Physical Properties of Nano-HAs/ZrO2 Coating on Surface of Titanium Materials Used in Dental-implants and its Biological Compatibility
Pang Xiao Feng
Affiliation
Institute of Physical Electron and Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China
Corresponding Author
Pang Xiao Feng, Professor, Institute of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 610054, China , Tel: (86) 28-83202595; E-mail: pangxf2006@yahoo.com.cn
Citation
Pang Xiao Feng. Physical Properties of Nano-HAs/ZrO2 Coating on Surface of Titanium Materials Used in Dental-implants and its Biological Compatibility. (2017) J Nanotechnol Material Sci 4(2): 67- 74.
Copy rights
© 2017 Pang Xiao Feng. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Nanohydroxyapatite; ZrO2; Titanium materials; Osteoblasts MG-63; Electrochemical deposition method; Composite coating; Biological compatibility
Abstract
A gradient composite coating on the surface of titanium materials, which are used in dental implants, is prepared using an electric-chemical method. The physical properties of the composite coating and its strength of combining with titanium material are studied by the scanning electron microscope, energy dispersive spectrum and X-ray diffraction analysis, etc. The results show that the complex of nanohydroxyapatite/ZrO2 is uniformly deposited and distributed on the surface of titanium materials; its strength of combining with titanium surface can reach 16.3 MPa, which is obtained using the tensile test. The immersion experiment shows that the new matter of carbonate-apatite is occurred and distributed uniformly on the surface of composite coating of nanohydroxyapatite/ZrO2. The cell experiment of cultivate exhibits that the osteoblasts MG-63 can be grown well on the surface of the composite coating. These results indicate that the nanohydroxyapatite/ ZrO2 composite coating on the surface of titanium materials has a good biological activity and compatibility, which could be used in the dental-implants.
Introduction
It is well known that the features of teeth in men and women can be changed with increasing age, in which some teeth could be damaged. Thus the dental-implants, which are implanted at the positions of damaged teeth to replace the latter, are developed and used widely in dentist medicine. At present, the dental-implants are, in general, manufactured by titanium materials. Clinic experiments show that the kind of dental-implants cannot be very well incorporated with the dental substrates, thus their biological functions will fail after about ten years. Therefore, it is very necessary to improve and lift the qualities of the dental-implants for enhancing the combined strength of the titanium materials with the dental substrates. The investigations show that nanomaterials, such as Hydroxyapatites (HAs), and their complexes with other nanomaterials, which are coated on the surface of titanium materials, can be used to enhance the incorporated strength with the dental substrates.
As it is known, the hydroxyapatite [Ca10(PO4)6(OH)2] (HA) is a mainly inorganic composition, which exists widely in bone and tooth in vertebrates and persons, and has an excellent biocompatibility. HAs can also incorporate with all biological tissues in virtue of interface reaction and hydrogen bonds[1-7]. HAs can release the free calcium and phosphorus through the degradation, when are in a solution of organism, thus HAs can be easily absorbed by the biological tissues to stimulate the growth of tissues, bone and tooth in living system. Therefore, HAs have a wide applications as a repairing material in biological hard tissues.
Yang Yunzhi, et al. prepared the HAs coating on the surface of titanium allay and measured its combined strength and toughness with the titanium allay and inspected further its biological functions through injecting it into the tissues[8]. Their results show that the combined strength of HAs with the biological tissues is increased relative to that of titanium allay. Hence, they thought that HA coating could be utilized in the repairing of bone and tooth. However, the experimental measurements display that the combined strength of HA with the titanium allay and its crystallization on the surface of the latter are lower[9,10], then HAs are easily fallen off under action of a force or dissolved through the erosion effect in solution. Therefore the lifetime of use of the coating is very short. This phenomenon is due to the fact that the mechanical properties of HA, such as the thermal expansion coefficient, compressive strength, modulus of elasticity, flexural strength, hardness and fracture toughness, match not with those of the titanium materials. However, if some ceramic powders, such as nano-ZrO2, are added into the coating to form a composite coating on the surface of titanium material, then the above shortcoming could be depressed or eliminated[11].
Experimental measurements show that the thermal expansion coefficient and fracture toughness of ZrO2 are just between those of HA and titanium material. At the same time, ZrO2 has a good abrasion resistance, physiological corrosion -resistance and biocompatibility, its modulus of elasticity, flexural strength and hardness are also higher relative to those of HA and titanium alloys, which are shown in Table 1. Therefore, ZrO2 can well combine with both the titanium materials and HA. If the HA/ZrO2 composite coating on the surface of titanium alloys can be prepared, then the combined strength of the composite coating with the titanium material will be greatly enhanced relative to that of HA coating with the titanium material. Thus the combined strength of dental-implant with the dental substrates also will be greatly increased, when it is used in the dental-implants[12-19]. Hence, it is quite necessary to study and prepare the HA/ZrO2 composite coating on the surface of titanium materials for developing new biological ceramic materials used in bone and tooth.
Table 1: The mechanical properties of materials of HA, Ti6Al4V allay and ZrO2 .
| material | modulus of elasticity (GPa) | compressive strength (MPa) | flexural strength (MPa) | hardness (Hv/GPa) | fracture toughness (MPam½) | expansion index (10-6/K) |
|---|---|---|---|---|---|---|
| HA | 81.4 | 462 ~ 509 | 103 ~ 113 | 3.43 | 0.69 ~ 0.96 | 11 ~ 14 |
| Ti6Al4V | 110 ~ 114 | 825 ~ 869 | 895 ~ 930 | 3.8 ~ 4.7 | 6 ~ 10 | 8.9 |
| ZrO2 | 210 | --- | 1000 ~ 1200 | 12.5 ~ 14.7 | 7 ~ 15 | 10.4 |
However, the experiments show that if ZrO2 is only mixed into HA coating, instead of is injected between the HA and surface of titanium materials, then the biological effect of the composite coating is also not effective. Therefore, we should prepare the composite coating, which is composed of HA/ZrO2, in which the ZrO2 is injected between the HA and surface of titanium materials to form a new layer. In this case it is very necessary to improve the prepared method of the coating.
In this paper we use an electrochemical method to prepare the gradient composite coating of HA/ZrO2 on the surface of titanium alloys through choosing appropriate electric voltage and current. This method has the following advantages.
(1) A gradient and dense composite coating of HA/ZrO2 can be formed by this method, in which HA and ZrO2 are orderly deposited on the surface of the titanium, i.e., ZrO2 is uniformly between the HA and titanium.
(2) The temperature of the solution, in which the chemical reaction occurs, is lower, thus the phase transition and brittle cracking of the coating occurred in the prepared process can be avoided and eliminated by accommodating the electric voltage and current.
(3) The electric current and voltage of instrument, pH value of the solution, the thickness and uniformity of coating can be easily controlled in prepared process for a good and excellent bio-active coating.
(4) The materials, which are used in the chemical reaction, can be injected continuously, thus there are not pollutants in the experimental process. Therefore, it can eliminate the difficulties occurred in other methods.
Materials and Methods
Materials and Instruments
Materials used in this research, including LK2005Atype electrochemical workstation made by Nanlinke Co. CN, Scanning Electron Microscopy (SEM) with EDAX spectrum (Sanyo, JPN), X-ray diffractometer (GFL. Germany), 670-FT-IR spectrometer (Nicolet Nexus, USA) and electronic tensile tester (Shanghai, CN), are as follows: the calcium nitrate with four water molecules (Sigma, USA), ammonium dihydrogen phosphate (Sigma, USA), Hydrated nitric acid oxygen zirconium (Sigma, USA), Osteoblast cells (MG63) (Shanghai, CN), pure titanium material with purity of > 99.9% (Baoji British knight medical titanium Co. LTD, CN).
Experimental Method
The treatment of titanium pieces: The pure titanium materials are processed as some titanium slices of 1 cm × 1 cm × 0.1 cm with smooth surface, which are again washed and cleaned with ethanol, acetone, deionized water and ultrasonic wave about 15 mins, respectively. After this the titanium slices are further corroded in acid solution of HF:HNO3:H20 = 1:1:15 about 20 ~ 40 mins, we extract them from the solution to clean by the deionized water and ultrasonic wave for 8 mins.
Preparation of gradient and dense composite coating of HA/ZrO2 on surface of the titanium: The processed titanium slices, platinum chips and saturation Hg2Cl2 are served as the worked electrode, pair electrode and reference electrode in the electrochemical equipment, respectively. The nitric acid zirconium oxygen is first dissolved as a deposited solution of zirconium with the zirconium concentration of 0.0125 mol/L by a dilute nitric acid solution, the pH value of deposited solution should be regulated to 2.3 by adding ammonia water, thus the electrolyte deposited solution is prepared, which is used in the electrochemical workstation. When the strength of electric current in the worked electrode is adjusted to 11.1 mA and the temperature of the electrolyte deposited solution is controlled to 25°C, then Zr(OH)4 can be well deposited on the surface of the titanium slices. The prepared titanium slices containing the Zr(OH)4 coating are extracted from this solution after the deposited time of 400s and are dried further. Subsequently, we finish second electrochemical deposition of HAs on the surface of Zr(OH)4 in the electrolyte solution at the temperature of 65°C to form HA/Zr(OH)4 composite coatings on the surface of the titanium slices, where the strength of electric-current of the worked electrode is adjusted to 1.1 mA and the deposited time is controlled to 900s. In this process the electrolyte solution is prepared by the calcium nitrate with four water molecules, dihydrogen phosphate and distilled water, in which the pH value is adjusted to 4.1, the ratio of Ca vs. P is Ca : P = 1.67, the concentrations of Ca2+ and PO43- ions are controlled to 0.021 mol/L and 0.0125 mol/L, respectively.
The prepared HA/Zr(OH)4 composite coatings on the surface of the titanium slices are placed into the sintering furnace with vacuum condition to sinter at high temperature of 650°C after they are washed by the distilled water and dried further by far-infrared heating. Thus we can obtain the nano-HA/ZrO2 composite coatings on the surface of titanium slices after the sintering times of 2h, which are required and can be used in the dental-implants.
Measurement of combined strength of the composite coating with the surface of titanium: We used the electronic-tensile tester to detect the combined strength of the nano-HA/ZrO2 composite coating with the surface of titanium, which is an average of measured values obtained from three points, its unit is Pa.
The immersion experiment: The nano-HA/ZrO2 composite coating on the surface of titanium material is soaked in a simulated solution of life (SSL) with 40 mL, which is placed in a closed and disinfectant centrifugal tube, the temperature and pH value of the solution are controlled to 36.5°C and 7.4, respectively. The components and their concentrations in the SSL are basically same with those in the Human plasma (HBP), which is shown in Table 2. The immersed composite-coatings extracted from SSL after 7 days and 14 days are washed and cleaned with de-ionized water and further dried, respectively, their properties are inspected by the bio-chemical method and SEM, respectively.
Table 2: The components and their concentrations in (SSL) and HBP(mM).
| Ion | Na+ | K+ | Mg2+ | Ca2+ | Cl- | HCO3- | HPO42- | SO42- |
|---|---|---|---|---|---|---|---|---|
| SSL | 142.0 | 5.0 | 1.5 | 2.5 | 147.8 | 4.2 | 1.0 | 0.5 |
| HBP | 142.0 | 5.0 | 1.5 | 2.5 | 103.0 | 27 | 1.0 | 0.5 |
The observation of morphology of coating surface adhering the osteoblast MG63:
(A) The culture of cells. The MG63 cells are grown in a 5% CO2 enriched incubator at 25°C in RPMI1640 media (Hyclone, American) supplemented with 5% fetal calf serum (FCS, Biological Industries, BaiAn, China). Using microscopic inspected method we can verify that there are no contaminating cells in the third passage of cell culture.
(B) The experimental process. In all experiments, 4 × 105 cells per well were seeded into 60 well micro-plates and allowed to grow continually under the conditions described above. The 100 μL/well foster liquids containing the fetal calf serum and insulin liquid of concentration of 1 mL/250 mL are added into each well. Subsequently, the titanium slices adhering the HA /ZrO2n composite coating are placed into the wells. The whole micro-plates with titanium slices are again shifted into the CO2 enriched incubator at 37°C to promote the proliferation and adherence of the MG63 cells on the surfaces of the composite coatings. After 24 hours the biological samples extracted from the enriched incubator are washed by the phosphate cushion fluid, dried by heating, fixed again by 2.5% glutaraldehyde about 24 hours and dehydrated further by the ethanol of 30 - 100% concentration about 20 min, then we can inspect the features of vaccination and adhesion of the MG63 cells on the surface of the composite coatings by SEM. Thus we eventually can determine the biological activity of the composite coatings from the measured results.
Results and Discussion
The influences of deposited time on the features of Zr(OH)4/HA coating
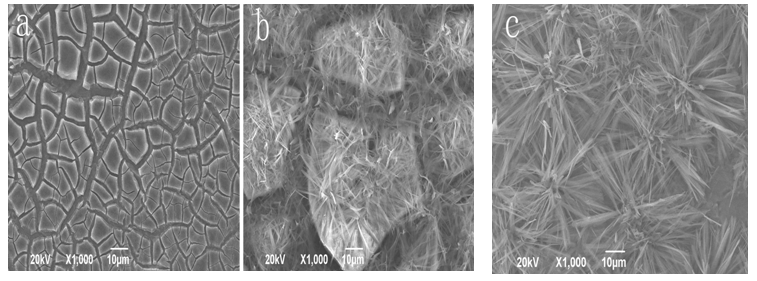
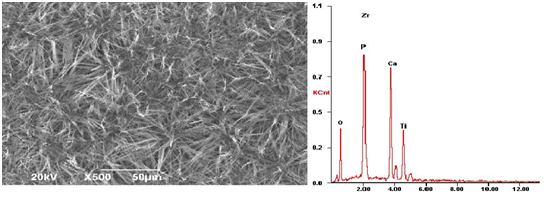
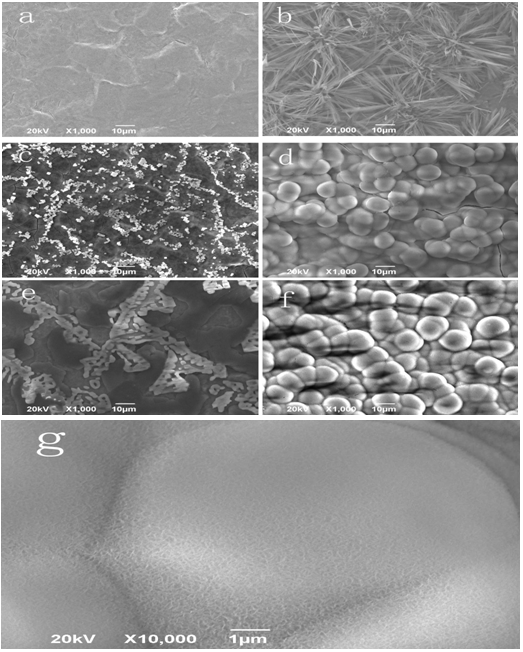
When the electric current of the worked electrode in LK2005A-type electrochemical workstation is adjusted to 11.1mA, the nano-HAs can be deposited on the surface of Zr(OH)4, which was, first of all, adhered on the surface of titanium material, to form a HA/ Zr(OH)4 composite coating in the electrolyte solution of calcium phosphate. Figure 1a shows the outline of the HA/ Zr(OH)4 composite coating at the deposited time of 800s, which is obtained by SEM. Obviously, the features of composite coating depend on the relative weight of components, pH value, temperature of the electrolyte solution, deposited time and size of electric current of the worked electrode. Figures 1(b) and 1(c) show the outlines of the composite coating at the electric-current of 11.1 mA and 1.1 mA and the deposited times of 700s and 900s, respectively. From these results we see that the nano-HAs have not covered the whole surface of Zr(OH)4, if the deposited time is too short and current is greater, which are shown in Figure 1(b), but if the deposited time is longer and current is smaller, then a good composite coating can be obtained as shown in Figure 1(c), where a dense needle structure containing some gaps are occurred. This kind of composite coating is helpful for the growth and adhesion of the osteoblast. Thus it could be used in the dental-implants[20]. In Figure 2(a) we exhibit the outline of HA/Zr(OH)4 composite coating on the surface of titanium material at 1.1mA and 850s. The compositions containing in the composite coating are shown in Figure 2(b), which is obtained by Energy Dispersive Spectrometer (EDS). We see from this figure that there are really Ca, P, Zr, O elements in the composite coating. Thus we can affirm that the HA/Zr(OH)4 composite coating is truly formed on the surface of titanium material in this condition.
Figure 1: The outlines of HA/Zr(OH)4 composite coating on the surface of titanium material at 11.1 mA, 800 s (a), 11.1 mA, 700 s (b) and 1.1 mA, 900 s (c), respectively.
Figure 2: The outline of HA/Zr(OH)4 composite coating on the surface of titanium material formed in the conditions of 1.1 mA and 850 s and its compositions obtained by EDS.
The influence of deposited temperature on the features of HA/Zr(OH)4 composited coating Experiments display that the features of HA/Zr(OH)4 composite coating on the surface of titanium materials depend closely on the temperature of the electrolyte solution. We collect the changes of feature of HA/Zr(OH)4 composite coating with increasing the temperature by SEM, which are shown in Figure 3. Figure 3(a) exhibits that the nano-HAs cover not the whole surface of Zr(OH)4, which is early adhered on the surface of titanium materials, where the nano-HAs are non-uniformly gathered together to form some groups on the surface of the Zr(OH)4 coating at lower temperature of 25°C. However, when the deposited temperature is lifted to 45°C, although the nano-HAs are covered the whole surface of Zr(OH)4 coating as shown in Figure 3(b), its density is too great to depress its bio-activity. Figure 3(d) shows that the needle structure of nano-HAs is covered the whole surface of Zr(OH)4 coating, but the clusters of nano-HAs having great area occurs also in the surface at the temperature of 80°C. However, an uniform and dense HA/Zr(OH)4 composite coating having the needle structure on the surface of titanium materials is obtained at the temperature 65°C, which is shown in Figure 3(c). This structure is very advantageous for the osteoblast’s growth and adhesion on its surface.
Figure 3: The SEM images of HA/Zr(OH)4 composite coating at different temperatures of 25°C (a), 45°C (b), 65°C (c) and 80°C (d), respectively.
The changes of combined strength of HA/ZrO2 composite coating with the surface of titanium material after sintering
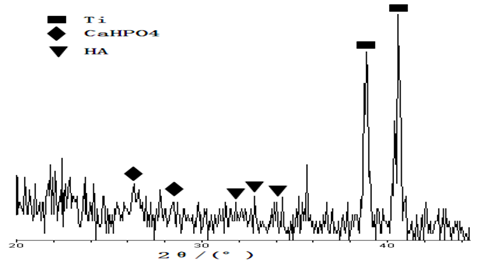
Experiments show that HA/Zr(OH)4 composite coatings can becomes HA/ZrO2 composite coating and its combined strength with the surface of titanium material is increased after are thermally sintered. We used the electronic tensile tester to detect the combined strength of HA/ZrO2 composite coating with the surface of titanium material after sintering at 650°C, which is 16.3MPa, but the combined strength of nano-HA coating with the surface of titanium material after sintering at 650°C is only 5.8MPa .The values are an average of measured values obtained three points as shown in Table 3. This shows clearly that ZrO2, which is served as a transition layer, can well ease and offset the non-matched effect of thermal expansion coefficient of HA with that of the titanium, thus the combined strength of HA/ZrO2 composite coating is significantly enhanced due to the match of thermal expansion coefficient between them. In Figure 4 we exhibit the XRD pattern of HA/ZrO2 composite coating on the surface of titanium material after thermal sintering at 650°C, which shows clearly that the nano-HAs are already covered on the surface of ZrO2 coating. In Figure 4 there are two strong peaks, which is due to the large thinner of the composite coating about 3 μm obtained by the ladder meter, and some weaker peaks, which denote the existence of precursors CaHPO4 of HAs in the coating arising from the lower of sintered temperature, in the titanium substrates.
Table 3:
| Sample | HA coating | HA/ZrO 2 coating (after sintering) |
|---|---|---|
| 1 | 4.7 | 14.6 |
| 2 | 5.6 | 16.1 |
| 3 | 7.1 | 18.3 |
| average | 5.8 | 16.3 |
Figure 4: XRD pattern of composite coating after sintering.
The biological activity of the composite coating
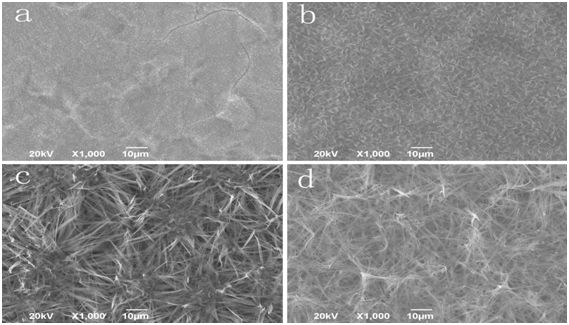
We use the immersion method and SEM to study and inspect the biological activity of the nano-HA/ZrO2 composite coating on the surface of titanium. Figures 5a and 5b are the outlines of Zr(OH)4 coating and nano-HA/ZrO2 gradient coating before the immersion, respectively, figures 5c and 5e are the features of Zr(OH)4 coating at different immersion times of 7 days and 14 days, respectively, which show that NaCl grains are formed and accumulated on the surface of the Zr(OH)4 coating, and the accumulation of NaCl is also increased with increasing the immersion time, in which but there are not growth of apatite particles induced by SSL, thus the apatite particles are not covered on the surface of the coating. Figures 5d, 5f and 5g exhibit the outlines of nano-HA/ZrO2 gradient coating at different immersion time of 7 days and 14 days, respectively. Figure 5d shows that there are some clusters formed by the apatite particles of white ball in the composite coating, the size of apatite particle is about 10 μm, they covers completely over the whole surface of ZrO2 coating. However, there are also some small cracks in the coating due to contraction of the surface arising from the drying of the coating after they are extracted from the immersion solution. Figure 5f indicates that the sizes of apatite particles and thickness of coating are increased in the coating in this condition. In the meanwhile, the blocky structure composed of small apatite balls is also appeared in the coating, when the immersion time is increased, which is 14 days. Figure 5g is a magnified result of pattern of HA/ZrO2 gradient coating after immersion time of 14 days, which is obtained by SEM with high resolution. These results indicates clearly that plenty of small needle HAs can be grown from the surface of a great spherical-bodies, which is accumulated by a lot of nano-HAs. Thus the mineralization and growth of apatite particles induced by SSL occur. These results manifest that the nano-HA/ZrO2 gradient coating has a good biological activity.
Figure 5: SEM images of Zr(OH)4 coating and gradient coating after different immersion times.
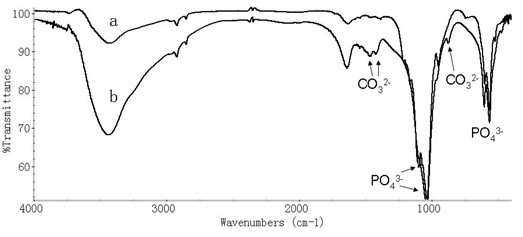
Figure 6: Infrared spectrum of absorption of nano-HA/ZrO2 coating after the immersion time of 7 days in SSL.
We collected also the infrared spectrum of absorption of nano-HA/ZrO2 composite coating on the surface of titanium after the immersion time of 7 days in SSL using 670-FT- IR spectrometer, which is shown in Figure 6. We see from this figure that some new peaks at 1489.38, 1412.92, 871.59 cm-1 occur after the immersion, which correspond to the characteristic peaks of CO32-. This means that the new matter of carbonate- apatites, such as like-bone apatites, which are formed in the composite coating arising from SSL in the immersion process, which induces the mineralization and growth of bone apatites[21,22]. This demonstrates again that nano-HA/ZrO2 gradient coating is existed in this case.
The biological compatibility of nano-HA/ZrO2 composite coating
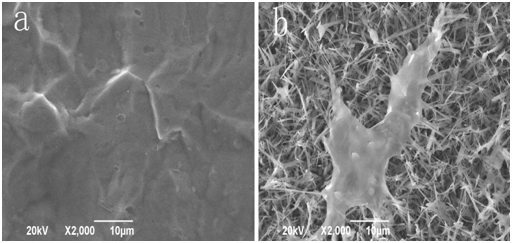
We further study the biological compatibility of nano-HA/ZrO2 composite coating in virtue of inspecting of growth states of osteoblast MG63 on its surface. In this experiment the nano-HA/ZrO2 coatings on the titanium pieces are together placed in the culture solution of MG63 cells. Figure 7 shows that the states of proliferation and adherence of osteoblasts MG63 on the surfaces of the pure titanium and nano-HA/ZrO2 coatings after culture of 24h, respectively. Figure 7b shows that MG63 cells are adhered well on the surface of nano-HA/ZrO2 coatings, the MG63 cells are proliferated along these needle structures, which provide more adherent positions for the proliferations of cells. Meanwhile, we see that the adhered areas of cells are gradually extended to form a structure of anchor shape in these gaps in the composite coating. This indicates that the nano-HA/ZrO2 composite coating has a good biological compatibility. However, the MG63 cells cannot proliferate on the surface of pure titanium pieces as shown in Figure 7a. This verified again that the dental- implants made by the pure titanium pieces have not a good biological compatibility as mentioned in introduction.
Modern cell biology and medicine indicate that when the dental-implants are implanted on the substrates of teeth, the plasma proteins will be adhered on surface of the dental- implants to form a molecular layer, which can absorb the osteoblasts and can extend their proliferation on the surface of dental-implant in virtue of the plasma proteins. Thus the dental-implants are fixed on the substrates of teeth. This represent clearly that the dental-implants made by the titanium adhering the nano-HA/ZrO2 composite coating are easily and firmly fixed on the substrates of teeth because the composite coating can provide more adherent positions for the cells through these needle structure containing some gaps. Therefore, the titanium adhering the nano-HA/ZrO2 composite coating prepared by us have a good biological compatibility, thus a wide application in dental medicine.
Figure 7: SEM images of proliferation of MG 63 cells on the surfaces of pure titanium coating (a) and composite coating (b), respectively
Conclusion
The HA/ZrO2 composite coating on the surface of titanium materials, which are used in dental implants in dentist medicine, are prepared by the electrolyte solution composed of ZrO(NO3)2, Ca(NO3)2 and NH4H2PO4 using an electric-chemical method, the physical properties including the combined strength and biological activity and compatibility of the composite coatings are studied by methods of SEM, EDS spectrum, X-ray diffraction and infrared spectrum of absorption, etc.. From these investigations we can draw the following conclusions.
(1) The nano-HA/ZrO2 composites can be uniformly deposited and adhered on the surface of titanium materials, its combined strength with the surface of titanium is very high, and can reach 16.3 MPa.
(2) The immersion experiment in SSL shows that the carbonateapatites can be uniformly deposited on the surface of HA/ZrO2 composite coating. This means that the composite coating has a good biological activity.
(3) The cell cultivate experiment denotes that the MG63 cells can be adhered well on the surface of nano-HA/ZrO2 coatings, where the MG63 cells can also proliferate along these needle structures, which provide more adherent positions for the proliferation of cells. Meanwhile, the adhered areas of cells are gradually extended to form a structure of anchor shape in these gaps in the composite coating. This indicates that the nano-HA/ZrO2 composite coating has a good biological compatibility. Thus, we can predict that the titanium adhering the nano-HA/ZrO2 composite coating prepared by us have a wide application in dental medicine.
Acknowledge:
Authors would like to acknowledge the Major State Basic Research Development Program (973 program) of China for the financial support (Grant No. 2007CB936103).
References
- 1. Kalita, S. J., Bhardwaj, A., Bhatt, H. A. Nanocrystalline calcium phosphate ceramics in biomedical engineering. (2007) Mater Sci Eng 27(3): 441-449.
Pubmed || Crossref || Others - 2. Suchanek, W., Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue. (1998) Jour Mater Res 13(1): 94-117.
Pubmed || Crossref || Others - 3. Wang, Z. C., Huang, L. M. Research progress of hydroxyapatite coating material with metal basis having biological activity. (2006) Bulletin Chin Ceramic Soc 1: 57.
Pubmed || Crossref || Others - 4. Lynn, A. K., DuQuesnay D. L. Hydroxyapatite-coated Ti-6Al-4V part 1: The effect of coating thickness on fatigue behavior. (2002) Biomaterials 23(9): 1937-1946.
Pubmed || Crossref || Others - 5. Pang, X. F., Zeng, H. J. Investigation of biological activities of nano- hydroxyapatite powder. (2009) Mater Eng 45: 14.
Pubmed || Crossref || Others - 6. Pang, X. F., Zeng, H. J., Liu, J. L., et al. The properties of nanohydroxyapatite materials and its biological effects. (2010) Mater Sci Appl 1: 81-90.
Pubmed || Crossref || Others - 7. Pang, X. F. Biophysics, 2007, The Press of Sichuan Sci. Tech., Chengdu. pp202-277.
Pubmed || Crossref || Others - 8. Yang, Y. Z., Kim, K. H., Joo, L. A review on Calcium phosphate coatings produced using a sputtering process - An alternative to plasma spraying. (2005) Biomaterials 26(3): 327-337.
Pubmed || Crossref || Others - 9. Roop, K. R., Wang, M. Functionally graded bioactive coatings of hydroxyapatite/titanium oxide composite system. (2002) Mater Lett 55(3): 133-137.
Pubmed || Crossref || Others - 10. Xion, X. B., Li, H. J., Huang, J. F., et al. Bioactive hydroxypatite coating on carbon/carbon composite prepared by sonoelectrodeposition and alkaline-heat treatment. (2005) Rare Metal Mater Eng 34: 515.
Pubmed || Crossref || Others - 11. Piconici, C., Maccauro, G. Zirconia as a ceramic biomaterial. (1999) Biomaterials 20(1): 1-25.
Pubmed || Crossref || Others - 12. Chou, B. Y., Chang, E. Influence of deposition temperature on mechanical properties of plasma-sprayed hydroxyapatite coating on titanium alloy with ZrO2 intermediate layer. (2003) J Thermal Spray Tech 12(2): 199-207.
Pubmed || Crossref || Others - 13. Khalil, A. K., Sug, W. K., Hak, Y.K. Consolidation and mechanical properties of nanostructured hydroxyapatite-(ZrO2 + 3 mol% Y2O3) bioceramics by high-frequency induction heat sintering. (2007) Mater Sci Eng 456(1-2): 368-372.
Pubmed || Crossref || Others - 14. Chevalier, J.J., Deville, S., Etienne, M., et al. Critical effect of cubic phase on aging in 3 mol% yttria-stabilized zirconia ceramics for hip replacement prosthesis. (2004) Biomaterials 25(24): 5539-5545.
Pubmed || Crossref || Others - 15. Inuzuka, M., Nakamura, S., Kishi, S., et al. Hydroxyapatite-deped zirconia for preparation of biomedical composites ceramics. (2004) Solid State Ionics. 172(1-4): 509-513.
Pubmed || Crossref || Others - 16. Yun, M.S., Dae, H. K. Crystallization characteristics of yttria-stabilized zirconia/hydroxyapatite composite nanopowder. (2003) J Cry Growth 254: 411.
Pubmed || Crossref || Others - 17. Yoshida, K., Hashimoto, K. Fabrication of structure-controlled hydroxyapatite/zirconia composite. (2006) J Euro Ceramic Soc 26: 515.
Pubmed || Crossref || Others - 18. Wang, Y. B., Lu, X., Zhao, J., et al. Preparation of n-HA/ZrO2 composite coating by pulsed electrochemical deposition. (2009) Rare Metal Mater Eng 38: 1071.
Pubmed || Crossref || Others - 19. Wang, Y. B., Lu, X., Feng, B., et al. Investigation of the stability and biocompatibility of HA/ZrO2 nanocomposite coatings prepared by pulsed electrochemical deposition. (2010) Chem J Chin Univer 31: 253.
Pubmed || Crossref || Others - 20. Anee, T. K., Ashok, M., Palanichamy, M., et al. A novel technique to synthesize hydroxyapatite at low temperature. (2003) Mater Chem Phys 80(3): 725-730.
Pubmed || Crossref || Others - 21. Li, P., Kangasniemi, I., Groot, K., et al. Bonelike hydroxyapatite induction by a gel-derived titania on a titanium substrate. (1994) J Amer Ceramic Soc 77(5): 1307-1312.
Pubmed || Crossref || Others - 22. Ding, S. J., Lee, T. L., Chu, Y. H. Environmental effect on bond strength of magnetron –sputtered hydroxyapatite/titanium coating. (2003) J Mater Sci Lett 22: 479.
Pubmed || Crossref || Others