Quantitative and Qualitative Estimation of Three Marketed Tablet Preparations of Ranitidine Hydrochloride, Tiemonium Methylsulfate and Domperidone Commonly Used in Bangladesh
Md. Kamrul Islam , Fahamida Islam, Redwan Hossain, Md. Hassan Kawsar
Affiliation
- 1Department of Pharmacy, State University of Bangladesh, Dhanmondi, Dhaka-1205, Bangladesh
- 2Incepta Pharmaceuticals Ltd, Dewan Idris Road, Zirabo, Savar, Dhaka, Bangladesh
Corresponding Author
Md. Didaruzzaman Sohel, Department of Pharmacy, State University of Bangladesh, Dhanmondi, Dhaka- 1205, E-mail: sohelphr15@gmail.com
Citation
Md. Didaruzzaman Sohel., et al. Quantitative and Qualitative Estimation of Three Marketed Tablet Preparations of Ranitidine Hydrochloride, Tiemonium Methylsulfate and Domperidone Commonly used in Bangladesh. (2017) J Pharm Pharmaceutics 4(1): 128- 132.
Copy rights
© 2017 Md. Didaruzzaman Sohel. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Ranitidine hydrochloride; Tiemonium methylsulfate; Domperidone; In vitro dissolution; Bangladesh.
Abstract
This study was aimed to evaluate the pharmaceutical quality of ranitidine hydrochloride, tiemonium methylsulfate and domperidone tablets manufactured in Bangladesh. Ten samples of Ranitidine Hydrochloride, Tiemonium Methylsulfate and Domperidone solid dosage forms available in Bangladesh drug market were assayed spectrophotometrically and their various physical parameters such as weight variation, friability, disintegration, dissolution and hardness were analyzed according to British Pharmacopoeia (BP) and United States Pharmacopoeia (USP). Among them seven ranitidine, seven timonium and seven domperidone samples were meet the BP specification (95 – 105% of claimed potency). Study revealed that eight ranitidine, ten tiemonium and seven domperidone formulations of different companies meet the USP specification for in vitro dissolution test in first 30 minutes.
Introduction
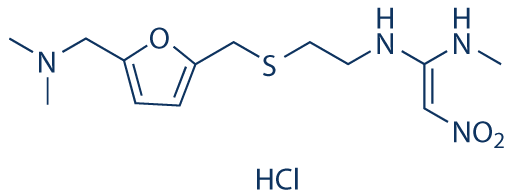
H2-receptor antagonists are also useful in the prevention of stress ulceration and recurrence of gastric and duodenal ulcer. Several H2 antagonists are currently available in the market as cimetidine, ranitidine, famotidine, nizatidine etc[1]. Ranitidine hydrochloride (Figure 1), chemically N, N-dimethyl- 5-[2-(1- methylamine-2-nitrovinyl)-ethylthiomethyl] furfurylamine hydrochloride (Figure 1) is a H2-receptor antagonist and is widely used in short term treatment of duodenal ulcer and in the management of hypersecretory conditions[2]. It is also prescribed in erosive esophagitis, gastric irritation. It is absorbed from upper GIT and has only 50% bioavailability. Colonic metabolism of ranitidine hydrochloride is partly responsible for poor bioavailability of drug. Hence it was thought that formulation residing at absorption window for prolonged period may be a useful approach to enhance bioavailability of ranitidine hydrochloride[3]. It is effective by both parenteral and oral routes of administration[4]. The recommended adult oral dosage of ranitidine is 150 mg twice daily or 300 mg once daily. The effective treatment of erosive esophagitis requires administration of 150 mg of ranitidine 4 times a day. A conventional dose of 150 mg can inhibit gastric acid secretion up to 5 hours but not up to 10 hours[5]. Ranitidine hydrochloride is also used for the treatment of Helicobacter pylori eradication, gastro esophageal reflux disease and erosive esophagitis[6,7]. Ranitidine can be found in many pharmaceutical firms such as tablets, injectable solutions and oral liquids. Peptic ulcer is a common disease worldwide, with an estimated 10% of the population effected. Research has shown that the infection of Helicobacter pylori is a key factor in the occurrence and reoccurrence of peptic ulcer[8,9].
Figure 1: Structure of Ranitidine Hydrochloride.
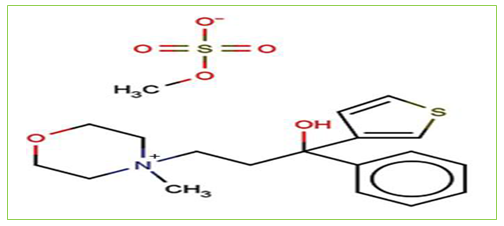
Tiemonium Methylsulfate (Figure 2) is chemically described as 4-(3-hydroxy-3-phenyl-3-(2-thienyl) propyl)-4-methyl morpholinium methylsulfate (salt). It is a quaternary ammonium antimuscarinic agent with peripheral effect similar to those of atropine and is used in the relief of visceral spasms. It prevents the effects of acetylcholine by blocking its binding to muscarinic cholinergic receptors at neuroeffector sites on smooth muscle of GI tract[10].
Figure 2: Structure of Tiemonium Methylsulfate.
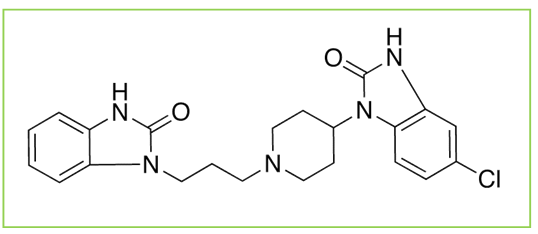
Domperidone (Figure 3), 5-chloro-1-[1-[3-(2, 3-dihydro-2-oxo-1Hbenzimidazol-1-yl)propyl]-4-piperidinyl]-1,3-dihydro-2 Hbenzimidazol-2-one (Figure 3), is a potent dopamine antagonist used for treatment of nausea and vomiting. Domperidone does not cross the blood-brain barrier and therefore has fewer adverse CNS effects than other dopamine antagonists[11].
Figure 3: Structure of Domperidone.
Materials
Ten samples of ranitidine hydrochloride, tiemonium methylsulfate and domperidone solid dosage forms were procured from various drug stores. Samples were properly checked for their Manufacturer name, Physical appearance, and Batch number, Date of manufacturing and Expiry date before purchasing. They were randomly coded, R1 to R10, T1 to T10 and D1 to D10 respectively. Pure ranitidine hydrochloride, tiemonium methylsulfate and domperidone used as reference standards and were collected from Pharmik Laboratories Limited, Chittagong, Bangladesh. Analytical grade hydrochloric acid was used. Distilled water (pH 6.8 ± 0.2 as measured at pH meter (Hanna-HI981.7, USA) was used throughout the work.
Physical Properties of Ranitidine Hydrochloride, TiemoniumMethylsulfate and Domperidone
Weight variation: The weight of tablets represents the net weight of content present per tablet. This test is important because every tablet should have the uniformity of content in order to maintain the dose. In these processes 20 tablets were weighed by a Mettler Toledo B303-S (Switzerland) weighing balance and the average weights were calculated[12].
Hardness test: Tablet hardness has been defined, as the force required breaking a tablet in a diametric compression test. The resistance of the tablets to chipping, abrasion or breakage depends on its hardness. The hardness test of tablet is a non-pharmacopoeia test. This crushing strength of tablets were determined using a Monsanto hardness tester (Campbell Electronics, India) three times and mean value and standard deviation was calculated[13].
Friability test: Tablet hardness is not an absolute indication of strength since some formulations, when compressed into very hard tablets tend to “cap” on attrition i.e. losing their crown portions. Therefore, another measure of a tablet’s strength i.e. its friability is tested. Tablets that tend to powder, chip, and fragment when handled, lack elegance and consumer acceptance. The friability test was performed by a Roche friabilator (Campbell Electronics, India). Ten tablets were weighed and placed in a friabilator and was exposed to rolling for 100 revolutions at a rate of 25 rpm. Then tablets were dedusted and reweighed. Percent of loss of weight is measured. A maximum weight loss of not more than 1% of the weight of the tablets being tested during the friability test[14].
Initial weight – Final weight × 100
Percentage friability = -------------------------------------------
Initial weight
Disintegration time test: The Disintegration Time (DT) of each sample of all 3 brand was determined by using USP standard Tablet DT machine (Indian equipment corporation, India) in simulated gastric fluid for three times and mean value and standard deviation was calculated[15].
In vitro dissolution studies: Simulated gastric medium (pH 1.2) was used as dissolution medium. Media (900 ml of simulated gastric juice) was maintained at 37 ± 0.5°C and rotation speed was set at 50 rpm[16]. Tablet Dissolution tester U.S.P. XXIII (Model- TDT 06, Indian Equipment Corporation, India) was used in our study. After 30 minutes, 5 ml of sample was withdrawn for each sampling. Samples were analyzed spectrophotometrically (UV/VIS Spectrophotometer, UV mini-1240, Shimadzu, Japan) at 314 nm, 214 nm and 286 nm for ranitidine hydrochloride[17], tiemonium methylsulfate[18] and domperidone[19] respectively. Preparation of simulated gastric medium (pH 1.2): 11.4 ml of Hydrochloric acid (32% w/v) was diluted with sufficient water to produce 1000 ml[20].
Potency determination of ranitidine hydrochloride: Transfer 0.125 gm of the sample powder was taken in a volumetric flask. Add 100 ml distilled water and sonicate for three minutes. Then dilute 1 ml to 100 ml with water to obtain final concentration of ranitidine hydrochloride. The diluted solution was filtered and measured the absorbance at 314 nm against ranitidine hydrochloride standard solution and calculates the potency[21].
Potency determination of tiemonium methylsulfate: Initially 0.1 gm tiemonium methylsulfate equivalent powder was taken in a volumetric flask. Then 100 ml distilled water was added. Then 1 ml dilute to 100 ml with water. After filtration then 1 ml of the solution was taken in the absorbance cell and the absorbance was measured at 214 nm. Repeat the whole procedure with standard tiemonium methylsulfate and calculate the potency[22].
Potency determination of domperidone: 0.125 gm of Domperidone equivalent powder was taken in a volumetric flask. Then 100 ml distilled water was added. 1 ml dilute to 100 ml with water. After filtration then 1ml of the solution was taken in the absorbance cell and the absorbance was measured at 286 nm. Repeat the whole procedure with standard tiemonium methylsulfate and calculate the potency[23].
Results and Discussion
Evaluation of Tablets
Weight variation: Weight variations of all thirty samples of 3 brands were determined and found all samples of three brands were satisfied the specification (Table 1)
Table 1: Weight variation of collected samples.
| Code | Average weight (gm) | Code | Average weight (gm) | Code | Average weight (gm) |
|---|---|---|---|---|---|
| R01 | 0.3535 ± 0.002 | T01 | 0.3638 ± 0.002 | D01 | 0.154 ± 0.002 |
| R02 | 0.3012 ± 0.002 | T02 | 0.3521 ± 0.002 | D02 | 0.192 ± 0.002 |
| R03 | 0.2212 ± 0.003 | T03 | 0.3495 ± 0.003 | D03 | 0.103 ± 0.003 |
| R04 | 0.2630 ± 0.004 | T04 | 0.3354 ± 0.004 | D04 | 0.132 ± 0.004 |
| R05 | 0.2460 ± 0.003 | T05 | 0.3070 ± 0.003 | D05 | 0.157 ± 0.003 |
| R06 | 0.3120 ± 0.002 | T06 | 0.3114 ± 0.002 | D06 | 0.213 ± 0.002 |
| R07 | 0.3160 ± 0.003 | T07 | 0.3621 ± 0.003 | D07 | 0.105 ± 0.003 |
| R08 | 0.2770 ± 0.006 | T08 | 0.3102 ± 0.006 | D08 | 0.189 ± 0.006 |
| R09 | 0.3640 ± 0.005 | T09 | 0.3511 ± 0.005 | D09 | 0.152 ± 0.005 |
| R10 | 0.3046 ± 0.005 | T10 | 0.3160 ± 0.005 | D10 | 0.147 ± 0.005 |
Hardness and friability test: These two tests are vital for the tablets to determine the strength. Hardness and friability was determined by all the collected samples and the results are tabulated in the Table 2. It was found in Table 2 that all most all samples were maintained uniform hardness; however one sample of ranitidine hydrochloride (R05) showed much higher hardness than other samples. Others two brands were found good in respect to hardness test. But in friability test a good number of samples were found unsatisfactory (R04, R10, T02, T05, T08, T10, D02, D05, D06 and D09).
Table 2: Hardness and Friability test of collected samples.
| Code | Hardness (kg) | Friability (%) | Code | Hardness (kg) | Friability (%) | Code | Hardness (kg) | Friability (%) |
|---|---|---|---|---|---|---|---|---|
| R01 | 3.5 ± 0.5 | 0.130% | T01 | 4.5 ± 0.1 | 0.092% | D01 | 3.50 ± 0.4 | 0.064% |
| R02 | 3.5 ± 0.2 | 0.156% | T02 | 3.5 ± 0.3 | 2.097% | D02 | 2.50 ± 0.8 | 1.066% |
| R03 | 5.5 ± 0.2 | 0.115% | T03 | 2.5 ± 0.4 | 0.104% | D03 | 2.50 ± 0.1 | 0.118% |
| R04 | 3.7 ± 0.3 | 1.056% | T04 | 3.0 ± 0.1 | 0.098% | D04 | 2.75 ± 0.9 | 0.090% |
| R05 | 8.5 ± 1.5 | 0.111% | T05 | 2.5 ± 0.2 | 1.152% | D05 | 3.00 ± 0.2 | 1.071% |
| R06 | 4.0 ± 0.2 | 0.053% | T06 | 3.0 ± 0.9 | 0.069% | D06 | 1.50 ± 0.1 | 1.051% |
| R07 | 4.5 ± 0.1 | 0.085% | T07 | 3.0 ± 0.4 | 0.201% | D07 | 2.50 ± 0.7 | 0.028% |
| R08 | 5.5 ± 0.2 | 0.138% | T08 | 2.5 ± 0.2 | 3.022% | D08 | 2.75 ± 0.6 | 0.044% |
| R09 | 4.5 ± 0.5 | 0.046% | T09 | 2.5 ± 0.4 | 0.090% | D09 | 2.75 ± 0.5 | 2.093% |
| R10 | 5.6 ± 0.2 | 1.224% | T10 | 3.0 ± 0.2 | 1.110% | D10 | 4.50 ± 0.4 | 0.089% |
Disintegration time and dissolution studies of marketed samples: Disintegration is a vital factor for the release of active ingredient after administration. The active ingredient must be released from the tablet matrix as effectively as possible to allow rapid dissolution. The disintegration time and dissolution studies were thoroughly done with the collected samples and presented in the Table 3. Disintegration times of all the samples were found within the BP range. But dissolution studies showed some unsatisfactory results in two samples of ranitidine (R04 and R06) and three samples of dompiridone (D02, D05 and D08), where sample D02 and D05 were far below than the lower limit as per USP specification (more than 80% drug release, in first 30 minutes).
Table 3: Disintegration time (DT) and Dissolution studies of collected samples.
| Code | DT (min) | % drug release (after 30 min) | Code | DT (min) | % drug release (after 30 min) | Code | DT (min) | % drug release (after 30 min) |
|---|---|---|---|---|---|---|---|---|
| R01 | 7.0 | 85 | T01 | 3.5 | 82 | D01 | 2.0 | 93 |
| R02 | 6.0 | 88 | T02 | 4.5 | 95 | D02 | 2.0 | 65 |
| R03 | 7.0 | 82 | T03 | 4.0 | 99 | D03 | 5.0 | 85 |
| R04 | 6.0 | 78 | T04 | 6.0 | 93 | D04 | 1.0 | 86 |
| R05 | 20 | 83 | T05 | 7.0 | 88 | D05 | 8.0 | 67 |
| R06 | 9.0 | 72 | T06 | 7.0 | 87 | D06 | 4.0 | 82 |
| R07 | 9.0 | 81 | T07 | 5.0 | 98 | D07 | 6.0 | 96 |
| R08 | 10 | 88 | T08 | 5.0 | 91 | D08 | 5.0 | 72 |
| R09 | 6.0 | 84 | T09 | 6.0 | 92 | D09 | 4.0 | 98 |
| R10 | 8.0 | 82 | T10 | 4.0 | 89 | D10 | 3.0 | 85 |
Potency determination: To determine the amount of active ingredient present in the sample, potency determination of all samples were done and the results are given in the Table 4.
Table 4: Potency estimation of the collected samples.
| Code | Potency (%) | Code | Potency (%) | Code | Potency (%) |
|---|---|---|---|---|---|
| R01 | 98.75 | T01 | 93.21 | D01 | 83.16 |
| R02 | 99.02 | T02 | 88.21 | D02 | 88.29 |
| R03 | 101.51 | T03 | 97.54 | D03 | 100.01 |
| R04 | 86.43 | T04 | 100.91 | D04 | 99.47 |
| R05 | 88.94 | T05 | 100.24 | D05 | 96.11 |
| R06 | 99.01 | T06 | 97.44 | D06 | 69.48 |
| R07 | 100.81 | T07 | 68.71 | D07 | 96.33 |
| R08 | 101.27 | T08 | 98.91 | D08 | 101.07 |
| R09 | 89.26 | T09 | 102.08 | D09 | 97.24 |
| R10 | 97.08 | T10 | 99.85 | D10 | 103.42 |
Discussion
From the Table 4, it was found that among the tested samples only one ranitidine sample (R07), two timonium samples (T04 and T05) and one domperidone sample (D03) contained the claimed potency. However all together seven ranitidine samples, seven timonium samples and seven domperidone samples were within the BP specification (95 – 105% of claimed potency), which is 70% of tested brands. All the sub-standard samples were below the lower limit. Among these only one sample (T01) was close to the lower limit and other eight brands (R04, R05, R09, T02, T07, D01, D02 and D06) were far below than the lower limit.
Conclusion
At present 95% of the essential of drugs are produced in Bangladesh. The overall quality of the drugs is satisfactory but some spurious and substandard drugs are also available in the market. As these three drugs are widely used in our country, it should check carefully. The present study, although performed on a limited scale yet on the basis of professional judgement the data reported in this study can help Drug Control Authority to get an idea about the quality status of the marketed ranitidine, timonium and domperidone preparations in Bangladesh.
Acknowledge:
The authors wish to thank, Pharmaceutical Technology Laboratory, Department of Pharmacy, State University of Bangladesh for providing laboratory facilities to carry out the experiments.
Conflict of interest:
The authors declare that they have no conflict of interest.
Funding:
Self funded research work.
References
- 1. Pahwa, R., Sharma, S., Kumar, V., et al. Ranitidine hydrochloride: An update on analytical, clinical and pharmacological aspects. (2016) J Chem Pharm Res 8(7): 70-78.
Pubmed || Crossref || Others - 2. Maslarska, V. Determination of Ranitidine Hydrochloride in Pharmaceutical Preparations by Direct Potentiometriy. (2014) Int J Pharm Pharm Sci 6(1): 538-540.
Pubmed || Crossref || Others - 3. Kotagale, N.R., Parkhe, A.P., Jumde, A.B., et al. Ranitidine Hydrochloride-loaded Ethyl Cellulose and Eudragit RS 100 Buoyant Microspheres: Effect of pH Modifiers. (2011) Indian J Pharm Sci 73(6): 626-633.
Pubmed || Crossref || Others - 4. Pahwa, R., Sharma, S., Kumar, V., et al. Ranitidine hydrochloride: An update on analytical, clinical and pharmacological aspects. (2016) J Chem Pharm Res 8(7): 70-78.
Pubmed || Crossref || Others - 5. Brijesh, S.D., Avani, F.A., Madhabhai, M.P. Gastroretentive drug delivery system of ranitidine hydrochloride: Formulation and in vitro evaluation. (2004) AAPS Pharm Sci Tech 5(2): 77-82.
Pubmed || Crossref || Others - 6. Gharti, K., Thapa, P., Budhathoki, U., et al. Formulation and in vitro evaluation of floating tablets of hydroxypropyl methylcellulose and polyethylene oxide using ranitidine hydrochloride as a model drug. (2012) J Young Pharm 4(4): 201–208.
Pubmed || Crossref || Others - 7. Hagemann, R.C., Threlkeld, D.S. Drug Facts and Comparisons. 50th ed St Louis, MO: Wolters Kluwer Co. (1996) pp1862–1876.
Pubmed || Crossref || Others - 8. Rao, K.R., Prakash, K., Prasad, C.V.N. Bioanalytical method development and validation of ranitidine from plasma using high performance liquid chromatography. (2011) Int J Pharm Pharm Sci 3(2): 219-223.
Pubmed || Crossref || Others - 9. Peden, N.R., Saunders, J.H., Wormsley, K.G. Inhibition of pentagastrin-stimulated and nocturnal gastric secretion by ranitidine, a new H2-receptor antagonist. (1979) Lancet 1(8118): 690–692.
Pubmed || Crossref || Others - 10. Islam, M.S., Wahiduzzaman., Islam, M.S., et al. UV-Spectroscopic Method For Estimation of Tiemonium Methylsulfate 50 Mg Tablet In Bulk and Pharmaceutical Preparations. (2014) IJPSR 5(2): 548-555.
Pubmed || Crossref || Others - 11. Suneetha, G., Venkateswarlu, P., Prasad, P.S.S., et al. Determination of Ilaprazole and Domperidone in Individual Dosage form Tablets by RP-HPLC. (2013) Asian J Chem 25(7): 3989-3992.
Pubmed || Crossref || Others - 12. Lachman, L., Lieberman, H.A., Kanig, J.L. The theory and practice of industrial pharmacy. 3rd ed (1987) pp300.
Pubmed || Crossref || Others - 13. Mullaicharam, A.R., Jameela, A.J.A., Halligudi, N. Evaluation of pharmaceutical equivalents of different brands of ranitidine tablets from multinational brands in Oman. (2012) Int J Nutr Pharmacol Neurol Dis 2(1): 40-44.
Pubmed || Crossref || Others - 14. Marshall, K., Lachman, N., Liberman, H.A. The theory and practice of industrial pharmacy. 3rd ed (1987) pp66–69.
Pubmed || Crossref || Others - 15. Sethi, J.D. Quantitative analysis of drugs in pharmaceutical formulations. 2nd ed (1993) CBS Publishers pp46-47.
Pubmed || Crossref || Others - 16. Emshanova, S.V., Goncharova, N.V., Ivanova, M.E., et al. Use of the "dissolution" test for evaluation of the pharmaceutical equivalence of tablet formulations of phenazepam. (2008) Pharm Chem J 42(1): 48-50.
Pubmed || Crossref || Others - 17. British Pharmacopoeia. (2007) Published on the recommendation of the Medicines Commission, Pursuant to the Medicine Act 1968. London Hermajesty's Stationary Office p2889.
Pubmed || Crossref || Others - 18. Theodore, J., Bradley, A.M. A laboratory manual of qualitative chemical analysis. 4th ed Lea & Febiger, Publishers (2006) p184.
Pubmed || Crossref || Others - 19. British Pharmacopoeia 1988. (1988) Published on the recommendation of the Medicines Commission, Pursuant to the Medicine Act 1968 p61.
Pubmed || Crossref || Others - 20. British Pharmacopoeia 2007. (2007) Published on the recommendation of the Medicines Commission, Pursuant to the Medicine Act 1968 p2531.
Pubmed || Crossref || Others - 21. Jain, S., Srinath, M., Narendra, C., et al. Development of a floating dosage form of Ranitidine hydrochloride by statistical optimization technique. (2010) J Young Pharm 2(4): 342–349.
Pubmed || Crossref || Others - 22. U.S. Pharmacopoeia: The United State Pharmacopoeial Convention. (2012) USP 35 NF 30 1: 880.
Pubmed || Crossref || Others - 23. Patra, S., Sahoo, R., Panda, R.K., et al. In vitro evaluation of domperidone mouth dissolving tablets. (2010) Indian J Pharm Sci 72(6): 822-825.
Pubmed || Crossref || Others