immune response contributing to an increased bacterial invasion and growth[47]. Moreover, a low lymphocyte count indicates a state of immunosuppression and physiological stress that have adverse effects on the overall clinical outcome after surgery[20].
A clinical study with 211 patients[43] showed that relative lymphopenia is significantly associated with survival of patients with known or suspected stable coronary artery disease. A low relative lymphocyte count may reflect the cortisol-induced stress response[43]. Therefore, the relative lymphocyte count could be a marker of the systemic stress induced by the adrenal axis[43]. Furthermore, a study including 309 patients diagnosed with acute heart failure[49] showed, that a low absolute lymphocyte count was associated with greater in-hospital mortality[49] and that a low absolute lymphocyte count was an independent predictor of all-cause mortality[49].
The prognostic value of NLR has been compared with other inflammatory biomarkers. In prosthetic joint surgery, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were used to predict prosthetic joint infections. A study including 1087 patients[49] concluded that NLR together with C-reactive protein (CRP)and ESR would increase the accuracy of the diagnosis and prediction of prosthetic joint infection after total knee arthroplasty[49].
WBC count is included in scoring systems of systemic inflammatory response syndrome[50]. Like WBC, NLR has been shown to predict the risk of sepsis in critically ill patients[50]. A study including 3,227 patients with acute myocardial infarction reported that WBC count was an independent predictor of death or myocardial infarction within 7 years[51]. The best predictive values in the study were a high neutrophil count or a low lymphocyte count resulting in a high NLR[51].
Moreover, the NLR was shown to be a better predictor of bacteraemia than the currently used inflammatory markers (CRP, WBC and ESR), which had a poor prognostic value compared with the NLR[51]. These results were confirmed in a clinical study including 395 patients[52] diagnosed with community acquired pneumonia. NLR had a higher prognostic accuracy compared with neutrophil count, WBC count, lymphocyte count and CRP[52].
Additionally, a study[53] including 92 patients with suspected community-acquired bacteraemia showed NLR to be a superior predictor of bacteraemia compared to CRP, WBC and ESR[53]. Hence, NLR is an easy available and inexpensive method whose predictive value has great potential.
Strengths and limitations
The systematic review was performed according to the PRISMA guidelines. The systematic literature search was performed in four major medical databases. Registration on PROSPERO secured transparency of the study. We excluded studies on patients undergoing transplantation due to the potential confounding of immunosuppressive medicine. The studies reported a variety of predictive NLR cut-off values. In heart surgery, the predictive NLR cut-off value was around 3, while the cut-off value in vascular surgery was around 4.5. In a majority of studies, NLR > 5 was considered to be predictive of clinical outcomes after cancer- surgery[54,55]. The predictive NLR cut-off value seems to depend on the population and type of surgery. With regard to non-cardiac non-vascular surgery, further studies are needed to come closer to a NLR cut-off. Even though a larger number of studies were included in the systematic review a meta analysis was not performed due to heterogeneous clinical outcomes and a heterogeneous reporting of short- and long-term postoperative mortality. The studies in non-cardiac non-vascular surgery were minor studies. The non-significant findings could be due to a lack of power. Therefore, to corroborate or invalidate NLR as a prognostic biomarker in patients undergoing non-cardiac non-vascular surgery further studies with larger populations are needed.
Conclusion
NLR predicted short-term mortality after cardiac- and vascular surgery. In 11 out of 13 studies, a high NLR predicted postoperative cardiovascular morbidity. In orthopaedic surgery, a high NLR predicted postoperative myocardial injury and mortality after hip surgery and postoperative infections after knee surgery. In general surgery, NLR predicted postoperative complications in patients with Crohn’s disease undergoing bowel resection and postoperative infectious complications. NLR has potential as a prognostic biomarker in patients undergoing benign surgery, however, the prognostic impact of NLR should be further explored in patients undergoing non-cardiac non-vascular surgery.
Conflict of interest: none
References
- 1. Pine, J.K., Morris, E., Hutchins, G.G., et al. Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: the relationship to patient survival, tumour biology and local lymphocytic response to tumour. (2015) Br J Cancer 113(2): 204-211.
- 2. Gomez, D., Farid, S., Malik, H.Z., et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. (2008) World J Surg 32(8): 1757-1762.
- 3. An, X., Ding, P.R., Li, Y.H., et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. (2010) Biomarkers 15(6): 516-522.
- 4. Wang, Y., Liu, P., Xu, Y., et al. Preoperative neutrophil-to-lymphocyte ratio predicts response to first-line platinum-based chemotherapy and prognosis in serous ovarian cancer. (2015) Cancer Chemother Pharmacol 75(2): 255-262.
- 5. Yang, X., Huang, Y., Feng, J-F., et al. Prognostic significance of neutrophil-to-lymphocyte ratio in esophageal cancer: A meta-analysis. (2015) Onco Targets Ther 8: 789-794.
- 6. Templeton, A.J., McNamara, M.G., Šeruga, B., et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. (2014) J Natl Cancer Inst 106(6): dju124.
- 7. Mohri, Y., Tanaka, K., Toiyama, Y., et al. Impact of Preoperative Neutrophil to Lymphocyte Ratio and Postoperative Infectious Complications on Survival After Curative Gastrectomy for Gastric Cancer: A Single Institutional Cohort Study. (2016) Medicine (Baltimore) 95(11): e3125.
- 8. Bhindi, B., Hermanns, T., Wei, Y., et al. Identification of the best complete blood count-based predictors for bladder cancer outcomes in patients undergoing radical cystectomy. (2015) Br J Cancer 114(2): 207-212.
- 9. Azab, B., Jaglall, N., Atallah, J.P., et al. Neutrophil-Lymphocyte Ratio as a Predictor of Adverse Outcomes of Acute Pancreatitis. (2011) Pancreatology 11(4): 445-452.
- 10. Liberati, A., Altman, D.G., Tetzlaff, J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. (2009) J Clin Epidemiol 62(10): e1-34.
- 11. Sterne, J., Higgins, J., Reeves, B.C., et al. A Cochrane Risk of Bias Assessment Tool: for Non- Randomized Studies of Interventions (ACROBAT-NRSI).
- 12. Azab, B., Shariff, M.A., Bachir, R., et al. Elevated preoperative neutrophil/lymphocyte ratio as a predictor of increased long-term survival in minimal invasive coronary artery bypass surgery compared to sternotomy. (2013) J Cardiothorac Surg 8: 193.
- 13. Gibson, P.H., Croal, B.L., Cuthbertson, B.H., et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. (2007) Am Heart J 154(5): 995-1002.
- 14. Gibson, P.H., Cuthbertson, B.H., Croal, B.L., et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. (2010) Am J Cardiol 105(2): 186-191.
- 15. Taşoğlu, I., Turak, O., Nazli, Y., et al. Preoperative neutrophil-lymphocyte ratio and saphenous vein graft patency after coronary artery bypass grafting. (2014) Clin Appl Thromb Hemost 20(8): 819-824.
- 16. Kim, W.H., Park, J.Y., Ok, S-H., et al. Association between the neutrophil/lymphocyte ratio and acute kidney injury after cardiovascular surgery: a retrospective observational study. (2015) Medicine (Baltimore) 94(43): e1867.
- 17. Yost, G.L., Joseph, C.R., Tatooles, A.J., et al. Neutrophil-to-Lymphocyte Ratio Predicts Outcomes in Patients Implanted with Left Ventricular Assist Devices. (2015) ASAIO J 61(6): 664-669.
- 18. Appleton, N.D., Bailey, D.M., Morris-Stiff, G., et al. Neutrophil to lymphocyte ratio predicts perioperative mortality following open elective repair of abdominal aortic aneurysms. (2014) Vasc Endovascular Surg 48(4): 311-316.
- 19. Halazun, H.J., Mergeche, J.L., Mallon, K.A., et al. Neutrophil-lymphocyte ratio as a predictor of cognitive dysfunction in carotid endarterectomy patients. (2014) J Vasc Surg 59(3): 768-773.
- 20. Kordzadeh, A., Malietzis, G., Browne, T., et al. Neutrophil to lymphocyte ratio (NLR) of five predicts 30-day morbidity in ruptured abdominal aortic aneurysms (rAAA): A retrospective cohort study. (2015) Int J Surg 15: 45-48.
- 21. Lafci, G., Ciçek, Ö.F., Uzun, H.A., et al. Relationship of admission neutrophil-to-lymphocyte ratio with in-hospital mortality in patients with acute type I aortic dissection. (2014) Turk J Med Sci 44(2): 186-192.
PubMed||CrossRef||Others
- 22. Kullar, P., Weerakoddy, R., Walsh, S. Neutrophil-lymphocyte ratio predicts graft patency following lower limb revascularisation. (2012) Acta Chir Belg 112(5): 365-368.
PubMed||CrossRef||Others
- 23. Taşoǧlu, I., Çiçek, O.F., Lafci, G., et al. Usefulness of neutrophil/lymphocyte ratio as a predictor of amputation after embolectomy for acute limb ischemia. (2014) Ann Vasc Surg 28(3): 606-613.
- 24. Gölge, U.H., Kaymaz, B., Pazarcı, Ö., et al. Neutrophil to lymphocyte ratio may be a diagnostic marker for prosthetic joint infection . (2016) J Clin Anal Med 7(2): 218-221.
PubMed||CrossRef||Others
- 25. Sedlar, M., Kvasnicka, J., Krska, Z., et al. Early and subacute inflammatory response and long-term survival after hip trauma and surgery. (2015) Arch Gerontol Geriatr 60(3): 431-436.
- 26. Forget, P., Dinant, V., De Kock, M. Is the neutrophil-to-lymphocyte ratio more correlated than C-reactive protein with postoperative complications after major abdominal surgery? (2015) Peer J 3: e713.
- 27. Alkhamis, T., Ivic, D., Wagner, J., et al. Postoperative immunosuppression markers and the occurrence of sepsis in patients with benign and malignant disease. (2014) Wien Klin Wochenschr 126(23-24): 774-784.
- 28. Fisher, A., Srikusalanukul, W., Fisher, L., et al. The neutrophil to lymphocyte ratio on admission and short-term outcomes in orthogeriatric patients. (2016) Int J Med Sci 13(8): 588-602.
- 29. Aydınlı, B., Demir, A., Güçlü, Ç.Y., et al. Hematological predictors and clinical outcomes in cardiac surgery. (2016) J Anesth 30(5): 770-778.
- 30. Kalkan, M.E., Kalkan, A.K., Gündeş, A., et al. Neutrophil to lymphocyte ratio : a novel marker for predicting hospital mortality of patients with acute type A aortic dissection. (2017) Perfusion 32(4): 321-327.
- 31. Sevuk, U., Bilgic, A., Altindag, R., et al. Value of the neutrophil-to-lymphocyte ratio in predicting post-pericardiotomy. (2016) Eur Rev Med Pharmacol Sci 20(5): 906-911.
- 32. Saskın, H., Düzyol, Ç., Özcan, K.S., et al. Preoperative Platelet to Lymphocyte Ratio Is Associated with Early Morbidity and Mortality after Coronary Artery Bypass Grafting. (2015) Heart Surg Forum 18(6): 255-262.
PubMed||CrossRef||Others
- 33. Wang, Q., Lui, H., Sun, S., et al. Neutrophil-tolymphocyte ratio is effective prognostic indicator for post-amputation patients with critical limb. (2017) Saudi Med J 38(1): 24-29.
- 34. Bolat, D., Kadir, Y., Ozgu, T., et al. Neutrophil to Lymphocyte Ratio as a predictor of early penile prosthesis implant infection. (2017) Int Urol Nephrol 49(6): 947-953.
- 35. Metineren, H., Cihan, T. Comparison of the Neutrophil / Lymphocyte Ratio and C-Reactive Protein Levels in Patients With Amputation for Diabetic Foot Ulcers. (2017) Int J Low Extrem Wounds 16(1): 23-28.
- 36. Kang, W., Zhu, C., Yang, X., et al. Application of the Onodera prognostic nutrition index and neutrophil-to-lymphocyte ratio in risk evaluation of postoperative complications in Crohn’s disease. (2017) Sci Rep 1-11.
PubMed||CrossRef||Others
- 37. Lafci, G., Ciçek, Ö.F., Uzun, H.A., et al. Relationship of admission neutrophil-to-lymphocyte ratio with in-hospital mortality in patients with acute type I aortic dissection. (2014) Turkish J Med Sci 44(2): 186-192.
PubMed||CrossRef||Others
- 38. Şaşkın, H., Düzyol, Ç., Özcan, K.S., et al. Preoperative platelet to lymphocyte ratio is associated with early morbidity and mortality after coronary artery bypass grafting. (2015) Heart Surg Forum 18(6): E255-E262.
- 39. Kim, W.H., Park, J.Y., Ok, S.H., et al. Association between the neutrophil/lymphocyte ratio and acute kidney injury after cardiovascular surgery: a retrospective observational study. (2015) Medicine (Baltimore) 94(43): e1867.
- 40. Yost, G.L., Joseph, C.R., Tatooles, A.J., et al. Neutrophil-to-Lymphocyte Ratio Predicts Outcomes in Patients Implanted with Left Ventricular Assist Devices. (2015) ASAIO J 61(6): 664-669.
- 41. McCourt, M., Wang, J.H., Sookhai, S., et al. Proinflammatory Mediators Stimulate Neutrophil-Directed Angiogenesis. (1999) Arch Surg 134(12): 1325.
- 42. Di Carlo, E., Forni, G., Musiani, P. Neutrophils in the Antitumoral Immune Response. In: The Neutrophil. (2003). Basel: KARGER 83: 182-203.
- 43. Ommen, S.R., Gibbons, R.J., Hodge, D.O., et al. Usefulness of the Lymphocyte Concentration as a Prognostic Marker in Coronary Artery Disease. (2015) Am J Cardiol 79(6): 812-814.
- 44. Yu, C., Chen, M., Chen, Z., et al. Predictive and prognostic value of admission neutrophil-to-lymphocyte ratio in patients with CHD. (2016) Herz 41(7): 605-613.
- 45. Kim, S.C., Sun, K.H., Choi, D.H., et al. Prediction of Long-Term Mortality Based on Neutrophil-Lymphocyte Ratio After Percutaneous Coronary Intervention. (2016) Am J Med Sci 351(5): 467-472.
- 46. Pearson, T.A., Mensah, G.A., Alexander, R.W., et al. Markers of Inflammation and Cardiovascular Disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. (2003) Circulation 107(3): 499-511.
PubMed||CrossRef||Others
- 47. Shau, H.Y., Kim, A. Suppression of lymphokine-activated killer induction by neutrophils. (1988) J Immunol 141(12): 4395-4402.
- 48. Madjid, M., Awan, I., Willerson, J.T., et al. Leukocyte count and coronary heart disease: Implications for risk assessment. (2004) J Am Coll Cardiol 44(10): 1945-1956.
- 49. Carubelli, V., Bonadei, I., Castrini, A.I., et al. Prognostic value of the absolute lymphocyte count in patients admitted for acute heart failure. (2017) J Cardiovasc Med (Hagerstown) 18(11): 859-865.
- 50. Akilli, N.B., Yortanlı, M., Mutlu, H., et al. Prognostic importance of neutrophil-lymphocyte ratio in critically ill patients: short- and long-term outcomes. (2014) Am J Emerg Med 32(12): 1476-1480.
- 51. Horne, B.D., Anderson, J.L., John, J.M., et al. Which White Blood Cell Subtypes Predict Increased Cardiovascular Risk? (2005) J Am Coll Cardiol 45(10): 1638-1643.
- 52. de Jager, C.P., Wever, P.C., Gemen, E.F., et al. The Neutrophil-Lymphocyte Count Ratio in Patients with Community-Acquired Pneumonia. (2012) PLoS One 7(10): e46561.
- 53. de Jager, C.P., van Wijk, P.T., Mathoera, R.B., et al. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. (2010) Crit Care 14(5): R192.
- 54. Gomez, D, Morris-Stiff, G., Toogood, G.J., et al. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. (2008) J Surg Oncol 97(6): 513-518.
- 55. Gu, X-B., Tian, T., Tian, X-J., et al. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: A meta-analysis. (2015) Sci Rep 5: 12493.