Nootropic effects of quince leaf (Cydonia Oblonga Miller.) decoct in mice: A neurobehavioral approach complemented with kinetics and molecular docking studies of encephalic acetylcholinesterase inhibition
Zahraminoosh Siavashhaghighi, Airin Najaf, Lora A Becker
Affiliation
- 1Department of Biology, Faculty of Science, Razi University, Kermanshah, Islamic Republic of Iran
- 2Department of Basic Veterinary Sciences, Laboratory of Molecular and Cellular Biology 1214, Faculty of Veterinary Medicine, Razi University, Kermanshah, Islamic Republic of Iran
- 3Department of Pathobiology, Faculty of Veterinary Medicine, Razi University, Kermanshah, Islamic Republic of Iran
- 4Department of Psychology, University of Evansville, Evansville, IN 47722, USA
Corresponding Author
Isaac Karimi; Department of Biology, Faculty of Science, Razi University, Kermanshah, Iran. E-mail: isaac_karimi2000@yahoo.com / karimiisaac@razi.ac.ir
Citation
Isaac Karimi, et al. Nootropic Effects of Quince Leaf (Cydonia Oblonga Miller.) Decoct in Mice: A Neurobehavioral Approach Complemented With Kinetics and Molecular Docking Studies of Encephalic Acetyl Cholinesterase Inhibition (2017) Bioinfo Proteom Img Anal 3(2): 196- 202.
Copy rights
© 2017 Isaac Karimi. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Quince leaves; Nootropics; Behavioral toxicology; Acetylcholinesterase; Molecular docking
Abstract
Cydonia oblonga Miller (Quince) is an herbal medicine that is reported to prevent amnesia in Kurdish ethnomedicine. The aim of this study was to evaluate nootropic effects of quince leaf decoction (QLD). Forty mice subdivided into four equal groups i.e., control group was gavaged daily with distilled water while other three groups were gavaged with 0.92, 1.85, and 3.70 g/dl of fresh QLD for 28 days. Behavioral toxicology, encephalic acetylcholinesterase (AChE) activity and molecular docking of quince phyto-compounds against AChE were measured. The anxiety, exploration and learning and spatial memory were not altered after intake of QLD in mice. The encephalic AChE activity decreased hormetically in QLD-treated mice compared with control group, which connotes nootropic property of QLD. Based on the results of molecular docking of earlier reported phyto-chemicals of QLD, quercetin-3-O-galactoside and 3- and 5-caffeoylquinic acid showed considerable binding energies to AChE and may involve in AChE inhibitory effects of QLD in mice. QLD intake did not lead to behavioral toxicity and exerted AChE inhibition and would be rationalized as a putative phyto-nootropic remedy.
Introduction
Growing body of evidence implicates that disturbances within the neurochemical milieu play pivotal role in the pathogenesis of neurodegenerative diseases like Alzheimer’s disease (AD). Cholinesterase inhibitors are commonly used to improve memory in AD-affected patients suffering from mild dementia[1]. In this regard, studies showed that hippocampus mediated learning and memory functions are associated with an increase in hippocampal level of extracellular neurotransmitter acetylcholine (ACh)[2,3]. During neurotransmission, vesicle-stored ACh is released from the nerve terminal into the synaptic cleft and binds to ACh receptors on the post-synaptic membrane, thereby relaying the signal from the presynaptic nerve to the postsynaptic nerve. Acetylcholinesterase (AChE, acetycholine acetylhydrolase, E.C. 3.1.1.7), also embedded in the postsynaptic membrane, terminates the signal transmission by hydrolyzing ACh. Choline liberated from ACh decomposition is taken up by the presynaptic nerve and the neurotransmitter is synthesized by combining with acetyl-CoA through the action of choline acetyltransferase[4]. Acetylcholinesterase exists in multiple molecular forms that possess similar catalytic properties but differ in their oligomeric assembly and mode of cell attachment to the cell surface. In the mammalian brain the majority of AChE occurs as a tetrameric G4 form with much smaller amounts of a monomeric G1 form[4].
Cholinergic nootropics include ACh precursors and cofactors, and AChE inhibitors[5]. To improve ACh activity in the brain, a group of drugs known as cholinesterase inhibitors act by blocking the actions of the enzyme, AChE, thus allowing ACh to act longer at the synapses between cells. The herbal drugs acting on the brain to enhance memory and learning are called “nootropic herbs” or “phyto-nootropics” and their isolated constituents referred as smart drugs. An array of nootropic herbs (e.g., quince) have been used in Kurdish ethnomedicine through millennia, however results to confirm their evidence-based medicine are scarce. Quince (Cydonia oblonga Miller.), deciduous tree belongs to the Rosaceae family, is a prehistoric plant cultivated in countries extending from Iran to India[6]. Various parts of this plant (“Beh” in Persian) have been employed against various diseases (e.g., diabetes mellitus and neuropathies) in both
traditional and orthodox medicine (for a review see[7]). Hence, this study was aimed to investigate the memory enhancing effects of quince leaf decoct with a focus on encephalic AChE activity and behavioral toxicology of normal mice. In silico molecular docking has been also implemented to elucidate possible mechanisms governed by main components of quince leaves that interact with AChE.
Materials and Methods
Plant materials and preparation of decoction
The fresh yellow leaves of quince (Cydonia oblonga Miller.) were collected from Anbar Bozan village (35° 27′ N, 46° 90′ E and 1812 m above sea level), Abidar rural district, Kurdistan, Iran in October 2014 and authenticated by botanist. The voucher specimens were stored at the herbarium of our university. The harvested leaves were washed with distilled water (DW) and then air-dried in the shade. Daily preparation of the quince leaf decoction (QLD), consisted of dried leaves boiled in DW pot (600 ml) for two hours that preserved in a water bath (100°C).
Animal subjects
Male Naval Medical Research Institute (NMRI) mice, Mus musculus, were maintained under 45% – 55% relative humidity, 12 h light: 12 h dark photocycle and temperature 25 ± 2 °C. The animals (20 – 30 g) were divided to 4 groups (n = 10 for each group) housed in colony cages with free access to pelleted feed (Gharbdaneh Co., Iran) and tap water except throughout behavioral tests. Animals in control group receive pelleted feed and gavaged daily by DW while animals in other three groups treated with fresh QLD at dosages of 5, 10, and 20% of QLD equivalent to Q0.92, Q1.85, and Q3.7, respectively when converted dosages to grams per deciliter via gavage for 28 days. The Animal Ethical Committee of Razi University reviewed and approved this study.
Behavioral endpoints
All tests were performed from 08:00 to 15:00 in a homogeneously lightened room.
Open field test (OFT): The open field wooden apparatus (36 × 36 × 36 cm³) consisted of a bright blue square floor marked with 16 equal blocks and one red lined square in the center. At the beginning of the test, mice were positioned in the open field apparatus facing one of the four corners. Mouse movement (locomotion and rearing) was video recorded for 5 min[8]. Video analysis of mouse behavior in the OFT consisted of counting the number of squares crossed through, total time in movement, time spent rearing (standing on feet) and the time spent in the central red square of the apparatus. The motobehaviors of mice was explored after QLD intake using an OFT[8,9].
Elevated plus maze (EPM): The wooden apparatus comprised two open arms (50 cm long × 10 cm wide) and two closed arms (50 cm long × 10 cm wide × 40 cm height) perpendicular to each other and situated 50 cm above the ground. Mice were placed in the center square and their behaviors were video–taped for 5 min[9]. Recordings were evaluated for total time spent in the open arms, total time spent in the closed arms, and entries into the open and closed arms. The anxiety–associated behavior of mice was explored after QLD intake using an EPM[9].
Object recognition test (ORT): ORT consisted of three steps. On day one mice were habituated in an empty arena (36 × 36 × 36 cm³) for 5 min. Twenty four hours after habituation, two identical objects were presented in the arena, and mice were allowed to explore the arena freely for 20 min. On day three, the mice were allowed to explore the arena for 3 minutes in the presence of the familiar object and a novel object videotaped ORT behavior consisted of counting the number of times the mouse explored each object (exploration consisted of being within 0.7 cm around the object and/or touching it with the nose). Time spent exploring the familiar object was used as a measure of long–term recognition memory. To identify time spent attending to the novel object the following discrimination ratio was calculated: B – A/B + A wherein A = the time spent exploring old object, B = the time spent exploring novel object. The ORT was used to assess the effect of QLD in associative learning and recognition memory[10].
Light–dark box (LDB): The LDB apparatus consisted of two vitreous boxes: one light chamber (27 × 27 × 27 cm³) brightly illuminated and the other was dark chamber (27 × 27 × 18 cm³). The mice were allowed to move from one box to the other through an interfacial gate. A mouse was put into the dark box facing the hole for three seconds, after which the gate was opened allowing the mouse to transition at will between the light and dark boxes for 5 minutes. The transition between the light and the dark boxes and time spent in the light box were analyzed from video recording[11]. The LDB is a conventional test for assessment of anxiety–like behavior, specifically approach-avoidance conflict anxiety in laboratory mice[11].
Morris water maze (MWM): The Morris water navigation task apparatus consisted of a plastic child swimming pool (111 cm in diameter and 35 cm in height) divided into four equal quadrants. The pool was filled with drinking yoghurt (23°C) to the depth of 15 cm and an invisible platform (5 × 14 cm²) was submerged 1 cm below the opaque surface in the center of one of the quadrants[12]. The pool was located in a test room with external cues positioned around the mazes that were visible from within the maze. These cues were accessible to the mice as markers for spatial orientation.
Each mouse was exposed to the pool for 2 min one day before training trials. The five training days consisted of each animal receiving 4, 60-second trials per day, with 5 minutes inter–trial intervals. A successful escape from the maze was counted when the mouse was able to locate and climb on the invisible platform. Once the mouse located the platform, it was permitted to remain on it for 10 sec. If the mouse did not locate the platform within 90 sec, it was placed on the platform for 15 sec and then removed from the pool. The point of entry of the mouse into the pool and the location of the platform for escape remained unchanged between training trials. During the probe trial (sixth day) the platform was moved to a new quadrant and the mouse was released into the quadrant opposite to the one that had previously contained the platform and allowed to swim for 1 min. During each trial, escape latencies (time to reach the platform) were videotaped for analysis. Escape latencies were averaged for each session of trials and for each mouse[12]. The decrease in escape latency from day to day represents long–term memory or reference memory while that from trial to trial, represents short–term memory or working memory[12]. Escape latency on the probe day represents the mouse’s ability to break free of their learned behavior to find the new platform position[12].
Biochemical assay
Brain preparation: The mice were killed under deep anesthesia with intraperitoneal injection of ketamine (100 mg/kg)/xylazine (30 mg/kg) cocktail at the end of study, then mice were decapitated and their whole brain removed, weighted and halved through longitudinal fissure. For preparation of brain homogenate, brain tissue was homogenized in 10 volumes of 0.9% w/v sodium chloride solution. The homogenate was centrifuged at 3000 × g for 10 min and the supernatant was used to assay total homogenate protein using bovine serum albumin as a standard[13], and brain AChE activity (vide infra).
AChE assay: The AChE activity was measured according to Ellman’s method[14] by providing an artificial substrate, ACh iodide (0.075 M dissolved in DW; Merck Co. Germany). Choline was released with the cleavage of ACh by AChE is allowed to react with –SH reagent 2, 2′–Dinitro–5, 5′–dithiodibenzoic acid (DTNB; 0.01 M; pH 8.0; Merck Co. Germany). The reduction produced yellow color with absorption maximum at 412 nm. The brain homogenate (0.4 ml) was added to a cuvette containing 2.6 ml phosphate buffer solution (0.1 M, pH 8.0) and 100 μl of DTNB, mixed thoroughly by bubbling air, when absorbance reached a stable value at 412 nm the basal reading was recorded. After that, 20 μl of substrate solution was added and change in absorbance was recorded at 2-minute intervals for duration of 16 minutes. The change in the absorbance (Δ Abs) per minute was determined. Activity was calculated as follows: Activity (mol/min/mg) = (Δ Abs × homogenate buffer vol.)/extinction coefficient × time (min) × homogenate protein (mg).
Molecular docking simulation of AChE inhibition
Simulations of the docking between the AChE and major bioactive compound of QLD were performed with PyRx software version 0.8[15] through setting up and running up AutoDockVINA[15] using default parameters. For the docking studies, crystal structures of the protein target were obtained from the Protein Data Bank, PDB ID 1EVE for Tetronarce californica AChE. The PDB format of target protein has been edited, optimized and trimmed in Molegro Virtual Docker[16] and Chimera 1.8.1 (http://www.rbvi.ucsf.edu/chimera before submission to PyRx (vide infra). The structures of the major known bioactive compounds of quince leaves were retrieved from ChemSpider (http://www.chemspider.com) and PubChem (https://pubchem.ncbi.nlm.nih.gov) databases. Binding affinity (kcal/mol) was determined after completion of the docking procedure. More negative the binding affinity means a better orientation of the ligand in the binding site. The selected conformer of ligand has been combined with 1EVE protein in Molegro Virtual Docker[16] or Chimera 1.8.1 (http://www.rbvi.ucsf.edu/chimera) and their graphical interface has been analyzed with LigPlot + software[17].
Statistical analysis
The results were expressed as means and standard error of the mean (SEM). The Shapiro –Wilk test showed that all data fitted a Gaussian distribution. When parametric analysis of variance revealed a significant difference, a post hoc Turkey’s test was used to check the difference between groups. Significance level was set at p < 0.05 and all statistical analyses were carried out using SPSS version 16.0 for Windows (SPSS, Chicago, Illinois, USA).
Results
Behavioral endpoints
OFT. In OFT, number of crossing squares as an index of locomotor activity/horizontal activity (F3,14 = 1.290; p = 0.326), the time of rearing as an index of exploratory activity/vertical activity (F3,14 = 1.089; p = 0.394), and center arena time as an index of anxiety (F3,14 = 0.292; p = 0.830); did not yield significant differences after QLD treatment (Table 1).
Table 1: Open field behavior after oral intake of quince leaf decoction in mice (n = 4 for each group).
| Group | ||||
| NC | Q0.92 | Q1.85 | Q3.7 | |
| Crossing square no. | 87.5 ± 27.1 | 99.2 ± 28.3 | 39.3 ± 7.6 | 122.5 ± 36.6 |
| Rearing time (s) | 19.2 ± 9.1 | 12.7 ± 6.6 | 1.0 ± 0.5 | 3.0 ± 1.4 |
| Center arenatime (s) | 3.0 ± 2.5 | 2.7 ± 1.7 | 1.0 ± 0.5 | 20.2 ± 9.5 |
Note: NC = Normal control group, Q0.92 = Quince treated group at dosage 0.92 g/dl, Q1.85 = Quince treated group at dosage 1.85 g/dl, Q3.7 = Quince treated group at dosage at 3.7 g/dl.
EPM
In EPM, time spent in open arm (F3,15 = 1.44; p = 0.280), number of entries into open arms (F3,15 = 1.706; p = 0.219), number of entries into close arms (F3,15 = 1.915; p = 0.181), sum of number of entries onto open and close arms (F3,15 = 1.813; p = 0.198) and number of entries into open arms as a percent of all entries (F3,15 = 1.044; p = 0.409) did not show significant difference (Table 2).
Table 2: Elevated plus–maze behavior after oral intake of quince leaf decoction in mice (n = 4 for each group).
| Group | ||||
| NC | Q0.92 | Q1.85 | Q3.7 | |
| OT | 32.7 ± 7.7 | 40.00 ± 4.49 | 33.00 ± 8.55 | 53.70 ± 10.7 |
| OE | 10.2 ± 2.0 | 9.25 ± 1.49 | 7.75 ± 2.28 | 16.00 ± 4.3 |
| CE | 11.2 ± 2.0 | 9.00 ± 1.58 | 7.75 ± 1.75 | 16.50 ± 4.6 |
| TE | 21.5 ± 4.1 | 18.25 ± 3.03 | 15.05 ± 4.03 | 32.50 ± 8.9 |
| OE % | 47.32 ± 0.64 | 51.04 ± 1.47 | 48.23 ± 2.41 | 49.01 ± 0.79 |
Note: NC = Normal control group, Q0.92 = Quince treated group at dosage 0.92 g/dl, Q1.85 = Quince treated group at dosage 1.85 g/dl, Q3.7 = Quince treated group at dosage at 3.7 g/dl. OT: time (s) spent in open arm, OE: number of entries into open arms, CE: number of entries into close arms, TE: sum of number of entries onto open and close arms, OE percent: OE/ OE+CE*100.
ORT
There were no significant differences in the total time spent exploring old (F3,15 = 2.586; p = 0.102) and novel (F3,15 = 0.396, p = 0.758) objects and discrimination ratio (F3,15 = 1.51; p = 0.262) between control and QLD–treated groups (Table 3).
Table 3: Object recognition test after oral intake of quince leaf decoction in mice (n = 4 for each group).
| Group | ||||
| NC | Q0.92 | Q1.85 | Q3.7 | |
| Old object recognition (s) | 34.5 ± 9.7 | 40.0 ± 7.1 | 139.0 ± 53.7 | 162.2 ± 61.4 |
| Novel object recognition (s) | 23.5 ± 9.7 | 35.2 ± 8.3 | 19.0 ± 10.2 | 29.2 ± 15.2 |
| Discriminative ratio | 3.50 ± 1.50 | 8.50 ± 3.37 | 2.25 ± 0.25 | 5.70 + 2.49 |
Note: NC = Normal control group, Q0.92 = Quince treated group at dosage 0.92 g/dl, Q1.85 = Quince treated group at dosage 1.85 g/dl, Q3.7 = Quince treated group at dosage at 3.7 g/dl.
LDB.
The total transfer between two chambers (F3,14 = 1.873; p = 0.193), the latency to enter the light chamber (F3,14 = 0.796; p = 0.521), and time spent in light chamber (F3,14 = 1.204; p = 0.354) and dark chamber (F3,14 = 1.204; p = 0.354) did not show significant difference among groups (Table 4)
Table 4: Anxiety–like behavior in light/dark box behavior after oral intake of quince leaf decoction in mice (n = 4 for each group).
| Group | ||||
| NC | Q0.92 | Q1.85 | Q3.7 | |
| Total transfer no. | 21.0 ± 4.6 | 29.3± 10.4 | 25.2± 7.3 | 8.5± 4.0 |
| Latency to enter the light chamber (s) | 8.0 ± 3.1 | 11.3 ± 5.3 | 10.7 ± 8.1 | 123.5 ± 120.1 |
| Time spent in light chamber (s) | 74.7 ± 21.5 | 88.0 ± 18.1 | 124.5 ± 40.4 | 48.5 ± 27.8 |
| Time spent in dark chamber (s) | 525.2 ± 21.5 | 512.0 ± 18.1 | 475.5 ± 40.4 | 551.5 ± 27.8 |
Note: NC = Normal control group, Q0.92 = Quince treated group at dosage 0.92 g/dl, Q1.85 = Quince treated group at dosage 1.85 g/dl, Q3.7 = Quince treated group at dosage at 3.7 g/dl.
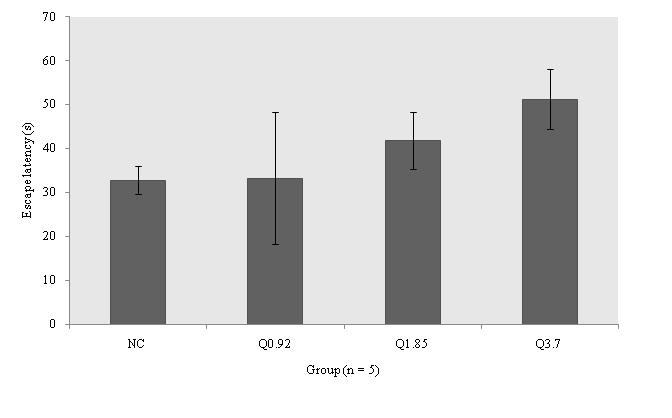
MWM: As shown in Figure. 1, the latencies to escape (s) after oral intake of QLD tended to be increased dose–dependently in Q3.7 (51.25 ± 6.73), Q1.85 (41.75 ± 6.51), and Q0.92 (33.25 ± 15.09) groups as compared to NC group (32.81 ± 3.18) at 6th day, however this difference was not significant (F3,12 = 1.078; p = 0.406). Quince decoction had no improving effect on spatial memory.
Figure 1: Effects of quince leaf decoction on water maze behaviours in pretrained mice (n = 4 or 5 for each group). Latency to escape was measured at each mouse. Values are mean ± SEM for four to five mice. NC = Normal control group, Q0.92 = Quince treated group at dosage 0.92 g/dl, Q1.85 = Quince treated group at dosage 1.85 g/dl, Q3.7 = Quince treated group at dosage at 3.7 g/dl.
AChE activity
The specific AChE activity (mol/min/mg) was significantly decreased in QLD–treated group compared to that of normal mice (F3,15 = 1.963; p = 0.007). In this regard, specific AChE activity in Q0.92 (16.31 ± 3.81; p = 0.001), Q1.85 (25.84 ± 2.66; p = 0.008), and Q3.7 (27.62 ± 2.71; p = 0.012) was less than that of NC (52.71 ± 10.8) group, however no differences were found in AChE activity between the QLD–treated groups.
Molecular docking
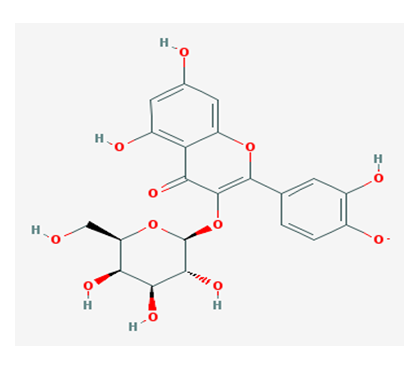
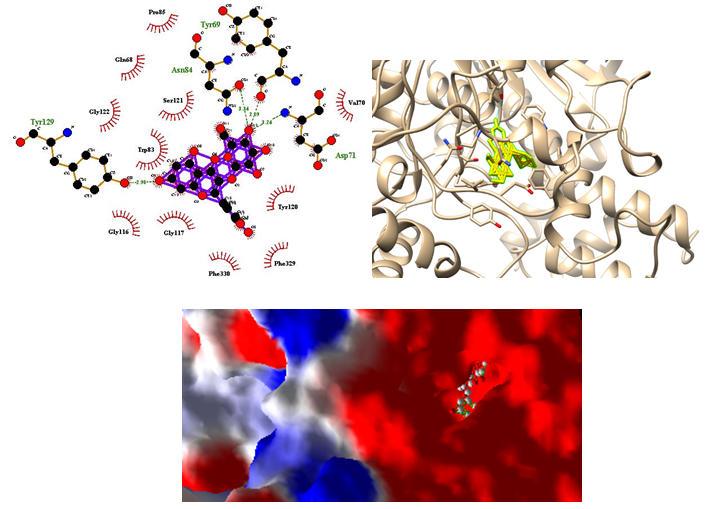
According to the docking results of major reported components of QLD[18-21], hyperoside (quercetin–3–O–galactoside; Figure 2) and 3– and 5–caffeoylquinic acid showed considerable binding energies to AChE (Table 5). The Torpedo california AChE interacted residues were indicated (in parentheses) throughout the paper[22]. We also reported the number of hydrogen bonds between the quercetin–3–O–galactoside (Figure. 3) bounded to AChE. In the complex of quercetin–3–O–galactoside and AChE, H bondings were observed between quercetin–3–O– galactoside and Tyr129 (Tyr130), Asp71 (Asp72), Tyr69 (Tyr70) and Asp84 (Asp85) residues during simulation. Several hydrophobic interactions occurred between several residues of AChE and quercetin–3–O–galactoside as shown in Figure. 3.
Table 5: Molecular docking simulation of acetylcholinesterase (PDB ID: 1EVE) inhibition by major components of quince leaves.
| Ligand code | Ligand name | Binding Affinity (kcal/mol) |
| PC: 12310830 | 5–Caffeoylquinic acid | –8.8 |
| PC: 1794427 | 3–Caffeoylquinic acid | –8.4 |
| PC: 5280805 | Quercetin–3–O–rutinoside | –3.7 |
| CS: 553148 | Hydroxycinnamic acid | –6.8 |
| PC: 6474310 | 3,5–Dicaffeoylquinic acid | –5.5 |
| PC: 90657624 | Quercetin–3–O–galactoside | –13.2 |
Note: PC: PubChem ID; CS: ChemSpider ID
Figure 2: Chemical structures of quercetin–3–O–galactoside
Figure 3: Molecular docking simulations of binding between Tetronarce californica acetylcholinesterase (PDB ID 1EVE) and quercetin–3–O–galactoside are depicted. The right photo corresponding surface structure of protein interacting with quercetin–3–O–galactoside. The cyanic parts show the residues of protein, which bind with quercetin–3–O–galactoside to develop a gorge. The coarser stick structure in yellow was used to represent quercetin–3–O–galactoside, in right photo which is buried in the gorge as shown in lower photo. The interactions between quercetin–3–O–galactoside and protein are shown in left photo. The coarser stick structure mainly in purple on the center was used to represent quercetin–3–O–galactoside while the coarser stick with orange colors shows the residues of proteins. Hydrogen bonds are shown in olive green breaking line while hydrophobic bonds are shown in brick red.
Discussion
There was no statistically significant difference in terms of anxiety, exploration and spatial memory among groups. The activity of AChE was significantly decreased in mice receiving QLD compared with the control mice, which indicates putative nootropic property of QLD at molecular level. On the other hand, the AChE enzyme activity in groups receiving QLD was increasingly less inhibited with increasing dosages, although activity was it was still lower than that of control group. This refers to the hormetic effect of QLD whereby a beneficial effect (AChE inhibition) results from exposure to low dose of QLD and toxic or lethal effects will be occurred when given at higher doses.
Based on behavioral tests, our results showed that QLD intake has no overt impact on locomotor activity, anxiety, learning memory, spatial memory, short-term memory, and anxiety-like behavior. Although intake of QLD did not lead to the overt behavioral improvement like memory enhancement, our study shows that QLD will not cause any kinds of behavioral toxicities like depression or anxiety disorders. Encephalic AChE activity declined after intake of QLD, however QLD–treated groups did not show significant differences in AChE activity. Once AChE activity decreases, the level of ACh increases in the synaptic clefts in the brain, thus allowing ACh to act longer at the synapses between cells and it could potentially enhance memory[3,5]. In the present study, AChE activity in the groups received QLD was less than 50 percent of control group, so a strong AChE inhibition occurred in mice treated with QLD. More inhibition of AChE increases ACh levels in the synapses thus results in down–regulation of neuronal or glial cholinergic receptors, so transmission pathway had been stopped[23]. Although we did not see any memory improvement based on results of behavioral tests, it seems that repetition of this study in an amnesic model treated with scopolamine[24] may theoretically show memory improvement at the behavioral level.
Major phyto-chemicals of QLD have been reported previously[18-21,25,26]. Among major phenolic compounds of QLD, 5–O–caffeoylquinic acid (36.2%) and quercetin 3–O–rutinoside (21.1%)[18], the former showed more negative binding affinity to AChE. Chlorogenic acid (3–O–caffeoylquinic acid) has antioxidant ability[27] and its anti–amnestic effects against scopolamine–induced amnesia was mediated via inhibiting AChE and reducing oxidative stress and lipid peroxidation[28]. The results of our study also showed that 3–O–caffeoylquinic acid produced acceptable binding affinity with AChE. In this continuum, quince leaves presented the highest relative contents of kaempferol derivatives, especially of kaempferol–3–O–rutinoside, which represented 12.5% of the total phenolics content[18], however to hydroxycinnamic acid and 3,5–dicaffeoylquinic acid did not dock with AChE. Other major components of QLD like quercetin–3–O–rutinoside did not dock firmly with AChE. Among major phyto-compounds found in quince leaves, hyperoside (quercetin–3–O–galactoside) showed the most negative binding affinity with AChE.
The AChE has a catalytic site and peripheral anionic site (PAS) for its activity[22]. The PAS binds ACh and allosterically modulates catalysis[29] along with binding specific inhibitory compounds. The PAS has also been identified that promotes amyloidosis through an interaction with the amyloid-β-peptide in AD[30]. The catalytic or acylation site of AChE (Ser200, His440 and Glu327) lies deep within the molecule at the base of a narrow 20Å deep gorge, lined predominantly with aromatic residues[22]. An array of subsites have been differentiated within the gorge that are also important in the catalytic process[31,32]. The “anionic” subsite (Trp84, Tyr130, Tyr330 and Phe331) binds the quaternary trimethylammonium choline moiety of the substrate, largely through π-cation interactions[33], optimally positioning the ester at the acylation site. In the present study, Tyr129 (Tyr130) interacted with quercetin–3–O–galactoside with hydrogen bondings while Phe330 (Phe331) and Trp83 (Trp84) interacted hydrophobically with quercetin–3–O–galactoside. The oxyanion hole, Gly118, Gly119 and Ala201, provides hydrogen bond donors to stabilize substrate[34]. Quercetin–3–O–galactoside interacted hydrophobically with Gly117 (Gly118) in the oxyanion hole. The PAS consists of 5 residues (Tyr70, Asp72, Tyr121, Trp279 and Tyr334) gathered around the entry of the active site gorge[31,35]. uercetin–3–O–galactoside formed hydrogen bondings only with Tyr69 (Tyr70) and Asp71 (Asp 72) while it interacted with Tyr120 (Tyr121) via hydrophobic interaction. The binding of the quercetin–3–O–galactoside to the PAS has been described above. To sum, quercetin–3–O–galactoside had a reasonable binding with PAS in AChE therefore it may be a good lead molecule for drug designing used against AD. Moreover, the AChE inhibitory of QLD may be mediated by the inhibitory effects of quercetin–3–O–galactoside against PAS of AChE. This bioactive compound has also been isolated from other plants[36,37] and its neuroprotective property has been reported in PC12 cells against cytotoxicity induced by hydrogen peroxide and tert-butyl hydroperoxide[38].
Conclusion
In conclusion, AChE activity in QLD-treated groups was less than 50 percent of activity in the control group, although we did not observe any memory improvement in QLD-treated groups. Quince decoction did not induce any toxic behavioral effects in mice. High dosages of quince decoction followed a hormetic dose response relationship and had inhibitory effects on AChE. In this study, in silico molecular docking showed that quercetin–3–O–galactoside, reported in QLD, has reasonable binding affinity for AChR and may become a novel therapeutic candidate for the treatment of AD. To our knowledge, there is not suitable database about the posology and pharmacokinetics of QLD and this study is one of the first ones to reveal the pharmacotherapeutic potential of leaves of quince.
Acknowledge:
This paper emanates from Doctor of Veterinary Medicine thesis of third author, School of Veterinary Medicine, Razi University, Kermanshah, Iran. Authors acknowledge David Alimoradian and Mohammadmehdi Zanganeh for technical assistance. The in vivo part of this study was supported by a grant from Razi University while in silco part was supported by personal money of corresponding author.
Disclosures:
The authors declared no conflicts of interest.
References
- 1. Jellen, L.C., Aliper, A., Buzdin, A., et al. Screening and personalizing nootropic drugs and cognitive modulator regimens in silico. (2015) Front Sys Neurosci 9: 4.
Pubmed || Crossref - 2. Parent, M.B., Baxter, M.G. Septohippocampal acetylcholine: involved in but not necessary for learning and memory. (2004) Learn Memory11 (1): 9-20.
Pubmed || Crossref - 3. Hasselmo M.E. The role of acetylcholine in learning and memory. (2006) Curr Opin Neurobiol 16(6): 710-715.
Pubmed || Crossref - 4. Colovic, M.B., Krstic, D.Z., Lazarevic-Pasti, T.D., et al. Acetyl cholinesterase inhibitors: pharmacology and toxicology. (2013) Curr Neuro pharmacol 11(3): 315-335.
Pubmed || Crossref - 5. Joshi Pranav, J.C. A review on natural memory enhancers (nootropics). (2013) Unique J Engin Advanced Sci(01): 8-18.
Crossref - 6. Vaez, H., Hamidi, S. Potential of Cydonia Oblonga leaves in cardiovascular disease. (2014) Hypothesis 12(1), e4
Crossref - 7. Sajid, S.M., Zubair, M., Waqas, M., et al. A review on quince (Cydonia oblonga): a useful medicinal plant. (2015) Glob Vet 14(4): 517–524.
Crossref - 8. Gholamine, B., Karimi, I., Salimi,A., et al. Neurobehavioral toxicity of carbon nanotubes in mice: Focus on brain-derived neurotrophic factor messenger RNA and protein. (2016) ToxicolInd Health May 26. pii: 0748233716644381.
- 9. Ivani, S., Karimi, I., Tabatabaei, S.R., et al. Effects of prenatal exposure to single-wall carbon nanotubes on reproductive performance and neurodevelopment in mice. (2014)ToxicolInd Health 32(7): 1293-1301.
Pubmed || Crossref - 10. Miraghaee, S.S., Karimi, I., Becker, L.A. Psychobiological assessment of smoke of agarwood (Aquilaria spp.) in Male Rats. (2011) JABS 2: 045-053.
- 11. Takao, K., Miyakawa, T. Light/dark transition test for mice. (2006) J Vis Exp: JoVE (1): 104.
- 12. Ivani, S., Karimi, I., Tabatabaei, S.R. Biosafety of multi walled carbon nanotube in mice: a behavioral toxicological approach. (2012) J Toxicol Sci 37(6): 1191-1205.
Pubmed || Crossref - 13. Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. (1976) Anal Biochem 72: 248–254.
Pubmed || Crossref - 14. Ellman, G.L., Courtney, K.D., Andres, V., et al. A new and rapid colorimetric determination of acetyl cholinesterase activity. (1961) Biochem Pharmacol 7: 88–95.
Pubmed || Crossref - 15. Dallakyan, S., Olson, A.J. Small–molecule library screening by docking with PyRx. (2015) Methods Mol Biol 1263: 243–450.
Pubmed || Crossref - 16. Thomsen, R., Christensen, M.H.MolDock: a new technique for high–accuracy molecular docking. (2006) J Med Chem 49(11): 3315–3321.
Pubmed || Crossref - 17. Laskowski, R.A., Swindells, M.B. LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery. (2011) J ChemInfModel 51(10): 2778–2786.
Pubmed || Crossref - 18. Oliveira, A.P., Pereira, J.A., Andrade, P.B., et al. Phenolic profile of Cydonia Oblonga Miller leaves. (2007) J Agr Food Chem 55(19): 7926-7930.
Pubmed || Crossref - 19. Oliveira, A.P., Pereira, J.A., Andrade, P.B., et al. Organic acids composition of Cydonia Oblonga Miller leaf. (2008) J Agr Food Chem 111(2): 393-399.
Pubmed || Crossref - 20. Costa, R.M., Magalhães, A.S., Pereira, J.A., et al. Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydonia Oblonga) leaf: a comparative study with green tea (Camellia sinensis). (2009) Food Chem Toxicol 47(4): 860-865.
Pubmed || Crossref - 21. Carvalho, M., Silva, B.M., Silva, R. et al. First report on Cydonia Oblonga Miller anticancer potential: differential anti proliferative effect against human kidney and colon cancer cells. (2010) J Agr Food Chem 58(6): 3366-3370.
Pubmed || Crossref - 22. Sussman, J.L., Harel, M., Frolow, F., et al. Atomic structure of acetyl cholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. (1991) Science 253: 872-879.
Pubmed || Crossref - 23. Wonnacott, S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. (1990) Trends PharmacolSci11 (6): 216-219.
Pubmed || Crossref - 24. Flood, J.F., Cherkin, A. Scopolamine effects on memory retention in mice: a model of dementia? (1986) Behav Neural Biol 45(2): 169-184.
Pubmed || Crossref - 25. De Tommasi, N., De Simone, F., Pizza, C., et al. New tetracyclic sesterterpenes from Cydonia vulgaris. (1996) J Nat Prod 59(3): 267-270.
Crossref - 26. Osman, A.G., Koutb, M., Sayed, A.E.D. Use of hematological parameters to assess the efficiency of quince (Cydonia Oblonga Miller) leaf extract in alleviation of the effect of ultraviolet–A radiation on African catfish Clariasgariepinus (Burchell, 1822). (2010) J Photo chem Photobio B 99(1): 1-8.
Pubmed || Crossref - 27. Granado–Serrano, A.B., Martín, M.A., Izquierdo–Pulido, M., et al. Molecular mechanisms of (−) –epicatechin and chlorogenic acid on the regulation of the apoptotic and survival/proliferation pathways in a human hepatoma cell line. (2007) J Agr Food Chem 55(5): 2020–2027.
Pubmed || Crossref - 28. Kwon, S.H., Lee, H.K., Kim, J.A., et al. Neuroprotective effects of chlorogenic acid on scopolamine–induced amnesia via anti–acetyl cholinesterase and anti–oxidative activities in mice. (2010)Eur J Pharmacol 649(1–3): 210–217.
Pubmed || Crossref - 29. Mallender, W.D., Szegletes, T., Rosenberry, T.L. Acetylthiocholine binds to asp74 at the peripheral site of human acetyl cholinesterase as the first step in the catalytic pathway. (2000) Biochemistry39: 7753-7763.
Crossref - 30. Inestrosa, N.C., Alvarez, A., Perez, C.A., et al. Acetyl cholinesterase accelerates assembly of amyloid beta-peptides into Alzheimer's fibrils; possible role of the peripheral site of the enzyme. (1996) Neuron 16: 881-891.
Pubmed || Crossref - 31. Ordentlich, A., Kronman, C., Flashner, Y., et al. Dissection of the human acetyl cholinesterase active center determinants of substrate specificity. Identification of residues constituting the active site, the hydrophobic site and the acyl pocket. (1993) J Biol Chem 268: 17083-17095.
Pubmed - 32. Radic, Z., Pickering, N.A., Vellom, D.C., et al. Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors. (1993) Biochemistry 32: 12074-12084.
Pubmed || Crossref - 33. Harel, M., Schalk, I., Ehrat-Sabatier, L., et al. Quaternary ligand binding to aromatic residues in the active site gorge of acetyl cholinesterase. (1993) Proc Natl Acad Sci USA 90: 9031-9035.
Pubmed || Crossref - 34. Ordentlich, A., Barak, D., Kronman, C., et al. Functional characteristics of the oxyanion hole in human acetyl cholinesterase. (1998) J Biol Chem 273: 19509-19517.
Pubmed || Crossref - 35. Shafferman, A., Kronman, C., Flashner, Y., et al. Mutagenesis of human acetyl cholinesterase. Identification of residues involved in catalytic activity and in polypeptide folding. (1992) J Biol Chem 261: 17640-17648.
- 36. Conforti, F., Statti, G.A., Tundis, R., et al. Antioxidant activity of methanolic extract of Hypericum triquetrifoliumT urra aerial part. (2002) Fitoterapia 73(6): 479-483.
Pubmed || Crossref - 37. Mahfoudhi, A., Grosso, C., Gonçalves, R.F., et al. Evaluation of antioxidant, anti cholinesterase, and antidiabetic potential of dry leaves and stems in Tamarixaphylla growing wild in Tunisia. (2016) Chem Biodivers 13(12): 1747-1755.
Pubmed || Crossref - 38. Liu, Z., Tao, X., Zhang, C., et al. Protective effects of hyperoside (quercetin-3-o-galactoside) to PC12 cells against cytotoxicity induced by hydrogen peroxide and tert- butyl hydroperoxide. (2005) Biomed Pharmacother 59 (9): 481-490.
Pubmed || Crossref