Quantification of Free and Bound Phenolics in Bio-Waste Pomegranate Peel and Formulation of Punicalagin Rich Rice Extruded Snacks
Citation
Robin, J., et al. Quantification of Free and Bound Phenolics in Bio- Waste Pomegranate Peel and Formulation of Punicalagin Rich Rice Extruded Snacks. (2017) Int J Food Nutr Sci 4(2):98- 104.
Copy rights
© 2017 Robin, J., et al. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Pomegranate peel; Free and bound phenolics; Antioxidant analysis; Extruder snacks; HPLC
Abstract
Free and bound phenolics were studied in pomegranate peel and incorporation of punicalagin in rice extruder snacks. Total phenolic content was calculated higher in pomegranate phenolic incorporated rice extruded snacks in free or soluble form as 97.62 mg GAE/g and in residual bound form as 92.56 mg GAE/g. It seems to be higher because of the total of phenolics of rice and pomegranate peel. Similarly, higher value of total flavonoids content also seen in its rice extruder snacks. In DPPH test, the IC50 value of free and bound phenolics of fresh pomegranate peel were 7.05 and 4.95 μg/mL, respectively. HPLC analysis showed the presence of punicalagin in pomegranate peel extract and was also present in extruded snacks in its free (0.01 mg/g) and bound (0.09 mg/g) forms. Other phenolics like p-coumaric acid (1.28 mg/g), gallic acid (0.08 mg/g), cinnamic acid (0.48 mg/g) and quercetin (0.06 mg/g) present in bound extracts of extruders product. The higher values explicit the utilization of pomegranate peels as a source of antioxidants in food.
Introduction
Pomegranate (Punica granatum L.) is an edible fruit receiving interest due to its increased medicinal benefits[1,2]. The pomegranate originates in the Middle East and India, belongs to the family Punicaceae is an important fruit of tropical and subtropical regions. Pomegranate is a good source of many vitamins, pectin, carotenoids and bioactive phytochemicals such as tannins and other phenolics[3,4]. It works as a whole fruit such as juice, peel and seeds composed of valuable compounds. Numerous studies have been published that the pomegranate peel possessed highest antioxidant activity among the peel, seeds and pulp[3]. Large variety of different phytochemicals compounds are also reported in pomegranate peel. Ellagic acid, gallic acid, punicalagins as gallotannins are the potent antioxidant in peels which exhibits antivirus, anticancer, anti-inflammatory and antihypertensive activities[5,6,7]. Various studies indicated the health benefits of phenolic compounds on human health and also considered as a free radical scavenging capabilities[8]. Thus, researchers faced towards the different phenolic compounds because of the general health benefits and therapeutic properties of the antioxidants[9]. These previous researches showed that pomegranate peels phenolic compounds could be used as a bioactive ingredient in the food product.
Phenolic compounds in plants exist either in the free or in the bound form. Generally, proanthocyanidins or flavonoids are considered as the free phenolic compounds which are easily extracted by the solvent, while the bound phenolic compounds are ester-linked to cell-wall polymers and cannot extracted into organic solvents[10]. It is been investigated that about 4 - 57% of the phenolic compounds present in fruits existed in their bound forms which are not extracted by any solvent extraction method[11].
Recent studies have been focused on reincarnation of the utilization of fruits byproducts into useful products because of loss of valuable components during processing[12]. It also has been reported that these fruit byproducts are rich source of antioxidant poly phenols[13]. Pomegranate peels representing up to 40% of the whole fruit as the main byproduct of pomegranate processing industries after production of pomegranate juice. This results a major waste disposal problem for the industry[14]. In spite of high nutritional value in pomegranate peel it is not been used properly as a valuable food product. Alteration of fruit’s byproducts into high value food product with the addition of polyphenols has recently raised the attention of researchers since they have the ability to scavenge the free radicals, i.e. antioxidant power[15,16]. Consumption of higher level of plant phytochemicals has been associated with health benefits, such as reduction in the incidence of hypertension, atherosclerosis and various forms of cancer[17]. Extraction is the key step for the isolation of antioxidants. Hence, free phenolics can be extracted by using a solvent or mixture of solvents[18]. Alkaline, acidic or enzymatic hydrolysis methods can be used to release bound phenolic compound. In most of the studies conducted in this area, alkaline hydrolysis method is used to release the bound phenolic compounds[19].
To the best of our knowledge, data regarding free and bound phenolics of pomegranate peel in food product are very less. As far as we know, there are no data in the literature about free and bound phenolics of pomegranate peel in extruded product. Hence, the free and bound phenolics were determined in fresh pomegranate peel and in incorporated extruder food product. As rice also contain some phenolic content, hence net phenolics results higher in incorporated extruded product. The objective of this research was to utilize the pomegranate peel in the extruded product to increase the nutritional value of food product. In addition, analyze the restructuring of free and bound phenolics present in the pomegranate peel by mild alkaline hydrolysis. Finally, compare it with the enriched rice flour made extruded product with pomegranate peel as a model example to evaluate the restructured phenolics.
Materials and Methods
Plant material
Ripened pomegranate was purchased from the local market of Palampur, (H.P, India). Peels were separated from the pulp manually and washed under tap water to remove the dirt particles. Rinsed peels collected were cut into pieces and dried at 50°C in the hot air (Macro Scientific Work, Pvt. Ltd. India) and crushed into powder using a grinder (Philips Electronics, India) for further analysis.
Reagent and chemicals
All the reagents including ethanol, hydrochloric acid, sodium hydroxide, hexane, diethyl ether and ethyl acetate were purchased from Himedia laboratories, Mumbai. The compounds like 2, 2′-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid were purchased from Merck India, Mumbai. HPLC grade acetonitrile, methanol and phenolic standards like catechin, p-coumaric acid, pro catechuic acid, punicalagin, kaempferol, gallic acid, chlorogenic acid and quercetin standards were obtained from Sigma-Aldrich, Mumbai.
Extruder food product formulation
Extruded rice snacks were prepared under controlled moisture conditions by adding rice flour (95%) and pomegranate peel (5%). The product was made using single screw Brabender extruder with temperature controlled in three zones at 130°C, 160°C and 190°C. A screw diameter of 3 mm and die speed of 120 rpm. The final product was crushed before analysis.
Extraction of free phenolics
Extract from fresh peel powder and extruder food product was prepared according to the protocol of Li et al.[20] with slight modifications. Both the samples were extracted twice with 60% ethanol followed by overnight shaking at room temperature and homogenized. Supernatant containing free phenols was taken out and concentrated using a rotary-evaporator (Labconco, India) at 40°C followed by lyophilization (Buchi, Switzerland) reported byVerardo et al.
Extraction of bound phenolics
Residual part obtained from free phenolic extraction process was digested with 300 mL of 4 M sodium hydroxide at room temperature for 4 h by continues shaking under nitrogen gas atmosphere as reported by[21]. The mixture was followed by acidification with hydrochloric acid (pH- 2-3) in a cold condition and extracted using 200 mL hexane repeatedly three times for the removal of lipids. Again the final solution was treated with 100 mL of 1: 1 diethyl ether: ethyl acetate (v/v). The organic layer was pooled out and evaporated to dryness in a rotary evaporator at 40°C followed by lyophilization (figure 1).
Figure 1: Free and bound phenolics extraction process.
Total phenolics content
Total phenolics content in peel extract was determined by using Folin-Ciocalteu reagent, as described by Joshi et al.[22] with slight modifications. Diluted extracts (500 μL) were introduced into test tubes followed by addition of 1000 μL of Folin- Ciocaulteu reagent. After fifteen minutes of incubation at room temperature (in the dark), 25 μL of saturated sodium carbonate was added. The reaction mixtures were again incubated for half an hour at room temperature in the dark environment. Absorbance was recorded at 760 nm against blank (samples solvent). Gallic acid was taken as the standard employed under the same conditions and concentrations were expressed as mg gallic acid equivalents (GAE) per g extract.
Total flavonoid content
Total flavonoid content was carried out according to the method described by Joshi et al[22]. Diluted extract (1 mL), 0.5 mL of aluminium chloride and 0.5 mL of potassium acetate were added. The reaction mixture was incubated for half an hour at room temperature. Absorbance was recorded at 420 nm against blank (samples solvent). Quercetin can be used as a positive control and it was expressed in terms of standard equivalent (μg mg-1) of extracted compound.
DPPH scavenging activity
Antioxidant activity of pomegranate extract was assessed according to the method of Joshi et al.[22] in terms of radical scavenging ability with slight modifications. Antioxidant activities were determined by reacting the methanol extract of various concentrations (50 - 500 μL) with 1, 1- diphenyl-2-picryl- hydrazyl (DPPH) and kept for 30 min at room temperature in the dark. Absorbance of the samples was determined spectrophotometrically at 517 nm using a kinetic Bio-spectrophotometer (Eppendorf, India). The radical scavenging activity was expressed as % inhibition and was calculated as follows:
Percent (%) inhibition of DPPH activity = [(AB – AS) / AB] x 100
Where, AB and AS are the absorbance of the blank and test sample, respectively.
ABTS radical scavenging assay
The antioxidant capacity was estimated using ABTS solution followed by the method described by Joshi et al[22]. ABTS radical cation substrate working solution was prepared by mixing 2,2′-azinobis-(3-ethyl-benzothiazoline-6-sulphonate) (ABTS•+, 7 mM, 5 mL) with potassium per sulphate solution (88 μL, 140 mM) and the mixture was left to stand in dark at room temperature for 12 - 16 hours before use. ABTS solution is diluted with ethanol to give an absorbance of 0.70 ± 0.05 at 734 nm. Further, the extract (20 μL) was mixed with ABTS+ working solution (980 μL), followed by the incubation in the dark for 10 min at room temperature. Finally, absorbance was read at 734 nm using a kinetic Bio-spectrophotometer (Eppendorf, India). The ABTS radical-scavenging activity of the samples was expressed as:
Percent (%) inhibition of ABTS activity = [(AC – AS) / AC] x 100
Where, AC and AS are the absorbance of the control and test sample respectively.
Hydroxyl radical scavenging (OH) activity
The hydroxyl radical-scavenging activity was measured using the method as described by Ajibola et al.[23]. Extract (25 μL) was mixed with 25 μL of ferrous sulphate (3 mM) and 25 μL of 1, 10-phenanthroline (3 mM, dissolved in 0.1 M PB (pH 7.4). Furthermore, 0.01 % (v/v) hydrogen peroxide (25 μL) was added to initiate the reaction. Mixture was incubated for 1 h at 37°C and signal was measured at 536 nm using a UV/VIS spectrophotometer. Hydroxyl radical-scavenging capacity was calculated according to the following equation:
Hydroxyl radical scavenging activity (%) = [(AS – AB) / AC – AB] x 100
Where, AB, AC and AS are the absorbance of the blank, control and test sample respectively.
HPLC analysis
Phenolic compounds all the extracts including FFP, FBP, PFP and PBP were analyzed using a Shimadzu analytical HPLC with column oven (C40-10ASVP), auto-sampler (SIL-10AF), vacuum solvent degas module model-DGU-20A5 and diode-array detector model-CBM-20A, auto sampler model-SIL-20AC. The mobile phases were (A) 0.1% TFA (tri-floro acetic acid) in water and (B) acetonitrile.
Results and Discussion
Polyphenols were referring to as the secondary metabolites present throughout the plant. Presently, they are classified into bound and non-bound form on the basis of its covalent binding nature. Some are soluble in nature and can be extracted by the solvent extraction methods. While, bound phenols are present in covalently attached form and are extracted by alkaline and acid hydrolysis techniques[21].
Total phenolic and flavonoid content in free and bound fraction Total phenolic and flavonoid content of free and bound fractions in fresh pomegranate peel and in rice extruded extract are shown in Table-1. Higher phenolic content in free fraction (97.6 ± 1.2 mg GAE/g) and bound fraction (92.5 ± 0.8 mg GAE/g) were resulted in extruder snacks because rice itself has a phenolic content in free form as 0.02 mg GAE/g and 0.05 mg GAE/g in bound fraction[24] which was further enhanced by pomegranate peel powder. Similarly, total phenolic in free fraction (58.9 ± 1.2 mg GAE/g) and bound fraction (48.8 ± 1.4 mg GAE/g) content was detected in the fresh pomegranate peel. Total flavonoid content in extruder snack’s free fraction (68.9 ± 0.01 mg QE/g) and in bound part (61.0 ± 0.2 mg QE/g) was observed, which also satisfy the above result of total phenolic content. In case of fresh pomegranate peel extract, total flavonoid content was observed in free fraction (41.8 ± 0.1mg QE/g) and bound (33.7 ± 0.12 mg QE/g). Similar results were also noticed previously on pomegranate peel by Elfalleh et al[25]. It was cleared that phenolics and flavonoids were sustained in the extruder derived product in a satisfactory amount. But still there is a comparative difference between the free and bound parts.
Table 1: Total phenolic, flavonoid profile and hydroxyl radical inhibition of free and bound fractions of pomegranate peel extract and derived extruder product.
| Assays | Fresh pomegranate peel | Derived extruder snacks | ||
|---|---|---|---|---|
| Free | Bound | Free | Bound | |
| Total phenolic content (mg/g) | 58.96 ± 0.03 | 48.82 ± 0.02 | 97.62 ± 1.6 | 92.56 ± 0.8 |
| Total flavonoid content (mg/g) | 41.86 ± 0.10 | 33.73 ± 0.12 | 68.93 ± 0.01 | 61.06 ± 0.2 |
| OH reducing assay (% inhibition) | 54.21 | 42.54 | 96.39 | 87.36 |
*Results were expressed as the averages of triplicates ± standard deviation.
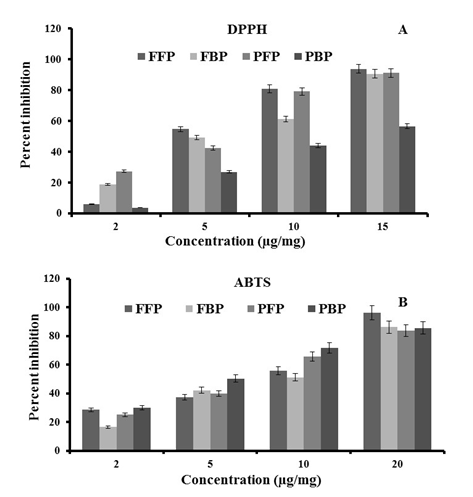
Effect on antioxidant properties The antioxidant scavenging activity was measured using DPPH and ABTS assays by decrease in the absorbance at 517 nm and 734 nm, respectively (Figure-2 and Figure-3). The phenolics present in the extracts were scavenging the free radicals by forming a stable complex with ABTS+ to form ABTS-H. IC50 calculated for ascorbic acid for DPPH (3.1 ± 0.02 μg/mL) and ABTS (3.0 ± 0.04 μg/mL) were used as a standard. Using DPPH, lower IC50 was noticed in bound extract of rice extruder snacks (4.9 ± 1.12 μg/mL) compared to free extract (7.9 ± 1.10 μg/mL). Similarly, lower IC50 represented by free fraction of fresh pomegranate peel (6.1 ± 1.05 μg/mL) compared to bound extract (12.4 ± 0.05 μg/mL). Higher radical-scavenging activity was shown by ABTS•+ assay in compare to DPPH assay and showed the variation of IC50 value of pomegranate peel. This study was verified by the previous study of Elfalleh et al.[25] on pomegranate peel extracts. It was also observed from the data that there is not much variation in antioxidant activity but quite higher in extruded snacks made by the incorporation of pomegranate peel. Hence, it promotes the utilization of bio-waste with a yield of healthy food item.
Figure 2: Graph between percent inhibition and concentration of the sample in the presence of A. DPPH and B. ABTS. Fresh free phenolics (FFP), Fresh bound phenolics (FBP), Product free phenolics (PFP), Product bound phenolics (PBP).
Figure 3: Antioxidant activity of fresh and derived food product (DPPH, ABTS and Correlation between phenolics Versus antioxidant).
Correlation between DPPH and ABTS antioxidant assay
Pomegranate peel extract of fresh and extruded food item were taken at a concentration of 2 μg/mL and the per cent inhibition at this concentration ranged between 3.84% and 30.28% for both the used methods including DPPH and ABTS (Table-2). A correlation between these two antioxidant methods was r2 = 0.34.
Table 2: Antioxidant activity and IC50 values of pomegranate peel extract using DPPH and ABTS assays.
| Samples | IC50 of radical scavenging activity (μg/mL) | Percent inhibition | ||
|---|---|---|---|---|
| DPPH | ABTS | DPPH | ABTS | |
| FFP | 6.12 ± 1.05 | 6.01 ± 1.05 | 27.06** | 25.21 |
| FBP | 12.47 ± 0.05 | 3.72 ± 2.05 | 3.84 | 30.28 |
| PFP | 7.05 ± 1.10 | 2.0 ± 1.62 | 8.02 | 28.34 |
| PBP | 4.95 ± 1.12* | 9.13 ± 2.10 | 18.18 | 16.52 |
Fresh free phenolics (FFP), Fresh bound phenolics (FBP), Product free phenolics (PFP), Product bound phenolics (PBP).
*Results were expressed as the averages of triplicates ± standard deviation.
**Inhibition at concentration 2.0 μg/mL
Correlation between phenolic content and antioxidant activity
A decent correlation was also understood in between the total phenolics to the ABTS and DPPH scavenging activities. The antioxidant activity of pomegranate peel extracts obtained by both DPPH and ABTS methods correlated well with the contents of total phenolics. Linear correlations obtained between total phenolics and antioxidant capacity of two methods were r2DPPH = 0.85 and r2ABTS = 0.76.
Effect on OH-radical scavenging activity
In this study scavenging activity of hydroxyl radical of all extracts were calculated and compared with present amount in 1 mg/mL extract. The highly reactive hydroxyl radical can make oxidative damage to DNA and proteins. Therefore, it is very essential to determine good scavenger molecules. All the free and bound extracts of fresh and extruder product revealed a strong concentration dependent scavenging capacities for the hydroxyl radical. Free phenolic part of derived extruder snacks were found to be the most dominant scavenger of the hydroxyl radical, with an inhibition of up to 96.3% at a concentration of 1 mg/mL and 87.3% inhibition observed in its bound form. Free phenolics in fresh pomegranate peel extract showed an inhibition of 54.2% at 1 mg/mL and in its bound form it was 42.5%. Results exemplified the scavenging ability of hydroxyl radical was increased in extruder food product and a large proportion has been incorporated in its intact form. Such results are correlated with the study performed by Bhandary et al.[26] on pomegranate peel, seed and whole fruit and also verified its hydroxyl radical scavenging property in a large extend.
Estimation of ascorbic acid using HPLC
Current research revealed that ascorbic acid is a building block of collagen, structural material for bone, skin and blood vessels. Hence, ascorbic acid content was determined by HPLC analysis (Figure 4). It was observed that ascorbic acid in the fresh pomegranate peel was 0.5 mg/g which was lower than bound form (0.6 mg/g). Similarly, in rice extruder snacks, it was estimated 1.5 mg/g in free and 0.63 mg/g in its bound form. From the results, it was verified that the amount of ascorbic acid was quite high in extruder snacks in its free and bound form and proved its enrichment of ascorbic acid in extruder snacks. Similar study was performed by Barros et al.[27] in which commercially enriched juice was made by pomegranate peel where amount of ascorbic acid was resembled from our current study and supports our present results.
Figure 4: HPLC chromatogram (a. Standard mixture, b. FFP, c. FBP, d. FBP, e. PFP, f. PBP, A. Gallic acid, B. p-Coumaric acid, C. Procatechuic acid, D. Caffeic acid, E. Catechin, F. Quercetin, G. Punicalagin).
Estimation of phenolics using HPLC
Identification of phenolic compounds was made through peak assignment in free and bound extracts of pomegranate peel and pomegranate peel + rice extruder snacks through HPLC analytical method. Results were made by comparing phenolics standard mixture retention time and area under peaks to the free and bounds extract of fresh pomegranate peel and extruder snacks. In free and bound extract of fresh pomegranate and incorporated extruder snacks showed a good amount of phenolics. Four phenolic compounds including p-coumaric acid, gallic acid, cinnamic acid and punicalagin were present in all the samples in a comparative good amount. On the other hand, quercetin, procatechuic acid and catechin were varied in all the fractions as mentioned in Table 3. Quercetin was present in free extruder snacks extract (0.08 mg/g) and bound extruder snacks extract (0.06 mg/g) but not detected in the fresh pomegranate peel extracts. Catechin present in pomegranate peel extract in free form is (0.04 mg/g) and in bound form is (0.03 mg/g) but results absent in extruder snacks. P-coumaric acid present in a good amount as 1.42 mg/g, 1.12 mg/g, 1.06 mg/g, 1.28 mg/g in free, bound pomegranate peel and extruded extract, respectively. One of the widely known phenolic compounds in pomegranate named punicalagin[28] showed its presence in the pomegranate peel rich rice extruder snacks in a good amount. Finally, procatechuic acid is present in all the results except in bound fraction of rice extruded snacks.
Table 3: HPLC quantification of free and bound phenolics and ascorbic acid content in fresh pomegranate peel and its derived extruder product.
| Extracts | Phenolics standards (mg/g) | Ascorbic acid (μg/mg) | ||||||
|---|---|---|---|---|---|---|---|---|
| p-Coumaric acid | Punicaligin | Quercetin | Procatechuic acid | Catechin | Gallic acid | Cinnamic acid | ||
| FFP | 1.42 ± 0.01 | 0.40 ± 0.05 | 0.11 ± 0.01 | 0.03 ± 0.02 | nd | 0.07 ± 0.06 | 0.04 ± 0.01 | 0.52 ± 0.01 |
| FBP | 1.12 ± 0.05 | 0.11 ± 0.07 | 0.08 ± 0.03 | 0.10 ± 0.05 | nd | 0.07 ± 0.01 | 0.03 ±0.02 | 0.67 ± 0.05 |
| PFP | 1.06 ± 0.05 | 0.13 ± 0.02 | 0.05 ± 0.07 | 0.01 ± 0.05 | 0.08 ± 0.02 | 0.07 ± 0.05 | nd | 1.59 ± 0.02 |
| PBP | 1.28 ± 0.12 | 0.08 ± 0.05 | 0.48 ± 0.01 | 0.09 ± 0.01 | 0.06 ±0.13 | nd | nd | 0.63 ± 0.05 |
Fresh free phenolics (FFP), Fresh bound phenolics (FBP), Product free phenolics (PFP), Product bound phenolics (PBP).
*Values are the mean of three replicates ± standard deviation, values with common letters in each column do not differ statistically according to Duccans’ Multiple Range Test at p ≤ 0.01; **nd = Not detected
Phenolic compounds were already reported in all the parts of pomegranate fruit[28] which showed its wide importance. Current study fulfills the concept of employing the incorporation of pomegranate peel extract which covers the maximum proportion of pomegranate fruit.
Conclusion
The results obtained demonstrated that pomegranate peel extracts have the good total phenolic content and antioxidant activity. There was a good correlation between total phenol content and antioxidant activity that support the idea of phenols as contributor of the antioxidant power of plants extracts. This also suggests that pomegranate peel extracts might be explored as a viable source of potent antioxidants.
Acknowledge:
We express our gratitude to the Director, Institute of Himalayan Bioresource Technology (CSIR), for valuable suggestions and encouragement. Authors acknowledge financial assistance received from CSIR networked project “Processes and products from Himalayan region and their toxicological evaluation (PROMOTE)” [BSC-0213].
Conflict of interest:
The authors report no conflicts of interest.
References
- 1. Radwan, S.F., Mohamed, S., Sekina, A.L. Phytochemical screening and polyphenol constituents of pomegranate peels and leave juices. (2014) Landmark Res. J. Agric. Soil Sci 2: 086-093.
Pubmed || Crossref || Others - 2. Bhowmik, D., Gopinath, H., Kumar, B.P., et.al. Medicinal uses of Punica granatum and its health benefits. (2013) J Pharmacognosy Phytochem 1(5): 2278-4136.
Pubmed || Crossref || Others - 3. Wang, Z., Pan, Z., Ma, H., et. al. Extract of phenolics from pomegranate peels. (2011) Open Food Sci J 5(1): 17-25.
Pubmed || Crossref || Others - 4. Spigno, G., Tramelli, L., De Faveri, D.M. Effect of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. (2007) J. Food Eng 81(1): 200-208.
Pubmed || Crossref || Others - 5. Mavlyanov, S.M., Islambekov, S.Y., Karimdzhanov, A.K., et.al. Polyphenols of the fruits of some varieties of pomegranate growing in Uzbekistan. (1997) Chem. Nat. Compd 81: 98-99.
Pubmed || Crossref || Others - 6. Reddy, M.K., Gupta, S.K., Jacob, M.R. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions ellagitannins and phenolic acids from Punica granatum L. (2007) Planta Medica 73(5): 461- 467.
Pubmed || Crossref || Others - 7. Syed, D.N., Chamcheu, J.C., Adhami, V.M., et. al. Pomegranate extracts and cancer prevention: Molecular and cellular activities. (2013) Anticancer Agents Med. Chem 13(8): 1149-1161.
Pubmed || Crossref || Others - 8. D’Archivio, M., Filesi, C., Vari, R. Bioavailability of the polyphenols: Status and controversies. (2010) Int. J. Mol. Sci 11(4): 1321-1342.
Pubmed || Crossref || Others - 9. Liu, B., Li, W., Hu, L., et.al. Mild alkaline hydrolysis is an efficient and low-cost method for improving the free phenolic content and health benefit of pomegranate peel extract. (2013) J. Food Process. Preserv 37(5): 694-700.
Pubmed || Crossref || Others - 10. Bonoli, M., Marconi, E., Caboni, M.F. Free and bound phenolic compounds in barley (Hordeum vulgare L.) flours. Evaluation of the extraction capability of different solvent mixtures and pressurized liquid methods by micellar electro kinetic chromatography and spectrophotometry. (2004) J. Chromatogr. A 1057(1-2): 1-12.
Pubmed || Crossref || Others - 11. Sun, J., Chu, Y.F., Wu, X., et al. Antioxidant and antiproliferative activities of common fruits. (2002) J. Agric. Food Chem 50(25): 7449-7454.
Pubmed || Crossref || Others - 12. Altan, A., McCarthy, K.L., Maskan, M. Effect of extrusion process on antioxidant activity, total phenolics and β-glucan content of extrudates developed from barley-fruit and vegetable by-products. (2009) Int J Food Sci Tech 44(6): 1263-1271.
Pubmed || Crossref || Others - 13. Balasundram, N., Sundaram, K., Samman, S. Phenolic compounds in plants agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. (2006) Food Chem 99(1):191-203.
Pubmed || Crossref || Others - 14. Kanatt, S.R., Chander, R., Sharma, A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. (2010) Int J Food Sci Tech 45(2): 216-222.
Pubmed || Crossref || Others - 15. Moure, A., Cruz, J.M., Franco, D. Natural antioxidants from residual sources. (2001) Food chem 72(2): 145-171.
Pubmed || Crossref || Others - 16. Mussatto, S.I., Dragone, G., Roberto, I.C. Ferulic and p-coumaric acids extraction by alkaline hydrolysis of brewer’s spent grain. (2007) Ind. Crops Prod 25(2): 231-237.
Pubmed || Crossref || Others - 17. Nara, K., Miyoshi, T., Honma, T., et.al. Antioxidative activity of bound-form phenolics in potato peel. (2006) Biosci Biotechnol Biochem 70(6): 1489-1491.
Pubmed || Crossref || Others - 18. Vichapong, J., Sookserm, M., Srijesdaruk, V. High performance liquid chromatographic analysis of phenolic compounds and their antioxidant activities in rice varieties. (2010) LWT Food Sci Technol 43(9): 1325-1330.
Pubmed || Crossref || Others - 19. Li, Y., Guo, C., Yang, J. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. (2006) Food Chem. 96(2): 254-260.
Pubmed || Crossref || Others - 20. Verardo, V., Arraez-Roman, D., Segura-Carretero, A. Determination of free and bound phenolic compounds in Buckwheat spaghetti by RP-HPLC-ESI-TOF-MS: Effect of thermal processing from farm to fork. (2011) J. Agric. Food Chem 59(14): 7700-7707.
Pubmed || Crossref || Others - 21. Joshi, R., Rana, A., Gulati, A. Studies on quality of orthodox teas made from anthocyanin-rich tea clones growing in Kangra valley, India. (2015) Food Chem, 176(1): 357-366.
Pubmed || Crossref || Others - 22. Ajibola, C.F., Fashakin, J.B., Fagbemi, T.N., et.al. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. (2011) Int. J. Mol. Sci 12(10): 6685-6702.
Pubmed || Crossref || Others - 23. Tian, S., Nakamura, K., Kayahara, H. Analysis of phenolic compounds in white rice, brown rice and germinated brown rice. (2004) J. Agric. Food Chem. 52(15): 4808-4813.
Pubmed || Crossref || Others - 24. Elfalleh, W., Hannachi, H., Tlili, N. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. (2012) J. Med. Plants Res. 6: 4724-4730.
Pubmed || Crossref || Others - 25. Bhandary, B.S.K., Kumari, N.S., Bhat, S.V., et.al. Assessment of antioxidant potential of various extracts of Punica granatum Linn: An in vitro study. (2013) Int. J. Pharm. Chem. Biol. Sci. 2(2): 2277-5005.
Pubmed || Crossref || Others - 26. Barros, Z.M.P., Salgado, J.M., Melo, P.S., et.al. Enrichment of commercially-prepared juice with Pomegranate (Punica granatum L.) peel extract as a source of antioxidants. (2014) J Food Res. 3(6): 1927-0887.
Pubmed || Crossref || Others - 27. Tyagi, S., Singh, A., Bhardwaj, P. Punicalagins-A. large polyphenol compounds found in pomegranates: A therapeutic review. (2012) Academ. J. Plant. Sci 5(2): 45-49.
Pubmed || Crossref || Others - 28. Akhtar, S., Ismail, T., Fraternale, D., et.al. Pomegranate peel and peel extracts: Chemistry and food features. (2015) Food Chem. 174: 417-425.
Pubmed || Crossref || Others