Relationship between the Plasma Levels of Leptin, Adiponectin and TNF-alpha in Diabetic Obesity and Non-Diabetic Obesity in Sohag Governorate, Egypt
Nagwa M. Elsawi1, Ali Taha A. Hassan2, Amira M. Ahmed1 and Hossam El-Din M. Omar3*
Affiliation
- 1Department of Chemistry, Faculty of Science, Sohage University, Sohage 82524, Egypt
- 2Department of Internal Medicine, Faculty of Medicine, Sohag University, Sohag 82524, Egypt
- 33Department of Zoology, Faculty of Science, Assiut University, Assiut 71516, Egypt
Corresponding Author
Hossam El-Din M. Omar, Department of Zoology, Faculty of Science, Assiut University, Assiut 71516, Egypt. E-mail: hossameldin.mo@gmail.com
Citation
Omar, H.M., et al. Relationship between the Plasma Levels of Leptin, Adiponectin and TNF-alpha in Diabetic Obesity and Non-Diabetic Obesity in Sohag Governorate, Egypt. (2015) J Dia Obes 2(1): 24- 27.
Copy rights
© 2015 Hossam El-din, M. O. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License
Keywords
Obesity; Leptin, adiponectin; TNF-α; Type 2 diabetes; Lipid profile
Abstract
Objectives: Obesity is associated with abnormal adipokines production and activation of inflammatory signal pathways in adipose tissue. Also, there is rising facts about the relation between the adiponectin function and pathogenesis of type 2 diabetes. The aim of this study is to investigate the relationship between the levels of leptin, adiponectin, tumor necrosis factor alpha (TNF- α) and lymphocytes count in diabetic and obese diabetic participants and its significance in the etiology of diabetes and obesity in Sohag Governorate.
Methods: 91 unrelated subjects include 34 normal, 49 obese and 8 obese with type 2 diabetes at Hospital of Sohag University were enrolled in this study. Serum leptin, adiponectin and TNF- α levels were measured using ELIZA kit. Fasting and postprandial 2 hours blood glucose level was measured by colorimetric method. Other measurements including blood pressure, BMI and CBR were measured in hospital laboratory.
Results: Both obese and obese diabetic subjects have hyperglycemia and hypertension in comparison with control, however, obese diabetic subjects have higher significant level than obese. Leptin level in plasma was significantly higher in obese and obese with type 2 diabetes subjects than control group, where adiponectin level was lower in these subjects than control group. In obese and obese with type 2 diabetes subjects, both of lymphocytes count and level of TNF- α were higher than control and in obese diabetic than obese non diabetic subjects.
Conclusion: The present results showed in obesity with or without type 2 diabetes serum levels of leptin and TNF- α and lymphocytes count were increased, however, adiponectin level was decreased. Moreover, the high level of TNF- α reflect risk factor for vascular diseases like hypertension.
Introduction
Obesity is carelessly associated with the premature development of atherosclerosis, increased risk of stroke, and development of congestive heart failure (Douketis & Sharma, 2005). Studies have indicated that the adiposity secretes a variety of adipokines involved in energy metabolism, inflammation, and cardiovascular functions such as leptin, adiponectin and TNF (Mohamed et al., 1998; Trayhurn and Wood, 2004). Leptin is an adipocyte-derived hormone that has attracted much attention as a key central and peripheral signal involved in energy homeostasis (Bates and Myers, 2003) by stimulation of glucose uptake and fatty acid oxidation in skeletal muscle to prevent lipid accumulation (Wauters et al., 2002; Minokoshi et al., 2002). LEPR, is a member of the class I cytokine receptor family that is expressed in the hypothalamus (Considine et al., 1996), plays a critical role in the regulation of appetite and metabolism (Zhang et al., 1994) and influence on lipid metabolism. Mutations resulting in a deficient leptin receptor cause obesity and diabetes in animals (Chen et al., 1996) and obesity in humans (Clement et al., 1998), because β- cell may be adversely affected by chronic increased leptin levels (Hukshorn et al., 2004).
From the most prominent adipokines produced by adipocyte is adiponectin. Adiponectin has been shown anti-inflammatory, anti-diabetic and anti-atherogenic effects through its ability to increase insulin sensitivity by regulating fatty acid β oxidization and glucose metabolism (Yamauchi et al., 2002 and 2003). It has been reported that low serum levels of adiponectin is associated with the risk of metabolic syndrome and coronary heart disease (Nakashima et al., 2010; Kajikawa et al., 2011). Tumor necrosis factor alpha (TNF- α) is a polypeptide cytokine produced primarily by mononuclear phagocytes and play a key role in the initiation of the inflammatory response but has relevant effects in many tissues that can regulate many cellular and biological processes such as immune function, cell differentiation, proliferation, apoptosis and energy metabolism (Bazzoni and Beutler, 1996). In adipose tissue TNF- α is produced by macrophages in response to inflammation (Tzanavari et al., 2010) to inhibit tyrosine kinase activity at the insulin receptor level and cause obesity induced insulin resistance (Dixon et al., 2004).
Therefore the present study aimed to assess the relationship between the plasma levels of leptin, adiponectin and TNF- α and lymphocytes counts in diabetic obesity and non-diabetic obesity participants at Sohag Governorate as epidemiological study for human diseases in Egypt.
Materials and Methods
Subjects
Ninety one participants over the age of 20 years were enrolled in study during 2013, 8 obese diabetic, 49 non-obese diabetic and 34 normal control. All subjects were non-somkers and had no clinical evidence of cancer, liver, renal or hematological disease or pregnancy for women. Hypertension and dyslipidemia occurred with type 2 diabetes in 37% (19/51) and 39% (20/51) of the subjects, respectively. Obesity was defined as a BMI of 30 kg/m² or greater. Non-diabetic obese and obese diabetic were selected from Internal Medicine Department in Sohag University Hospital.
Plasma parameters determination
Blood samples were collected in heparinized syringes and placed in pre chilled test tubes containing EDTA. The blood samples were centrifuged at 4°C and plasma was frozen at -80°C for subsequent biochemical analyses in the Biochemistry Laboratory, Faculty of Science, Sohag University. Plasma glucose was measured with the glucose oxidase method by commercial kit from Spectrum Diagnostics, Egyptian Company for Biotechnology. Count of lymphocytes in blood and CBC were performed in Hospital Laboratory, Faculty of Medicine, Sohag University. Serum leptin, adiponectin and TNF- α levels were determined by a kit obtained from Diagnostics Biochem Canada Inc. (London) at the Molecular Biology Unit-Assiut University
Statistics
Statistics was performed using the statistical graph pad prism 5. One way analysis of variables (ANOVA) was used posted by Newman-keuls test. All results are expressed as mean ± SE and the level of significance between groups were *p < 0.05, **p < 0.01, ***p < 0.0001.
Results
The study was conducted on 49 normal obese, 8 obese diabetic patients and 34 control participants. In the present study we have determined the degree of obesity according to BMI law (weight/m²) and the degree of abdominal obesity by Waist Circumference (cm). We found that BMI of obese subjects is 35.61 kg/m² and Waist Circumference 120 cm; however, BMI of obese with type 2 diabetes is 34.13 kg/m² and Waist Circumference 121.0 cm. we observed that both obese and diabetic obese subjects have elevated abdominal obesity than normal individuals. There were no significant differences in Waist Circumference and BMI between the diabetic obese and non-diabetic obese. By measurement the fasting blood glucose level we found that normal individuals had 85.18 mg/dl, obese individuals had 92.04 mg/dl, and obese diabetic patients had fasting blood glucose level 179.3 mg/dl. These data indicate that fasting blood glucose level in obese have significant differences than normal individuals. Also, obese diabetic patients have highly significant elevation in blood glucose than both obese and normal subjects Table1.
Table 1: Clinical and biochemical parameters in the studied groups regarding obesity index, hypertension and hyperglycemia
| Parameters | Control | Non-diabetic Obese | Diabetic obese |
|---|---|---|---|
| Number of subject | 34 | 49 | 8 |
| BMI (kg/m²) | 21.83 ± 0.2428 | 35.61 ± 0.6045***a | 34.13 ± 1.137 |
| Waist circumference (cm) | 84.26 ± 1.105 | 120.0 ± 1.434***a | 121.0 ± 1.732 |
| Systolic blood pressure (mmHg) | 125.1 ± 3.221 | 134.3 ± 2.253*a | 160.0 ± 7.498***b |
| Diastolic blood pressure (mmHg) | 85.18 ± 2.541 | 91.15 ± 1.781*a | 109.8 ± 2.498***b |
| Fasting blood glucose level | 84.32 ± 1.438 | 92.04 ± 1.394*a | 179.3 ± 12.47***b |
| Postprandial 2 hours blood glucose level | 108.8 ± 3.252 | 118.0 ± 2.811 | 257.3 ± 11.78***b |
| HbA1c | 5.118 ± 0.0917 | 5.548 ± 0.088**a | 8.025 ± 0.387***b |
| Leptin/adipnectin ratio | 0.663 ± 0.156 | 4.981 ± 0.403***a | 4.404 ± 0.509 |
Data are expressed as mean ± SE, letter a and b referred to the significant difference between control and non-diabetic obese and between non-diabetic obese and diabetic obese respectively, where the number of asterisk(*) indicates the levels of significant at p < 0.05, 0.01, 0.0001.
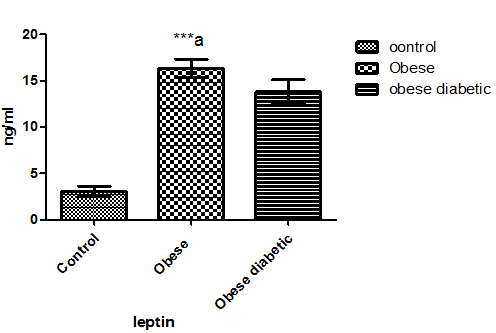
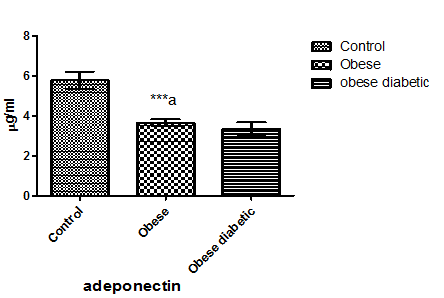
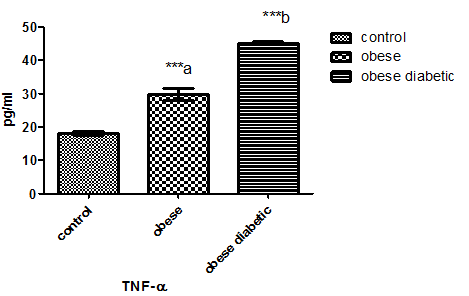
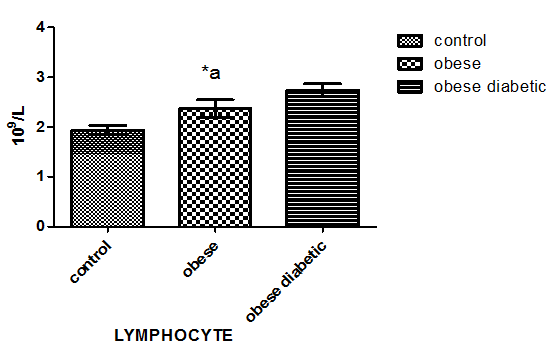
As in Figure 1 leptin level in non-diabetic obese and diabetic obese showed a significant increase in comparison with normal subjects. Plasma adiponectin level in Figure 2 was significantly decreased in both non-diabetic obese and diabetic obese than normal subjects. However, plasma level of TNF- α and lymphocytes count showed a significant increase in non-diabetic obese and diabetic obese in comparison with normal subjects as in Figure 3 and Figure 4 respectively.
Figure 1: Leptin level in plasma of the studied groups. Letter a referred to the significant difference between control and non-diabetic obese at p < 0.0001.
Figure 2: Adiponectin level in plasma of the studied groups. a significant difference between control and non-diabetic obese, b significant difference between non-diabetic obese and diabetic obese, were *p < 0.05, **p < 0.01, ***p < 0.0001.
Figure 3: TNF- α level in plasma of the studied groups. Letter a and b referred to the significant difference between control and non-diabetic obese and between non-diabetic obese and diabetic obese respectively at 0.0001.
Figure 4: Lymphocytes count in blood of the studied groups. Letter a referred to the significant difference between control and non-diabetic obese at p < 0.05.
Discussion
Waist circumference and waist/hip ratio have been used as measures of central obesity and BMI has been used as a measure of general obesity. Moreover, higher percent of type 2 diabetics have central obesity as compared to general obesity (Kamath et al., 2010). In the present study we observed that both non-diabetic obese and diabetic obese subjects have elevated abdominal obesity than normal individuals but there no significant differences in WC and BMI. Moreover, fasting blood glucose level in non-diabetic obese higher than normal individuals and in diabetic obese subjects have highly significant than both non-diabetic obese and normal subjects. Those finding were similar to the result recorded by Hamed et al. (2011). However, Kamath et al. (2010) found that blood glucose levels did not differ significantly between the obese and non-obese individuals in Southern India. Also, we observed that both systolic and diastolic blood pressure has high value in obese subjects than non-obese, and very high value in obese diabetic subjects than normal and obese subject's. In consistence, Vazquez et al. (2007) found a significant association between obesity and blood pressure.
Adipocytokines have clinical implications as useful markers and potential therapeutic targets of glucose, lipid and cardiovascular abnormalities in all age groups. Blood leptin, adiponectin and TNF- α levels may accurately predict the presence of hyperlipidemia and/or diabetes mellitus (Margoni et al., 2011). The present results showed a significant increase in plasma level of leptin and a significant decrease in adiponectin in both non-diabetic obese and diabetic obese in comparison with control subjects. These results were consistence with the previous results obtained by Arita et al. (1999), Hamed et al. (2011), Nayak et al. (2010) who found that blood leptin concentration was significantly increased and adiponectin level was significantly reduced among obese subjects in comparison with lean control subjects. However, Coimbra et al. (2014) found that adiponectin and leptin levels in elderly patients with type 2 diabetes seem to be closely linked to obesity and to the length of the disease. Moreover, adiponectin and leptin levels in type 2 diabetes patients are more associated with obesity and less with diabetes (Neuparth et al., 2013).
The increase in leptin level was positively correlates with BMI (Hardie et al., 1997; Haitao et al., 2014). However, serum adiponectin was negatively correlates with BMI (Yoshida et al., 2005). Hence, Kowalska et al. (2006) suggested that adiponectin could play a role in the pathogenesis of insulin resistance in lean offspring of type 2 diabetic subjects. Moreover, there is significant relationship between serum TNF-α and insulin resistance between diabetic and non–diabetic subjects, but there is no significant relation in obese type 2 diabetes alone (Al-Dhar and Jiffri, 2010). In the present study individuals diagnosed with obesity and type 2 diabetes were characterized by high level of TNF- α and lymphocytes count. These indicate that obese diabetic patients have high inflammation risk than obese patients; also obese subjects have risk of inflammation than normal individual (Lago et al., 2007; Mavridis et al., 2008; Gauthier and Ruderman, 2010). This overproduction of TNF- α by adipose tissue may be explaining the hyper insulinaemia and/or insulin resistances as major determinant of the hypoadiponectinemia in obesity and type diabetes (Assal et al., 2007).
Conclusion
The present results showed in obesity with or without type 2 diabetes plasma levels of leptin and TNF- α and lymphocytes count were increased, however, adiponectin level was decreased. Moreover, the high level of TNF- α reflect risk factor for vascular diseases like hypertension.
Acknowledgments
This work was funded by Academy of Scientific Research and Technology.
References
- 1. Douketis, J.D., Sharma, A.M. Obesity and cardiovascular disease: pathogenic mechanisms and potential benefits of weight reduction. (2005) SeminVasc Med 5(1): 25– 33.
- 2. Mohamed-Ali, V., Pinkney, J.H., Coppack, S.W. Adipose tissue as an endocrine and paracrine organ. (1998) Int J ObesRelatMetab Disord 22(12): 1145- 1158.
- 3. Trayhurn, P., Wood, I.S. Adipokines: inflammation and the pleiotropic role of white adipose tissue. (2004) Br J Nutr 92(3): 347– 355.
- 4. ates, S.H., Myers,Jr, M.G. The role of leptin receptor signaling in feeding and neuroendocrine function. (2003) Trends EndocrinolMetab 14(10): 447- 452.
- 5. Wauters, M., Mertens, I., Rankinen, T., et al.Leptin receptor gene polymorphisms are associated with insulin in obese women with impaired glucose tolerance. (2001) J ClinEndocrinolMetab 86(7): 3227- 3232.
- 6. Minokoshi, Y., Kim,Y.B., Peroni, O.D., et al.Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. (2002) Nature 415(6869): 339- 343.
- 7. Wauters, M., Considine, R.V., Van Gaal, L.F.: Human leptin: from an adipocyte hormone to an endocrine mediator. (2000) Eur J Endocrinol 143(3): 293- 311.
- 8. Zhang,Y., Proenca, R., Maffei, M., et al. Positional cloning of the mouse obese gene and its human homologue. (1994) Nature 372(6505): 425– 432.
- 9. Chen, H., Charlat, O., Tartaglia, L.A., et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. (1996) Cell 84(3): 491- 495.
- 10. Clement, K., Vaisse, C., Lahlou, N., et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. (1998) Nature 392(6674): 398- 401.
- 11. Hukshorn, C.J., Lindeman, J.H.N., Toet, K.H., et al.Leptin and the proinflammatory state associated with human obesity. (2004) J ClinEndocrinolMetab 89(4): 1773– 1778.
- 12. Yamauchi, T., Kamon, J., Minokoshi, Y., et al.Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMPactivated protein kinase. (2002) Nat Med 8(11): 1288– 1295.
- 13. Yamauchi, T., Kamon, J., Waki, H., et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. (2003) J BiolChem 278(4): 2461– 2468.
- 14. Nakashima, R., Yamane, K., Kamei, N., et al. Low serum levels of total and high molecular weight adiponectin predict the development of metabolic syndrome in Japanese-Americans. (2010) J EndocrinolInvest 34(8): 615- 619.
- 15. Kajikawa, Y., Ikeda, M., Takemoto, S., et al. Association of circulating levels of leptin and adiponectin with metabolic syndrome and coronary heart disease in patients with various coronary risk factors. (2011) Int Heart J 52(1): 17– 22.
- 16. Bazzoni, F., Beutler, B. The tumor necrosis factor ligand and receptor families. (1996) New Engl J Med 334(26): 1717– 1725.
- 17. Tzanavari, T., Giannogonosa, P., Karalis, K.P. TNF-alpha and obesity. (2010) CurrDirAutoimmun 11: 145-156.
- 18. Dixon, D., Goldberg, R., Schneiderman, N., et al. Gender differences in TNF-alpha levels among obese vs non obese Latino children. (2004) Eur J ClinNutr 58(4): 696- 699.
- 19. Kamath, A., Shivaprakash, G., Adhikari, P. Body mass index and Waist circumference in Type 2 Diabetes mellitus patients attending a diabetes clinic. (2011)Int J Biol Med Res 2(3): 636- 638.
- 20. Hamed, E.A., Zakary, M.M., Ahmed, N.S., et al.Circulating leptin and insulin in obese patients with and without type 2 diabetes mellitus: relation to ghrelin and oxidative stress. (2011) Diabetes Res ClinPract 94(3): 434- 441.
- 21. Vazquez, G., Duval, S., Jacobs, D.R., et al. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: A meta-analysis. (2007) Epidemiol Rev 29: 115– 128.
- 22. Margoni, A., Perrea, D.N., Vlachos, I., et al. Serum leptin, adiponectin and tumor necrosis factor-α in hyperlipidemic rats with/without concomitant diabetes mellitus. (2011) Mol Med 17(1-2): 36- 40.
- 23. Arita, Y., Kihara, S., Ouchi, N., et al. Paradoxical decrease of an adipocyte specific protein, adiponectin, in obesity. (1999) BiochemBiophys Res Commun 257(1): 79–83.
- 24. Nayak,B.S., Ramsingh, D., Gooding, S., et al. Plasma adiponectin levels are related to obesity, inflammation, blood lipids and insulin in type 2 diabetic and non-diabetic Trinidadians. (2010) Prim Care Diabetes 4(3): 187– 192.
- 25. Coimbra, S., Proença, J.B., Santos-Silva, A., et al.Adiponectin, leptin, and chemerin in elderly patients with type 2 diabetes mellitus: A close linkage with obesity and length of the disease. (2014) BioMed Research International 2014: 1- 8.
- 26. Neuparth, M.J., Proenca, J.B., Santos-Silva, A., Coimbra S (2013). Adipokines, oxidized low-density lipoprotein, and creactive protein levels in lean, overweight, and obese Portuguese patients with type 2 diabetes. (2013) ISRN Obesity 2013: 1- 7.
- 27. Hardie, L., Trayhurn, P., Abramovich, D., et al. Circulating leptin in women: a longitudinal study in the menstrual cycle and during pregnancy. (1997) ClinEndocrinol 47(1): 101- 116.
- 28. Pan, H., Guo, J.,Su, Z. Advances in understanding the interrelations between leptin resistance and obesity. (2014) PhysiolBehav 130: 157– 169.
- 29. Yoshida, H., Hirowatari, Y., Kurosawa, H., et al. Implications of decreased serum adiponectin for type IIb hyperlipidemia and increased cholesterol levels of very low density lipoprotein in in type 2 diabetes patients. (2005) ClinSci 109(3): 297-302.
- 30. Kowalska, I., Czkowski, M.S., Nikołajuk, A., et al. Plasma adiponectin concentration and tumor necrosis factor-a system activity in lean non-diabetic offspring of type 2 diabetic subjects. (2006) Eur JEndocrinol 154(2): 319– 324.
- 31. Al-Dhar, M.H.S., Jiffri, E.H. Adipose tissue expression of TNF-alpha and insulin resistance in obese subjects with type 2 Diabetes mellitus. (2010) World journal of Medical sciences 5(2): 30- 35.
- 32. Lago, F., Dieguez, C., Gَmez-Reino, J., et al. Adipokines as emerging mediators of immune response and inflammation. (2007) Nat ClinPractRheumatol 3(12): 716– 724.
- 33. Mavridis, G., Souliou, E., Symeonidis, G., et al. Inflammatory cytokines in insulin-treated patients with type 2 diabetes. (2008) NutrMetabCardiovasc Dis 18(7):471– 476.
- 34. Gauthier, M.S., Ruderman,N.B. Adipose tissue inflammation and insulin resistance: all obese humans are not created equal. (2010) Biochem J 430(2): 1– 4.
- 35. Assal, H.B., Fath-Allah, M., Elsherbiny, A. Serum leptin and adiponectin in obese diabetic and non-diabetic. (2007) J Med Sci 7(5): 865- 869.