Relative Clinical Heat Transfer Effectiveness: ForcedAir Warming Vs. Conductive Fabric Electric Warming, A Randomized Controlled Trial
Haruko Sugai1*, Tomoya Koizumi1, Shinzou Sumita2, Michiaki Yamakage1
Affiliation
1Department of Anesthesiology, Sapporo Medical University School of Medicine, Sapporo, Japan
2Asahikawa Red Cross Hospital, Asahikawa, Japan
Corresponding Author
Haruko Sugai, M.D, Department of Anesthesiology, Sapporo Medical University School of Medicine, S.1,W.16, CHUO-KU, Sapporo, Japan, Tel: +81-11-688-9663; E-mail: hayashi.haruko@sapmed.ac.jp
Citation
Sagai, H., et al. Relative Clinical Heat Transfer Effectiveness: Forced-Air Warming Vs. Conductive Fabric Electric Warming, A Randomized Controlled Trial. (2018) J Anesth Surg 5(2): 123- 126.
Copy rights
© 2018 Sagai, H. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Patient warming; forced-air warming; conductive warming
Abstract
Study Objective: Forced-air warming (FAW) relies on convection and is limited to the area under a single blanket. Conductive fabric warming (CFW) relies on conductive heat transfer. More important clinically is that CFW can roughly double the body surface area in contact with the heat by using both a blanket over the patient and a heated mattress under the patient. This study is designed to test the hypothesis that doubling the body surface area in conductive contact with heat will improve clinical heat transfer of warming systems.
Design: This study is a prospective randomized, controlled trial with a two group, parallel design.
Patients and setting: We randomized 41 ASA 1 & 2 patients undergoing open GI surgical procedures in the operating room, lasting more than 2 hours and no need for fluid warming.
Interventions: 1) FAW Group; treated with a WarmTouch® upper or lower body blanket. 2) CFW Group; treated with a HotDog® upper or lower body blanket plus an underbody heated mattress. All of the warming blankets and mattress temperatures were set at 39°C. All of the other relevant variables were held constant, including: warming temperature, warming duration, surgical exposure and patient demographics.
Measurements: We recorded the rewarming rate as a surrogate indicator of clinical heat transfer effectiveness.
Results: The FAW Group (n = 20) experienced a warming rate of 0.01°C/hr over 2 hours. The CFW Group (n = 21) experienced a warming rate of 0.35°C/hr over 2 hours. There were no adverse events due to patient warming in either group.
Conclusions: The CFW system showed significantly higher patient warming rates than the FAW system (0.35°C/hr. vs. 0.01°C/hr.), when all other relevant variables were held constant, including warming temperature. Under these controlled conditions, the clinical heat transfer effectiveness of CFW (HotDog®) is significantly greater than FAW (WarmTouch®)
Introduction
Over the past 25 years, forced-air warming (FAW) has become a Standard of Care for most surgical procedures. However, a recent editorial by Hopf[1] commenting on a study by Sun and Sessleret al.[2] noted, "…a critical implication of this study is that current standards and practice routinely lead to intraoperative hypothermia…" Sun et al. reviewed 59,000 surgical cases that had been treated with FAW at the Cleveland Clinic. They reported that 50% of the patients failed to reach 36°C within two hours of induction and 25% failed to reach normothermia within five hours of induction. These results can be characterized as a very high failure rate for a commonly used warming technology.
Given the nearly ubiquitous occurrence of redistribution hypothermia after the induction of anesthesia and the recognized need to minimize "the degree and duration of intraoperative hypothermia," the effectiveness of any warming technology is a critical metric. Sun et al. clearly showed that FAW is marginally effective at rewarming a surgical patient[2]. This is not the first study to report these results. The mean of the mean FAW intraoperative rewarming rates reported in 10 published studies, was only 0.1°C/hr (ranging from a low of -0.125°C/hr to a high of 0.23°C/hr)[3-12].
These studies show that the heat transfer effectiveness of FAW under clinical conditions is limited. Two factors severely limit the heat transfer effectiveness of FAW: 1) The relative inefficiency of convective heat transfer[13] and 2) The relatively small skin surface area in contact with the heat under a single FAW blanket.
Another issue underscores the importance of finding an effective alternative to FAW. The US Centers for Disease Control and Prevention recently issued a warning: "Nothing that blows air should be in an operating theater, if possible[14]."
The HotDog patient warming system, an air-free conductive fabric warming (CFW) solution with electric blankets and mattresses, has a theoretical heat transfer effectiveness advantage in that: 1) It uses relatively efficient conductive heat transfer[13], and 2) The system contains a heated blanket and a heated mattress, nearly doubling the body surface area exposed to the heat. This electric warming system allows simultaneous warming from above and below the patient with a single controller, creating a significant theoretical clinical heat transfer advantage for CFW.
Studies comparing the warming rate with a FAW blanket to a CFW blanket, have shown nearly identical results[15-18]. Studies comparing the warming rate with a FAW blanket to a CFW or electric mattress have also shown nearly identical results[19,20]. No published studies have looked at the comparative warming rates or clinical heat transfer effectiveness when CFW and FAW are used as systems—using both blankets and mattresses for CFW, compared to blankets-only for FAW. This is a comparison of the two warming systems as they are designed to be used. We hypothesize that doubling the body surface area in contact with heat by using CFW will improve clinical heat transfer over FAW.
Materials and Methods
After obtaining approval from the Sapporo Medical University Institutional Review Board and written informed consent from each patient, we prospectively randomized 41 patients undergoing open GI surgical procedures lasting more than 2 hours, into 2 groups: 1) FAW Group (n = 20); treated with a WarmTouch® (Medtronic/Covidien Inc., Minneapolis, MN, USA) upper or lower body blanket. 2) CFW Group (n = 21); treated with a HotDog® (Augustine Temperature Management, LLC, Eden Prairie, MN, USA) upper or lower body blanket plus an underbody heated mattress.
This study is a randomized, controlled trial with a two group, parallel design, to test the hypothesis that doubling the body surface area in contact with heat will improve clinical heat transfer. Patients were randomized by any one of the investigators drawing a card from an opaque envelop with the name of a warming method written on the card. Investigators were not blinded to the therapeutic group during the data collection.
The FAW Group was treated with an upper or lower body blanket that was energized when the patient was "draped." The CFW Group was treated with an upper or lower body blanket plus an underbody heated mattress that was energized when the patient entered the OR per hospital protocol. There were no protocol violations.
The FAW blower and CFW controller were set to 39°C. 39°C is the "medium" temperature for both types of blankets and is the highest temperature allowed for the CFW heated mattress. We relied on the manufacturer for the accuracy of the temperature of the heat output.
Tympanic temperatures (CE Thermo™, Nipro, Tokyo, Japan) were recorded at 15 minute intervals, starting when the blankets were energized (Time = 0). We determined the rewarming rate as a surrogate indicator of clinical heat transfer effectiveness, our primary outcome measure.
All patients received a similar anesthetic that included: propofol, fentanyl, an inhalation agent and Remifentanil®. Administration of anesthesia and fluids was similar according to protocol. Ambient room temperature was held constant. Warming was discontinued if the patient’s temperature exceeded 38°C.
All adult ASA 1 & 2 patients undergoing open GI surgical procedures lasting more than 2 hours, with BMIs between 17 and 30 kg/m2 and no need for fluid warming were eligible. Data were collected in the operating rooms of Sapporo Medical University (Sapporo, Japan).
The patient background data are presented as Mean ± S.D. Statistical significance was determined by the 2-way ANOVA and Bonferoni tests for the comparison between the data of two groups. P < 0.05 was considered as statistically significant. Patients were randomized by any one of the investigators drawing a card from an opaque envelop with the name of a warming method written on the card. Investigators were not blinded to the therapeutic group during the data collection.
Results
Randomization resulted in 20 patients in the FAW Group and 21 patients in the CFW Group. Table 1 show that there were no significant demographic differences between the two groups. Table 1 also shows that there were no significant differences between the two groups in surgical exposure (open vs. laparoscopic) or positioning (supine vs. lithotomy). There were no losses or exclusions after randomization. Age-eligible participants were recruited from 16 years to 90 years. Table 1.
Table 1: Patient demographics and surgical positioning.
| CFW (n = 21)a | FAW (n = 20)b | P-value | |
|---|---|---|---|
| Age (years) | 69 ± 10 | 69 ± 10 | 0.95 |
| BMI (kg/m2)c | 23.5 ± 2.7 | 21.9 ± 2.8 | 0.08 |
| Female/Male | 12/9 | 12/8 | 0.85 |
| ASA-RSd 1/2 | 4/17 | 4/16 | 0.75 |
| Open surgery/ Laparoscopic surgery | 6/15 | 7/13 | 0.65 |
| Supine/ Lithotomy Position | 14/7 | 14/6 | 0.81 |
| Shivering | 0 | 1 | -- |
| Flushing | 1 | 0 | -- |
a: Conductive Fabric Warming
b: Forced-air Warming
c: Body mass index
d: American Society of Anesthesiologists Risk Score
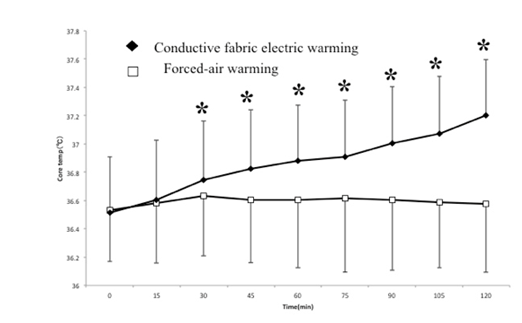
The FAW Group started at 36.53 ± 0.36°C and ended at 36.55 ± 0.49°C after 120 min. of warming—a warming rate of 0.01°C/hr. The CFW Group started at 36.51 ± 0.39°C and ended at 37.20 ± 0.49°C after 120 min. of warming—a warming rate of 0.35°C/hr. The temperature difference between the two groups was statistically significant at each data point after 30 min. (p < 0.05).(Figure. 1)
Figure 1:
On the "medium" 39°C temperature setting, the CFW system showed significantly higher patient warming rates than the FAW system (0.35°C/hr. vs. 0.01°C/hr.), when all other relevant variables were held constant, including warming temperature. Therefore, under these controlled conditions, the clinical heat transfer effectiveness of CFW (HotDog®) system is significantly greater than FAW (WarmTouch®) system. There were no adverse events due to patient warming in either group.
Discussion
The clinical effectiveness of patient warming products is usually reported as a rate of warming. The rate of warming and the resultant patient temperature are the most clinically relevant measurements of effectiveness and are a reflection of the relative clinical heat transfer effectiveness of the patient warming systems. Studies in the past have focused on the rate of warming that results from a FAW blanket compared to a CFW blanket or a FAW blanket compared to a CFW mattress. These narrow comparisons miss the essential point: FAW can only be applied above the patient (blankets only) but the CFW system on the other hand can simultaneously warm from both above and below the patient (blankets and mattresses).
Intraoperative warming rates are influenced by numerous factors including: the warming technology (FAW, CFW, water), the mechanism of heat transfer (convection, conduction radiation, passive), the warming temperature set point, warming start time and duration, the amount and location of the body surface area in contact with the heat, the environmental temperature, surgical exposure and surgery type, patient position and patient demographics.
In order to determine the relative heat transfer effectiveness of FAW vs. CFW systems, we attempted to hold all of the variables listed above constant, except those inherent in the given warming technology. Thus, the independent variables were: FAW used convective heat transfer and exposed the skin surface area under a single warming blanket to this heat. CFW used conductive heat transfer and exposed the skin surface area under a single blanket and a heated mattress to this heat.
Since HotDog CFW mattresses are limited to 39°C, we elected to set the blanket temperatures to 39°C as well. All of the warming blankets and mattresses were at the same temperature. The blankets for both FAW and CFW were not set at their highest temperature setting. Therefore, these results do not represent the highest patient warming rates for each technology. The study objective was to determine the relative heat transfer effectiveness of each warming system, not the highest warming rate.
When all other relevant variables were held constant, CFW showed a clinical heat transfer effectiveness that was significantly greater than FAW, as evidenced by significantly higher patient warming rates (0.35°C/hr. vs. 0.01°C/hr.). This superior heat transfer effectiveness for CFW is due to the combination of conductive heat transfer and the larger surface area of simultaneously heating from above and below the patient.
The superior heat transfer effectiveness of CFW over FAW is consistent with the relative energy efficiencies of these two technologies as reported by Bayazit and Sparrow[13]. These investigators showed that CFW (HotDog®) was 2.3 times more efficient than FAW (WarmTouch®) in heat transfer.
It is axiomatic that FAW cannot change its mechanism of heat transfer. Therefore, it seems that unless more skin surface area can be included under a FAW blanket, this technology may have reached its theoretical limit for heat transfer. In contrast, the CFW system is a new technology that will likely continue to improve its clinical heat transfer capabilities with improved blanket and mattress designs. CFW has an additional advantage because a second blanket can be added to the therapy at no extra cost, for any exceptionally challenging cases.
There is another issue that underscores the importance of finding an effective alternative to FAW: FAW systems have been shown to produce an unintended consequence of disrupting operating room airflow and contaminating the surgical field[21]. The clinical concern is especially severe in implant surgery where a single airborne bacterium can cause an infection. The US Centers for Disease Control and Prevention recently issued a warning: "Nothing that blows air should be in an operating theater, if possible[14]." Identifying an effective air-free alternative is paramount.
Further research will be needed to determine if the greater heat transfer effectiveness and higher warming rates of the CFW system found in this study are reproducible at the higher temperature settings. However, the present data suggest that CFW could "...reduce the degree and duration of intraoperative hypothermia…" as called for by Dr. Hopf, which certainly warrants further investigation.
Financial disclosures: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Clinical trial number: The study registered with the UMIN clinical trial registry (R000021761) on 04/04/2016.
Principal investigator is Haruko Sugai.
References
- 1. Hopf, H.W. Perioperative Temperature Management: Time for a New Standard of Care? (2015) Anesthesiology 122(2): 229-230.
- 2. Sun, Z., Honar, H., Sessler, D., et al. Intraoperative Core Temperature Patterns, Transfusion Requirement, and Hospital Duration in Patients Warmed with Forced Air. (2015) Anesthesiology 122(2): 276-285.
- 3. Negishi, C., Hasegawa, K., Mukai, S., et al. Resistive-heating and forced air warming are comparably effective. (2003) Anesth Analg 96(6):1683-1687.
- 4. Matsuzaki, Y., Matsukawa, T., Ohki, K., et al. Warming by resistive heating maintains perioperative normothermia as well as forced air warming. (2003) Br J Anaesh 90(5): 689-691.
- 5. Bennett, J., Ramachandra, V., Webster, J., et al. Prevention of hypothermia during hip surgery: effect of passive compared with active skin warming. (1994) Br J Anaesth 73(2): 180-183.
- 6. Hynson, J.M., Sessler, D. Intraoperative warming therapies: A comparison of three devices. (1992) J Clin Anesth 4(3): 194-199.
- 7. Kurz, A., Sessler, D., Lenhardt, R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. (1996) N Engl J Med 334(19): 1209-1215.
- 8. Kurz, A., Kurz, M., Poeschl, G., et al. Forced-air warming maintains intraoperative normothermia better than circulating-water mattresses. (1993) Anesth Analg 77(1): 89-95.
- 9. Borms, S.F., Engelen, S.L.E., Himpe, D.G.A., et al. Bair Hugger forced-air warming maintains normothermia more effectively that Thermo-Lite insulation. (1994) J Clin Anesth 6(4): 303-307.
- 10. Kabbara, A., Goldlust, S.A., Smith, C.E., et al. Randomized prospective comparison of forced air warming using hospital blankets versus commercial blankets in surgical patients. (2002) Anesthesiology 97(2): 338-344.
- 11. Camus, Y., Delva, E., Just, B., et al. Leg warming minimizes core hypothermia during abdominal surgery. (1993) Anesth Analg 77(5): 995-999.
- 12. Smith, I., Newson, C., White, P.F. Use of forced-air warming during and after outpatient arthroscopic surgery. (1994) Anesth Analg 78(5): 836-841.
- 13. Bayazit, Y., Sparrow, E.M. Energy efficiency comparison of forced-air versus resistance heating devices for perioperative hypothermia management. (2010) Energy 35: 1211-1215.
- 14. The Healthcare Infection Control Practices Advisory Committee (HICPAC) of the Centers for Disease Control and Prevention. November 2015. Record of the Proceedings.
PubMed||CrossRef||Others
- 15. Kimberger, O., Held, C., Stadelmann, K., et al. Resistive Polymer Vs Forced-air Warming: Comparable Heat Transfer and Core Rewarming Rates in Volunteers. (2008) Anesth Analg 107(5): 1621-1626.
- 16. Brandt, S., Oguz, R., Huttner, H., et al. Resistive-polymer Versus Forced-air Warming: Comparable Efficacy in Orthopedic Patients. (2010) Anesth Analg 110(3): 834-838.
- 17. Tanaka, N., Ohno, Y., Hori, M., et al. A randomized controlled trial of the resistive heating blanket versus the convective warming system for preventing hypothermia during major abdominal surgery. (2013) J Periop Pract 23(4): 82-86.
- 18. Galvao, C.M., Liang, Y., Clark, A.M. Effectiveness of cutaneous warming systems on temperature control: meta-analysis. (2010) J Adv Nurs 66(6): 1196-1106.
- 19. Egan, C., Bernstein, E., Reddy, D., et al. A randomized comparison of intraoperative PerfecTemp and forced-air warming during open abdominal surgery. (2011) Anesth Analg 113(5): 1076-1081.
- 20. Ng, V., Lai, A., Ho, V. Comparison of forced-air warming and electric heating pad for maintenance of body temperature during total knee replacement. (2006) Anaesthesia 61(11): 1100-1104.
- 21. Wood, A.M., Moss, C., Keenan, A., et al. Infection control hazards associated with the use of forced-air warming in operating theatres. (2014) J Hosp Infect 88(3): 132-140.