Potent Anti-Adipogenic Effect of Green Tea and Green Tea Extracts in Chicken
Alemu Regassa1#, Dong-hun Lee2#, Soo-Won Choi2, Chang-Seon Song2, Woo Kyun Kim3, Jong Hwan Kwak4
Affiliation
- 1Department of Animal Science, University of Manitoba, Winnipeg, Manitoba, Canada
- 2College of Veterinary Medicine, Konkuk University, Seoul, Republic of Korea
- 3Department of Poultry Science, University of Georgia, 303 Poultry Science Building, Athens 30602, GA, U.S.A
- 4College of Pharmacy, Sungkyunkwan University, Suwon 440-746, Korea
- #These authors contributed equally
Corresponding Author
Woo Kyun Kim, Department of Poultry Science, University of Georgia, 303 Poultry Science Building, Athens 30602, GA, U.S.A, E-mail: wkkim@uga.edu
Citation
Kyun Kim, K., et al. Potent Anti-Adipogenic Effect of Green Tea and Green Tea Extracts in Chicken. (2017) J diab Obes 4(3): 1- 6.
Copy rights
© 2017 Kyun Kim, K. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Chicken; Supplementation; Green tea; Obesity; Anti-adipogenic
Abstract
This study was conducted to examine the effect of green tea on body, adipose tissue, and liver weights and adipose tissue weight to body weight ratio and the adipogenic differentiation and expression of adipogenic transcripts in chicken (Gallus gallus) preadipocytes. In experiment one, chicks were weighed and randomly assigned to a control diet (CTRL) and green tea treatment (GT 1%, w/w) for 38 days. In experiment 2, preadipocytes were isolated from 20 wk old chicken and treated with an adipogenic cocktail (DMIOA) containing 500 nM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 20 μg/mL insulin, and 300 μM OA, DMIOA+30 μg of extract B, E, H, Mc, T, and W, respectively, for 48 h. Data were analysed using the General Liner Model procedure of the Statistics Analysis System (SAS) Institute version 9.4, and differences were considered significant at P < 0.05. Gene expression was measured using quantitative real-time PCR. GT 1% significantly reduced body (- 9%; P = 0.0447), liver (-20%; P = 0.0206), and abdominal fat weight (- 44%; P = 0.0055) compared with the control (CTRL) group (GT 0%). The abdominal fat/body weight ratio of green tea supplemented group (- 36%; P = 0.0125) was also significantly lower than that of control group. In cell culture study, all green tea extracts inhibited C/EBPα and β mRNA expression compared to DMIOA. DMIOA+B, T, or W reduced mRNA expression of FABP4 by three-fold compared to DMIOA. Although all green tea extracts reduced adipocyte formation, T and W had the strongest anti-adipogenic effects. These results demonstrate that supplementation of green tea could be an effective strategy in the control of obesity in chickens.
Introduction
Progress in body weight gain or meat production in broiler chickens has been accomplished through genetic selection. Genetic selection for growth parameters in broiler chickens resulted in breeder stock that tends to lack the ability to self-regulate feed intake[1-3]. This improvement in body-weight-for-age of modern broiler chickens, due to an increased growth rate and associated higher nutrient supply, is closely related with excessive fat deposition, metabolic, skeletal and reproductive disorders, and high mortality rates that eventually reduce the profitability of the poultry industry[4,5].
Modern commercial chickens exhibit excessive fat accumulation in the abdominal area that negatively affects their reproductive performance and causes fatty liver hemorrhagic syndrome. Owing to the potential health risk due to consumption of high fat meat, the demand for lean meat is increasing. Therefore, devising feeding strategies that would help to reduce excessive fat accumulation in modern poultry has a paramount importance. In line with this, understanding the mechanisms responsible for the regulation of lipid metabolism and identification of key enzymes and transcription factors involved in lipid metabolism could enhance production of lean meat.
Broiler chickens fed ad libitum feed likely consume energy at two or three times greater than their maintenance needs, and deposit body fat at faster rate[6]. A higher degree of feed restriction[7] and supplementation of anti-adipogenic compounds or feed additives in diets[8-11] have been noted as some of the strategies to regulate this rapid body weight gain and reduce health consequences of consuming high meat fat.
Green tea is a historically popular beverage, and is produced from the leaves of the evergreen plant, Camellia sinensis. Green tea contains several polyphenols including (-)-epigallocatechin gallate (EGCG), (-)-epicatechin, (-)-epigallocatechin, (-)-epicateching gallate[12]. However, the largest polyphenol in green tea is constituted by EGCG that accounts for more than 60% of the total polyphenols[13].
Results from numerous clinical trials have indicated that consumption of tea containing polyphenols and caffeine reduces body weight and fat metabolism in humans and in rodents fed high-fat diets[14-16]. In particular, green tea polyphenols (GTPs) have been shown to inhibit adipogenesis and induce apoptosis in mouse 3T3-L1 preadipocytes[17,18]. Similarly, GTPs have been shown to reduce obesity and serum lipid levels in broiler chickens by suppressing fatty acid synthesis and stimulating lipolysis[19]. This study was conducted to examine the effects of different green tea extracts (GTEs) on body and organ weight and expression of key adipogenic transcripts in broiler chickens.
Materials and Methods
This study had two experiments. In experiment one, day-old Ross 308 broiler chickens were used to examine the effect of green tea powder on body, liver, and abdominal fat weights. In experiment two, preadipocytes isolated from chicken fat tissues were used to examine the effect of different Green Tea Extracts (GTEs) on the adipogenic differentiation and expression of key adipogenic transcripts.
Animals and diet
This experiment was conducted for 38 days. Day old broilers (N = 51) arrived at the poultry facility of Konkuk University (Seoul, Republic of Korea), weighed and randomly allocated to the following dietary treatments. A control diet (CTRL) containing a corn and soybean meal diet with 20% crude protein and 3,100 kcal/kg metabolizable energy (n = 25) and green tea treatment (GT 1%, w/w green tea leaf powder) (n = 26). The experimental protocols were approved by the Committee for Animal Care and Use at Konkuk University and University of Manitoba. Total body weight, liver, and abdominal fat weights were measured on day 38.
Preparation and dissolution of green tea extracts
Green tea leaves (2.0 kg) were extracted 2 times with distilled water (20 L) at 90°C for 5 h, and the extract was lyophilized (at −50°C for 24 h) to obtain the water-green tea extract (30.1% yield). After the water extraction, the leaves were airdried and subsequently extracted with 60% ethanol (E) (14 L) at 42°C for 5 h to obtain ethanol-green tea extract (20.5% yield). The green tea extract was prepared by direct 60% ethanol extraction (at 42°C for 5 h) from original green tea leaves (25.1% yield). Another green tea by-product was extracted by soaking 1.4 kg of green tea powder in methanol (twice with 14 L, at 42°C for 5 h). The methanol extract was evaporated to dryness under reduced pressure, and a portion of the residue (150 g) was dissolved in methanol-H2O (9:1, 1.5 L). The resulting solution was partitioned with hexane (1.5 L × 3), and then the aqueous methanol solution was diluted with H2O to 30% of H2O in methanol and extracted with dichloromethane (1.5 L × 3). The aqueous methanol solution was concentrated in a vacuum, and the aqueous residue was diluted with H2O to 1.5 L, and then consecutively partitioned with ethyl acetate (1.5 L × 3), and n-butanol (1.5 L × 3). Each of the solvent fractions was evaporated under reduced pressure to prepare hexane (H) (26.3 g), dichloromethane (Mc) (31.8 g), ethyl acetate (E) (59.5 g), n-butanol (B) (15.1 g), methanol (T), and water (W) (16.0 g) extracts.
In order to prepare the extracts for cell culture treatments, 20 mg of each GTE was dissolved in one mL of Dimethyl Sulfoxide (DMSO) and dissolved by vortexing for several times to prepare 20 mg/mL of stock solution. 5 μL of the stock solution (100 μg GTE) was transferred to 5 mL of 1X DMEM and thoroughly mixed by vertexing and 1.5 μL (30 μg) of each extract was used to treat the cells.
Cell culture
Abdominal adipose tissue was collected from 20-week old Single Comb chicken (Gallas gallus) by sterile dissection as described[20]. The adipose tissue was minced into fine sections with scissors and incubated in 10 mL Dulbecco’s Modified Eagle’s Medium (DMEM) digestion buffer containing 0.1% collagenase, 2.8 mM glucose, 4% BSA for 45 min at 37°C in a shaking water bath. After the incubation, the contents were filtered through 100 and 40 μm Nylon meshes (Fisher Scientific, Ottawa, ON), and the filtrate was centrifuged at 1,800 rpm for 10 minutes to separate floating adipocytes from pellets of preadipocytes. The supernatant was discarded, and cell pellets were re-suspended in 10 mL 1X DMEM containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin and L-glutamate (Mediatech, Inc., Manassas, VA). Preadipocytes were then seeded in 100 mm Petri dishes (BD Biosciences, Mississauga, ON) and cultured in an incubator with 95% air and 5% CO2 at 37°C. Cells were checked for viability every day, and the media were changed every three days until confluence. Cells were washed twice in 5 mL phosphate buffered saline (PBS) and incubated in 3 mL 1X Tris-Ethylene diamine tetra acetic Acid (TE) buffer at 37°C for 2 minutes. Seven mL of 10% DMEM was added, and the cells were washed several times to remove adhering cells. The contents were centrifuged at 1,800 rpm for 5 minutes, the supernatant was discarded, and cell pellets were resuspended in 5 mL of 10% DMEM. Cell density was determined, and the cells were plated in six-well plates at 20,000 cells/cm² at confluence.
Treatment of preadipocytes using different green tea extracts
In order to examine the effect of GTE on the expression of key adipogenic transcripts, preadipocytes were treated with different GTE for 48 hrs in 1X DMEM. The experiment had eight treatments: 1) FBS = Non-treated preadipocytes (Preadipocytes cultured with 1X DMEM alone), 2) DMIOA = Preadipocytes treated with an adipogenic cocktail (DMIOA) containing 500 nM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 20 μg/mL insulin, and 300 μM OA, 3) DMIOA+B = Preadipocytes treated with DMIOA + 30 μg GTE B, 4) DMIOA+E = Preadipocytes treated with DMIOA + 30 μg GTE E, 5) DMIOA+H = Preadipocytes treated with DMIOA + 30 μg GTEH,6) DMIOA+Mc = Preadipocytes treated with DMIOA + 30 μg GTEMc, 7) DMIOA+T = Preadipocytes treated with DMIOA + 30 μg GTET and 8) DMIOA + W = Preadipocytes treated with DMIOA + 30 μg GTEW.
Oil Red O staining
In order to examine the effect of different green tea extracts on adipogenic differentiation, preadipocytes representing all treatments were stained with Oil Red O stain according to supplier’s protocol to assess accumulation of triglycerides (Lonza, Walkersville, USA).
RNA isolation, cDNA synthesis and quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
At the end of each experiment, growth media were removed, and total RNA was extracted using one mL of TRIzol (Invitrogen, Burlington, ON) according to the manufacturer’s protocol. First strand cDNA was synthesized using a high capacity cDNA synthesis kit following the supplier’s protocol (Applied Biosystems, Burlington, ON). Pairs of primers for each gene were designed using the National Centre for Biotechnology Information (NCBI). Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) was performed in duplicate reactions including nuclease free water, the forward and reverse primers of each gene, template cDNA and SYBR Green as a detector using CFX Connect™ Real-Time PCR Detection System (Life Science Research, Bio-Rad). Data were generated using ΔΔCt method by normalizing the expression of the target gene to a housekeeping gene (GAPDH), and the values were reported as fold changes of the expression of the target genes in the experimental groups compared with the control group. Pairs of primers used for qRT-PCR assay are shown in Table 1.
Table 1: Pairs of primers that were used for quantitative real-time qRT-PCR assay.
| Name | Forward | Reverse | Product length (base pair) | Annealing temperature ( °C) |
|---|---|---|---|---|
| FABP4 | GAGTTTGATGAGACCACAGCAGA | ATAACAGTCTCTTTGCCATCCCA | 106 | 57 |
| PPARγ² | TGAATGTCGTGTGTGTGGGG | GCATTCGCCCAAACCTGATG | 229 | 55 |
| C/EBPα | CCTACGGCTACAGAGAGGCT | GAAATCGAAATCCCCGGCCA | 205 | 55 |
| C/EBPβ | CCGCTCCATGACCGAACTTA | GCCGCTGCCTTTATAGTCCT | 204 | 55 |
| GAPDH | GCTAAGGCTGTGGGGAAAGT | TCAGCAGCAGCCTTCACTAC | 116 | 55 |
Statistical analysis
Gene expression data were analyzed using the General Linear Model Procedure of the Statistics Analysis System (SAS)[21]. Institute version 9.4. For body and organ weight of chickens, Tukey-test was carried out using GraphPad Prism. Probability value of p < 0.05 was considered significant.
Results
Effect of green tea supplementation on body and organ weight
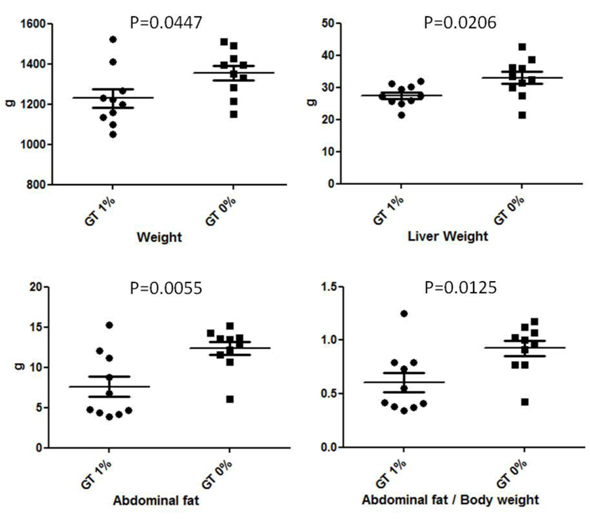
The effects of green tea supplementation on body, liver, and abdominal fat weight are shown in Figure 1. Green tea supplementation (GT 1%) significantly reduced body (-9%; P = 0.0447), liver (-20%; P = 0.0206), and abdominal fat weight (-44%; P = 0.0055) compared with the control (CTRL) group (GT 0%). The abdominal fat/body weight ratio of green tea supplemented group (-36%; P = 0.0125) was also significantly lower than that of control group.
Figure 1: Effect of green-tea supplementation on body and organ weight. GT 1% = Green tea 1%; GT 0% = Control (CTRL).
Effect of green tea on adipogenic differentiation
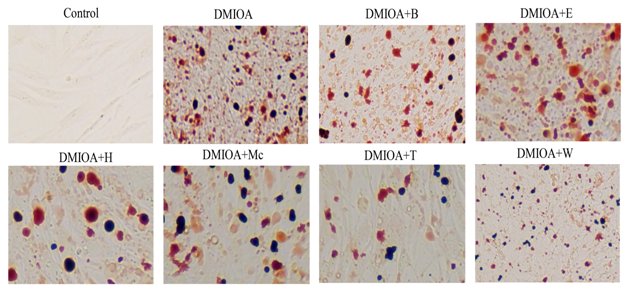
Results of the Oil-Red O staining indicated reduced adipocyte formation in cells treated with DMIOA + GTEs compared with cells treated with DMIOA alone. Especially, adipocyte formation was remarkably reduced in cells treated with DMIOA + extract B, T, and W (Figure 2).
Figure 2: Representative Oil Red O stained images of preadipocytes treated with an adipogenic cocktail (DMIOA) containing 500 nM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 20 μg/mL insulin, and 300 μM OA (DMIOA), and DMIOA +30 μg different green tea extracts. B = butane extract, E = ethanol extract, H = hexane extract, Mc = dichloromethane extract, T = methane extract, and W = water extract.
Effect of green tea on the expression of key adipogenic transcripts
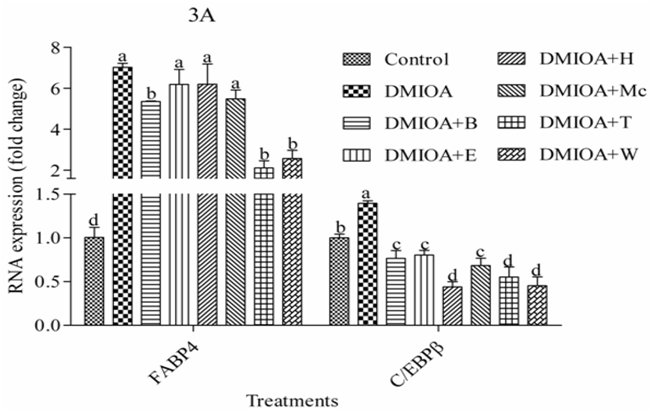
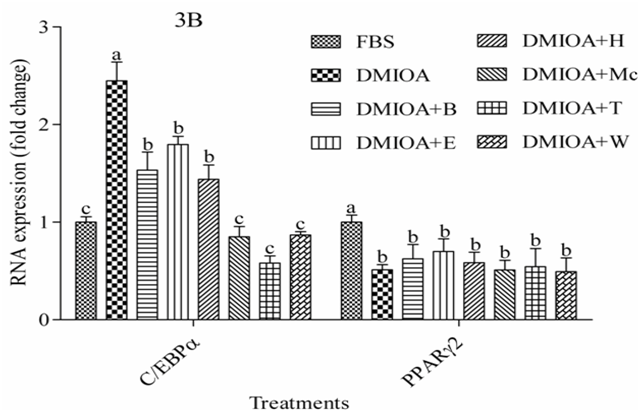
Results of gene expression study are presented in Figure 3A and 3B. The analysis indicated that treatment of preadipocytes with green tea extracts, T and W significantly reduced (P < 0.05) the expression of fatty acid binding protein 4 (FABP4) by three-fold compared with preadipocytes treated with DMIOA. Similarly, the expression of the core enhancer binding protein beta (C/EBPβ) and the core enhancer binding protein alpha (C/EBPα)was significantly reduced (P < 0.05) in preadipocytes treated with all GTEs compared with preadipocytes treated with DMIOA; Mc, T, and W inhibited C/EBPα and C/EBPβ mRNA expression over two-fold compared to DMIOA. However, the expression of peroxisome proliferator activator receptor gamma 2 (PPARγ²) was not affected by green tea treatments.
Figure 3a: The mRNA expression levels of fatty acid binding protein 4 (FABP4) and the core enhancer binding protein beta (C/EBPβ) in preadipocytes treated with an adipogenic cocktail (DMIOA) containing 500 nM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 20 μg/mL insulin, and 300 μM OA (DMIOA), and DMIOA +30 μg different green tea extracts for 48 hr. B = butane extract, E = ethanol extract, H = hexane extract, Mc = dichloromethane extract, T = methane extract, and W = water extract. Bars with different letters are significantly different (P < 0.05).
Figure 3b: The mRNA expression levels of the core enhancer binding protein alpha (C/EBPα) and peroxisome proliferator receptor gamma 2 (PPARγ²) in preadipocytes treated with an adipogenic cocktail (DMIOA) containing 500 nM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 20 μg/mL insulin, and 300 μM OA (DMIOA), and DMIOA + 30 μg different green tea extracts for 48 hr. B = butane extract, E = ethanol extract, H = hexane extract, Mc = dichloromethane extract, T = methane extract, and W = water extract. Bars with different letters are significantly different (P < 0.05).
Discussion
Excessive accumulation of adipose tissue mass relative to lean body mass, referred to as obesity, is a nutritional and metabolic disorder most prevalent in humans and other animal species. Obesity in chicken is becoming a growing production concern in that excess fat accumulation in laying hens reduces egg production, the fertility of the eggs and productive life of the animal and the production of parent stock[22].
Drinking green tea has been documented to be beneficial to health by reducing body weight and fat deposition in the body[23-27]. Significant weight loss and reduction of body mass index and waist circumference has been reported from human clinical trials where high-dose of EGCG was used[23]. A study on the effects of green tea EGCG supplementation on body weight gain, adipose tissue formation, and related gene expression in high fat diet-induced obese mice showed that 0.2 or 0.5% EGCG supplementation reduced body and adipose tissue weight, and plasma lipids and the expression of key adipogenic and lipogenic genes including PPARγ, C/EBPα, SREBP1c, FABP4, LPL and FAS[24]. In another study, it was reported that 0.5 or 1% EGCG supplementation significantly attenuated fat tissue formation and body weight gain in mice fed high-fat diet[25]. Similarly, EGCG has been shown to inhibit adipogenic differentiation of mouse 3T3-L1 cells by up-regulating phosphorylation of AMPK and its substrate, acetyl-CoA carboxylase[26]. Moreover, green tea EGCG significantly reduces triglyceride accumulation and inhibits the expression of PPARγ and C/EBPα in mouse 3T3-L1 preadipocytes[27].
The beneficial effects of EGCG may be attributed to its ability to inhibit adipogenesis and induce apoptosis, lipolysis and thermogenesis[28-30]. Inhibition of adipogenic differentiation by EGCG may also be attributed to the activation of Adenosine monophosphate-activated protein kinase (AMPK)[27], a conserved serine/threonine kinase which is responsible for energy homeostasis and a novel target for obesity and its related chronic disorders[27,30,31]. (-)-Epigallocatechin gallate exerts its anti-adipogenic effect by up-regulating the expression of key WNT/β-catenin signaling pathway related genes such as low density lipoprotein receptor-related protein (LRP) 5 and 6, disheveled (DVL) 2 and 3, and down-regulating key adipogenic genes, such as PPARγ, C/EBPα, FABP4, LPL, and FAS[32]. Interestingly, β-catenin siRNA reversed the anti-adipogenic effects of EGCG in 3T3-L1 preadipocytes[33], consolidating the notion that EGCG inhibits adipogenesis through WNT/β-catenin dependent pathway.
Consistent with previous findings, body and adipose tissue and liver weights and body weight to adipose tissue weight ratio were significantly reduced in chickens fed a diet supplemented with 1% green tea powder. Reduction of adipose tissue weight could be attributed to either increased lipolysis or reduced lipolytic activities. Additionally, treatment of preadipocytes with different GTEs differentially inhibited adipogenesis and the expression of key adipogenic transcripts in chicken preadipocytes. Whereas the expression of C/EBPα and C/EBPβ were significantly reduced in cells treated with all GTEs, expression of FABP4 was reduced in cells treated with extracts from butane, methane and water only. Expression of PPARγ² was not affected in any treatments. Recently, we have shown that C/EBPα, C/EBPβ and FABP4 expression was associated with oleic acid induced adipogenic differentiation of chicken preadipocytes whereas the expression of PPARγ² was not affected[34]. However, this inconsistent anti-adipogenic effect due to different GTEs is not clearly understood. Therefore, identification of bioactive compounds in each extract would help to understand the underlying mechanism.
Conclusion
Results of this study indicated that green tea has the potential to reduce adipogenesis and could be an important adjunct in the control of obesity in chickens.
Competing Interests:
The authors declare no conflict of interest.
References
- 1. Hocking, P.M. Unexpected consequences of genetic selection in broilers and turkeys: problems and solutions. (2014) Br Poult Sci 55(1): 1-12.
Pubmed || Crossref || Others - 2. Renema, R.A., Robinson, F.E. Defining normal: comparison of feed restriction and full feeding of female broiler breeders. (2004) World's Poult Sci J 60(4): 508-522.
Pubmed || Crossref || Others - 3. Richards, M.P., Rosebrough, R.W., Coon, C.N., et al. Feed intake regulation for the female broiler breeder: In theory and in practice. (2010) J Applied Poult Res 19(2): 182-193.
Pubmed || Crossref || Others - 4. Robinson, F.E., Classen, H.L., Hanson, J.A., et al. Growth performance, feed efficiency and the incidence of skeletal and metabolic disease in full-fed and feed-restricted broiler and roaster chickens. (1992) J Applied Poult Res 1(1): 33-41.
Pubmed || Crossref || Others - 5. Yu, M.W., Robinson, F.E. The application of short-term feed restriction to broiler chicken production: a review. (1992) J Applied Poult Res 1(1): 147-153.
Pubmed || Crossref || Others - 6. Boekholt, H.A., van der Grinten, P., Schreurs, V.V., et al. Effect of dietary energy restriction on retention of protein, fat and energy in broiler chickens. (1994) Br Poult Sci 35(4): 603-614.
Pubmed || Crossref || Others - 7. Urdaneta-Rincon, M., Leeson, S. Quantitative and qualitative feed restriction on growth characteristics of male broiler chickens. (2002) Poult Sci 81(5): 679-688.
Pubmed || Crossref || Others - 8. Fouad, A.M., El-Senousey, H.K., Yang, X.J., et al. Dietary L-arginine supplementation reduces abdominal fat content by modulating lipid metabolism in broiler chickens. (2013) Animal 7(8): 1239-1245.
Pubmed || Crossref || Others - 9. Rebolé, A., Rodríguez, M.L., Ortiz, L.T., et al. Effect of dietary high-oleic acid sunflower seed, palm oil and vitamin E supplementation on broiler performance, fatty acid composition and oxidation susceptibility of meat. (2006) Br Poult Sci 47(5): 581-591.
Pubmed || Crossref || Others - 10. Skrivan, M., Skrivanová, V., Marounek, M., et al. Influence of dietary fat source and copper supplementation on broiler performance, fatty acid profile of meat and depot fat, and on cholesterol content in meat. (2000) Br Poult Sci 41(): 608-614.
Pubmed || Crossref || Others - 11. Velasco, S., Ortiz, L.T., Alzueta, C., et al. Effect of inulin supplementation and dietary fat source on performance, blood serum metabolites, liver lipids, abdominal fat deposition, and tissue fatty acid composition in broiler chickens. (2010) Poult Sci 89(8): 1651-1662.
Pubmed || Crossref || Others - 12. Chen, Z., Zhu, Q.Y., Tsang, D., et al. Degradation of green tea catechins in tea drinks. (2001) J Agric Food Chem 49(1): 477-482.
Pubmed || Crossref || Others - 13. Bose, M., Lambert, J.D., Ju, J., et al. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. (2008) J Nutr 138(9): 1677-1683.
Pubmed || Crossref || Others - 14. Lu, C., Zhu, W., Shen, C.L., et al. Green tea polyphenols reduce body weight in rats by modulating obesity-related genes. (2012) PLoS One 7(6): e38332.
Pubmed || Crossref || Others - 15. Rains, T.M., Agarwal, S., Maki, K.C. Antiobesity effects of green tea catechins: a mechanistic review. (2011) J Nutr Biochem 22(1): 1-7.
Pubmed || Crossref || Others - 16. Wolfram, S., Wang, Y., Thielecke, F. Anti-obesity effects of green tea: from bedside to bench. (2006) Mol Nutr Food Res 50(2): 176-187.
Pubmed || Crossref || Others - 17. Lin, J., Della-Fera, M.A., Baile, C.A. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. (2005) Obes Res 13(6): 982-990.
Pubmed || Crossref || Others - 18. Wu, B.T., Hung, P.F., Chen, H.C., et al. The apoptotic effect of green tea (-)-epigallocatechin gallate on 3T3-L1 preadipocytes depends on the Cdk2 pathway. (2005) J Agric Food Chem 53(14): 5695-5701.
Pubmed || Crossref || Others - 19. Huang, J., Zhang, Y., Zhou, Y., et al. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. (2013) J Agric Food Chem 61(36): 8565-8572.
Pubmed || Crossref || Others - 20. Matsubara, Y., Sato, K., Ishii, H., et al. Changes in mRNA expression of regulatory factors involved in adipocyte differentiation during fatty acid induced adipogenesis in chicken. (2005) Comp Biochem Physiol A Mol Integr Physiol 141(1): 108-115.
Pubmed || Crossref || Others - 21. Statistics Analysis System (SAS) Institute version 9.4.
Pubmed || Crossref || Others - 22. Richards, M.P., Rosebrough, R.W., Coon, C.N., et al. Feed intake regulation for the female broiler breeder: In theory and in practice. (2010) J Applied Poult Res 19(2): 182-193.
Pubmed || Crossref || Others - 23. Chena, I., Chia-Yu, L., Jung-Peng, C., et al. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. (2016) Clin Nutr 35(3): 592–599.
Pubmed || Crossref || Others - 24. Lee, M.S., Kim, C.T., Kim, Y. Green tea (-)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. (2009) Ann Nutr Metab 54(2): 151-157.
Pubmed || Crossref || Others - 25. Klaus, S., Pültz, S., Thöne-Reineke, C., et al. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. (2005) Int J Obes (Lond) 29(6): 615-623.
Pubmed || Crossref || Others - 26. Hwang, J., Park, I., Shin, J., et al. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. (2005) Biochem Biophys Res Commun 338(2): 694-699.
Pubmed || Crossref || Others - 27. Chan, C.Y., Wei, L., Castro-Muñozledo, F., et al. (-)-Epigallocatechin-3-gallate blocks 3T3-L1 adipose conversion by inhibition of cell proliferation and suppression of adipose phenotype expression. (2011) Life Sci 89(21-22): 779-785.
Pubmed || Crossref || Others - 28. Söhle, J., Knott, A., Holtzmann, U., et al. White Tea extract induces lipolytic activity and inhibits adipogenesis in human subcutaneous (pre)-adipocytes. (2009) Nutr Metab (Lond) 6: 20.
Pubmed || Crossref || Others - 29. Lin, J., Della-Fera, M.A., Baile, C.A. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. (2005) Obes Res 13(6): 982-990.
Pubmed || Crossref || Others - 30. Yang, X., Yin, L., Li, T., et al. Green tea extracts reduce adipogenesis by decreasing expression of transcription factors C/EBPα and PPARγ. (2014) Int J Clin Exp Med 7(12): 4906-4914.
Pubmed || Crossref || Others - 31. Luo, Z., Saha, A.K., Xiang, X., et al. AMPK, the metabolic syndrome and cancer. (2005) Trends Pharmacol Sci 26(2): 69-76.
Pubmed || Crossref || Others - 32. Jones, R.G., Plas, D.R., Kubek, S., et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. (2005) Mol Cell 18(3): 283-293.
Pubmed || Crossref || Others - 33. Lee, H., Bae, S., Yoon, Y. The anti-adipogenic effects of (-)epigallocatechin gallate are dependent on the WNT/beta-catenin pathway. (2013) J Nutr Biochem 24(7): 1232-1240.
Pubmed || Crossref || Others - 34. Regassa, A., Kim, W.K. The effects of oleic acid and chicken serum on the expression of adipogenic transcription factors and adipogenic differentiation in hen preadipocytes. (2013) Cell Biol Int 37(9): 961-973.
Pubmed || Crossref || Others