Release Problems for Nifedipine in the Presence of Soluplus
Cristina Cavallari , Selenia Ternullo , Fabrizio Tarterini
Affiliation
- 1Department FABIT, University of Bologna, Italy
- 2Drug Transport and Delivery Research Group, Department of Pharmacy, University of Tromsø, Norway
- 3Department DIN, University of Bologna, Italy
Corresponding Author
Adamo Fini, Professor, Department FABIT, University of Bologna, Via San Donato 15, 20127 Bologna, Italy, Tel: +00390512095655; Fax: +00390512095652; E-mail: adamo.fini@unibo.it
Citation
Cavallari, C., et al. Release Problems for Nifedipine in the Presence of Soluplus. (2016) J Pharm Pharmaceutics 3(2): 70- 82.
Copy rights
© 2016 Fini, A. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Nifedipine; Soluplus; Co-precipitates; Slow release; Gel forming; Flower-like aggregates; FT-IR; Interaction
Abstract
Nifedipine/soluplus co-precipitates were prepared at 1:9 and 1:1 weight ratios, using the solvent method, with the aim to obtain an improvement of the drug release. The release, though accelerated with respect to physical mixtures and pure drug, resulted slow with respect to similar systems containing soluplus and are characterized by the appearance in suspension of micro-particulates that, at the optical microscope, revealed a large variety of shapes typical of flower-like aggregates, not previously reported. For comparison systems were also prepared with PVP K30 and sucrester P1670 and two mixtures, without any evidence of this last phenomenon. The systems were studied by thermal (differential scanning calorimetry – DSC, thermomicroscopy – HSM) and spectroscopic (SEM, FT-IR, micro-Raman) analysis. Slow release of nifedipine and formation of colloidal micro- particulates were hypothesized due to the gelling tendency of soluplus associated to drug/polymer multi-site-interactions: when one of these parameters is absent in the system the two phenomena could not be observed together, such as in the case of PVP K30 or sucrester P1670, when used as carriers for nifedipine release.
Introduction
Nifedipine, a dihydropyridine drug belonging to a class of calcium channel blockers, is used for the prophylaxis of angina symptoms by acting as an arterial vasodilator. In the biopharmaceutical classification nifedipine is a class 2-drug, for which bioavailability is restricted by low solubility and dissolution rather than low permeation. In fact, due to its low aqueous solubility (5 − 6 μg/ml at 25°C) that increases little when the temperature is increased (about 10 μg/mL at 37°C)[1], nifedipine is known to show low and irregular bioavailability after oral administration. Angina requires a rapid drug response to manage the patient conditions: the low water solubility of nifedipine should suggest the need of efficient formulations enabling rapid release, also to prevent the risk of administered doses higher than necessary. The availability enhancement for nifedipine by means of co-precipitates therefore could represent an important aspect of its development: a drug molecularly dispersed within a hydrophilic solid matrix undergoes a fast entering the solution.
Most of papers dealing with nifedipine co-precipitate concern a variety of carriers, such as phosphatidylcholine[2], mannitol[3], solutol[4], PEG[5], poloxamer[6], sodium starch glycollate, croscarmellose sodium, eudragit E-100[7], Gelucire 44/14[8], HPMC[9], Pluronic F68 and Gelucire 50/13[10] in the form of solid or surface dispersions and co-precipitates[12].
Few examples can be found for nifedipine tested with soluplus, a new attractive carrier[12-15], recently proposed and designed to prepare co-precipitate of drug by hot-melt extrusion[16]. It represents a hydrophilic non-ionic multipurpose carrier for its ability to act as polymer matrix former suitable for co-precipitates; and is considered as a member of the fourth generation of co-precipitate carriers[17]. Due to hydrophilic and hydrophobic functionalities in its chains, soluplus displays solubilising properties through micelle formation in solution (CMC 7.6 mg/l)[18]. But it also exhibits a lower critical solution temperature (LCST) near 40°C[19], above which it aggregates into larger micelles, displays gel-forming ability that makes turbid the releasing medium[16]. As a consequence the final result for this carrier, proposed to improve the release of the embedded drug, could depend on the relative weight of these opposite parameters.
In the case of the association nifedipine/soluplus literature has been mainly concerned with the study of interaction between the drug and the polymer, the miscibility limits, the effects of the preparing techniques and aging on dissolution behavior[12-15]. The original aim of our research was to compare a range of hydrophilic carriers, including soluplus, such as Gelucires, PEGs and Lutrols, in the nifedipine release improvement from co-precipitates. In the present paper we preferred a more in-depth examination of the soluplus system, since nifedipine experiments unexpected release problems in the presence of soluplus, unlike other carriers; moreover an unusual formation of particulates, different in shape and size from the starting particles, was observed during the release test. While few examples are reported in the literature for slow drug release in the presence of soluplus, presence of this type of particulates was not observed or reported in previous papers, which separately referred to nifedipine or to soluplus. To interpret this fact, for comparison, also PVP and sucrester P1670 were employed as carriers for nifedipine, alone or in mixture with soluplus. All the systems thus prepared were examined by thermal (DSC, TGA, HSM) and spectroscopic (FT-IR, Raman, SEM) analysis and a mechanism was hypothesized for the slow release and the formation flower-like particulates during the release tests.
Materials and Methods
Chemicals
Nifedipine was a commercial sample of pharmaceutical grade: its thermogram fits that of a commercial sample; soluplus, sucrester P1670 and PVP K30 were commercial samples of the highest degree available (Sigma, Milan, and Italy).
Preparation of the physical mixtures
Physical mixtures were prepared mixing powders of the same particle size range of nifedipine and each carrier in order to obtain the formulations shown in (Table 1).
Table 1: Weight percent composition of the formulations examined
| Nifedipine | Soluplus | PVP | Sucrester |
|---|---|---|---|
| 10 | 90 | ||
| 50 | 50 | ||
| 10 | 90 | ||
| 10 | 65 | 25 | |
| 10 | 45 | 45 | |
| 10 | 90 |
Preparation of the co-precipitates
Five grams of each physical mixture were dissolved in 10 ml ethanol at mild heating under stirring. The systems were stirred up to complete dissolution of both components and filtered. The solvent was evaporated at reduced pressure and the mass thus obtained was heated at 40°C up to complete release of the solvent. The samples were placed in a freezer at - 20°C for 24 h and then crushed, milled and sieved at room temperature. Particles in the size range 200 - 350 micron were used for further tests. Attention was paid to exclude the formation of solvates, since this ability has been reported for nifedipine[20].
Solubility of nifedipine in aqueous solution containing soluplus
The saturation solubility of nifedipine was measured at 25°C in 10 ml aqueous solutions containing increasing concentrations (0, 1, 5 and 10%, w/v) of soluplus. An excess of drug was suspended into these solutions under stirring for 24 h. After filtration (0.22 micron), the concentration of nifedipine was spectrophotometrically determined (UV spectrophotometer UNICAM Helyos Beta) at 234 nm.
In-vitro release study
Dissolution of nifedipine powder (10 mg) and co-precipitates (varying amounts equivalent to 10 mg nifedipine, size fraction 200 - 350 μm) was carried out using USP dissolution test apparatus at a temperature of 37°C, at 60 rpm using 900 ml dissolution medium. The dissolution study was carried out for two hours. The dissolution chamber operated in closed circuit with a flow rate of 12.5 ml/min (Gilson Minipuls 3 peristaltic pump) through a spectrophotometer the test for each sample was repeated and the average of three measurements was then calculated. The percentage of nifedipine dissolved was calculated from the regression equation generated from standard data of specific absorbance.
Samples of particulates formed during the release test were collected from the container walls by means of a steel spoonshaped micro spatula and examined by optical microscope (Olympus BH-2, equipped with a photographic recorder Olympus C-35AD-4).
Scanning Electron Microscopy (SEM)
The shape and surface characteristics of the particulate were observed by SEM. Microparticles were sputter-coated with Au/Pd using an Edwards Auto 306 (Milan, Italy) electron-beam evaporator and examined using a scanning electron microscope (Philips 500, Eindhoven, NH) at 10 kV accelerating voltage.
Differential Scanning Calorimetry (DSC): Thermograms were obtained by Mettler equipment (FP 80HT control unit, FP 85TA cell furnace and FP 89 control software). Samples of about 10 mg were accurately weighed and analysed in pierced Al crucibles in the range of 30 - 220°C, at a heating rate of 10°C min-1.
Thermomicroscopy (HSM): Hot-stage microscopy was carried out by means of a hot plate Mettler FP 82HT, coupled to an optical microscope Olympus BH-2, equipped with a photographic recorder (Olympus C-35AD-4). A control unit Mettler FP 80HT was used to control the heating rate of the hot plate in the range of 30 – 300°C.
Thermogravimetry (TGA): Loss of solvent from the crystals was characterized by thermogravimetric analysis with a Mettler Toledo automatic thermal analyser system TGA/SDTA851e/SF/1100). TG traces were recorded at heating rates of 10°C/min under a nitrogen purge.
X-ray diffraction (XRD): Some of the systems were analysed by X-ray powder diffraction technique, using Philips PW 3719 diffractometer controlled by a computer. Experimental conditions: Cu Ka radiation (λ = 1.78896 Å); 40 kV and 30 mA. Scanning interval: 0 - 50°2θ; Time per step: 1 s; Graphite monochromator on the diffracted beam.
Micro-Raman and FT-IR spectroscopy
Micro-Raman spectra were recorded by means of a Renishaw Raman Invia configured with a Leica DMLM microscope (spatial resolution 1 – 60 μm²), a notch filter to cut off Rayleigh scattering, a monochromator (1800 lines/mm) and a Charge-Coupled Device (CCD) thermoelectrically cooled (203 K) detector. The light sources available were an Ar+ laser (514.5 nm) and a diode-laser (780.0 nm). Experimental details: Ar+ laser, (λ = 514.5 nm), time of each scan: 20 s, number of scans: 4, Pout laser: 1.5 mW. FT-IR spectra were recorded by a Jasco 650 - 4000 cm-1 spectrophotometer.
Results
Co-precipitate components
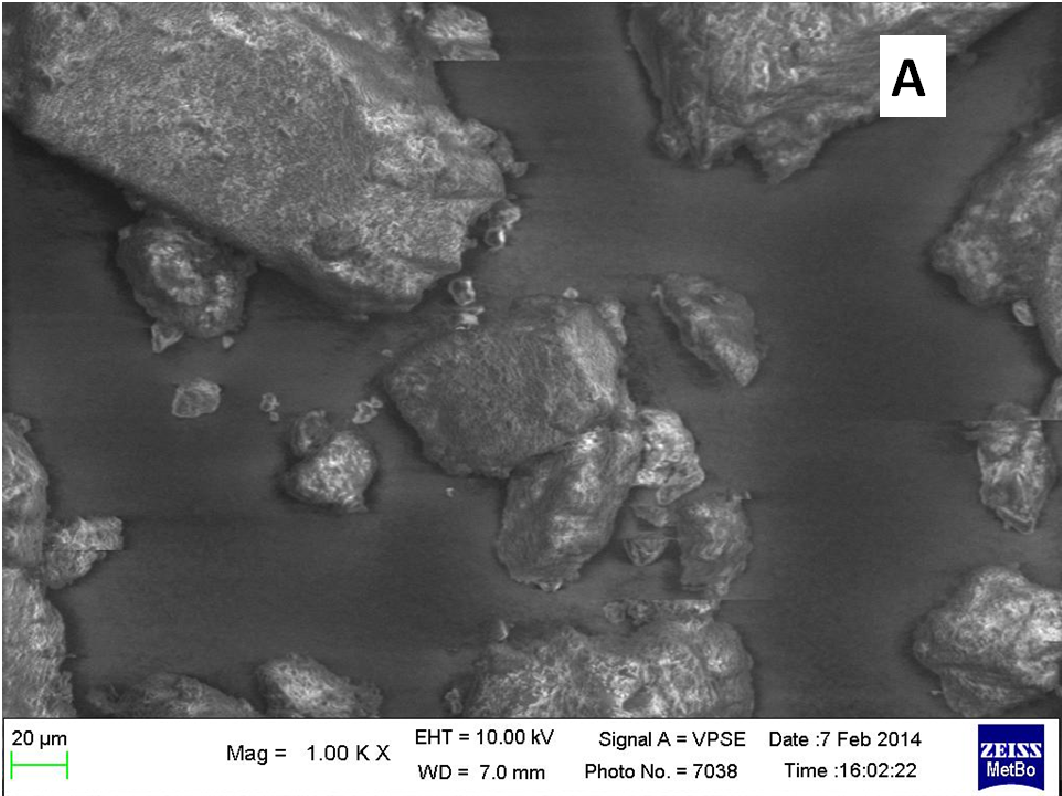
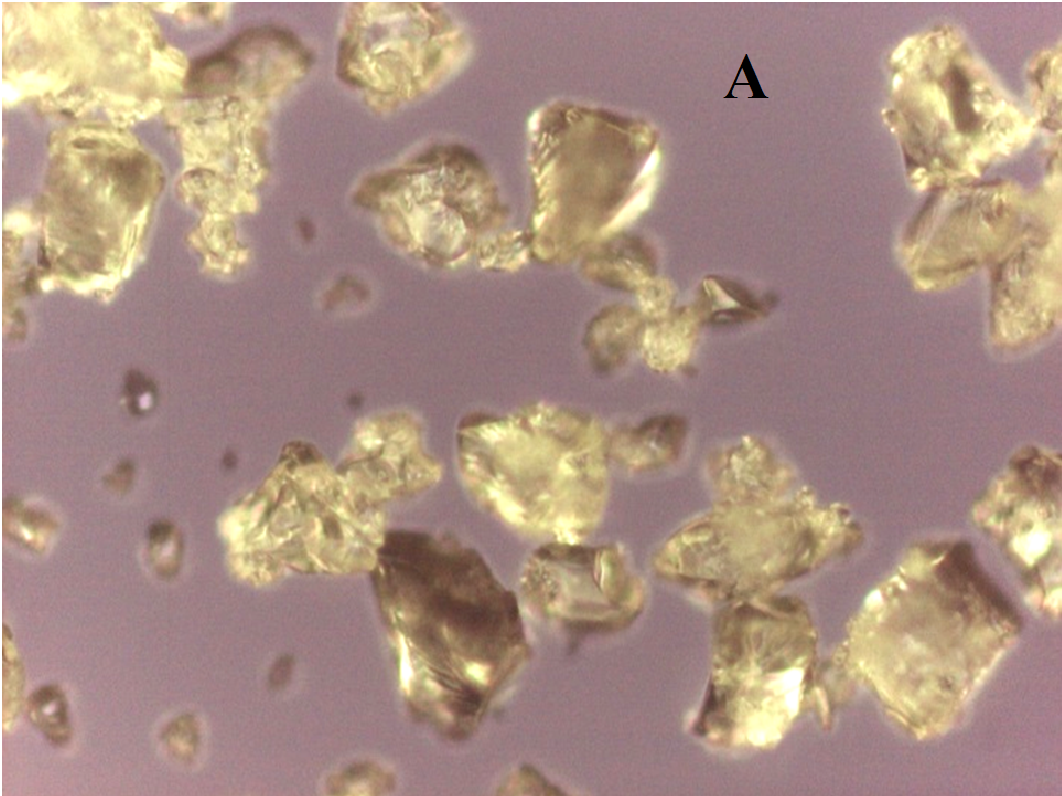
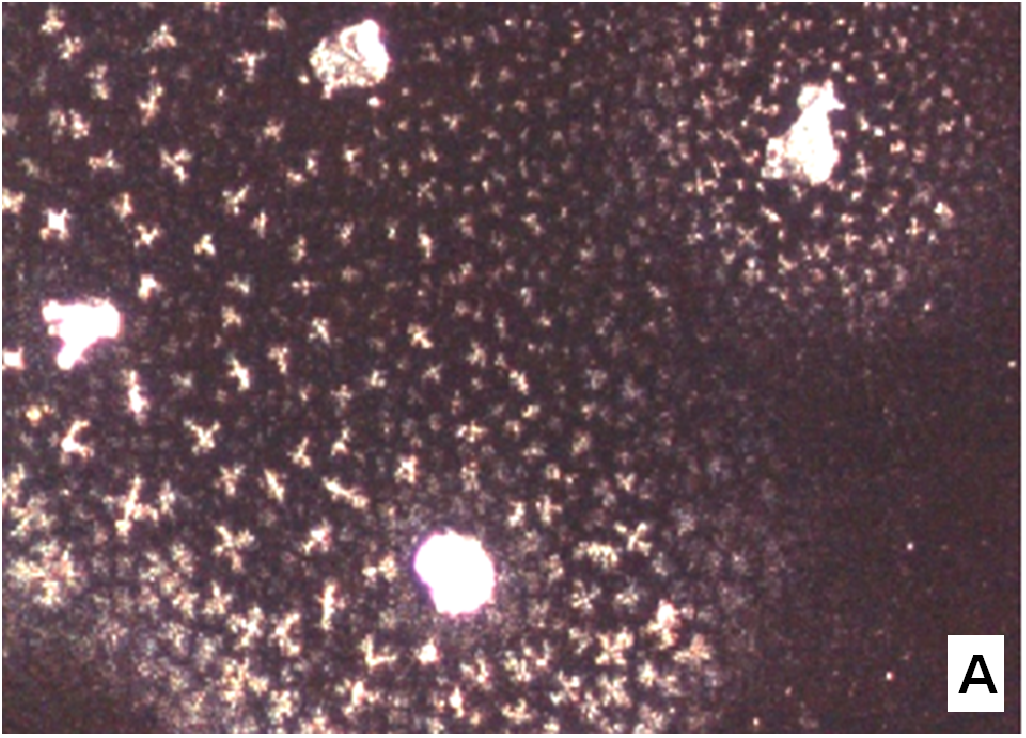
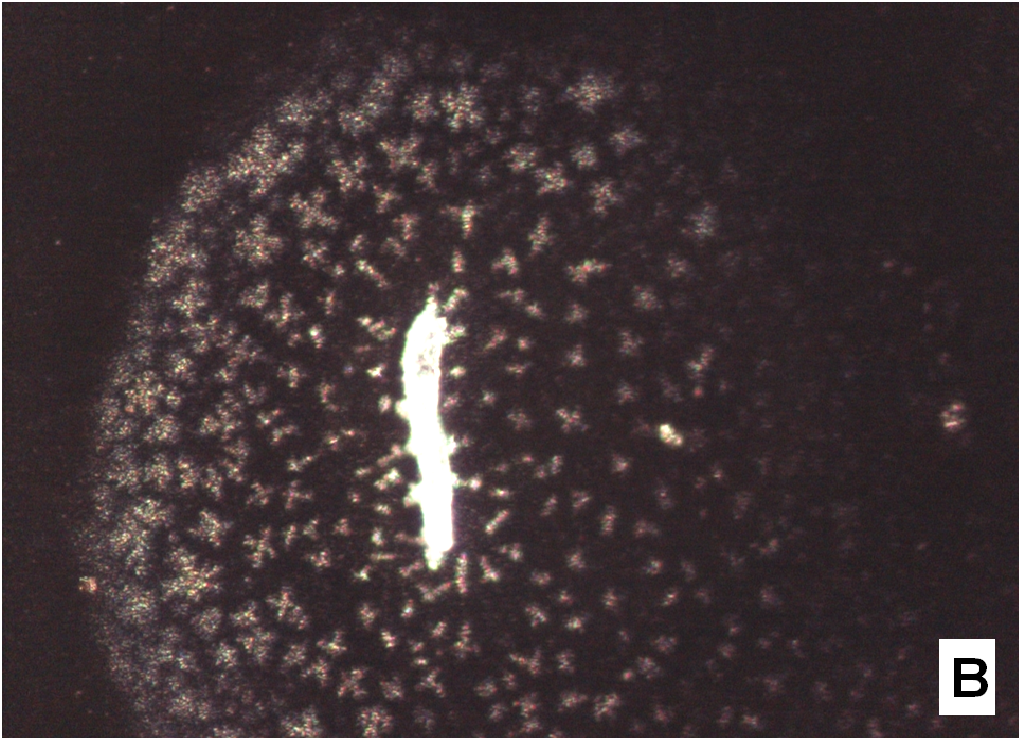
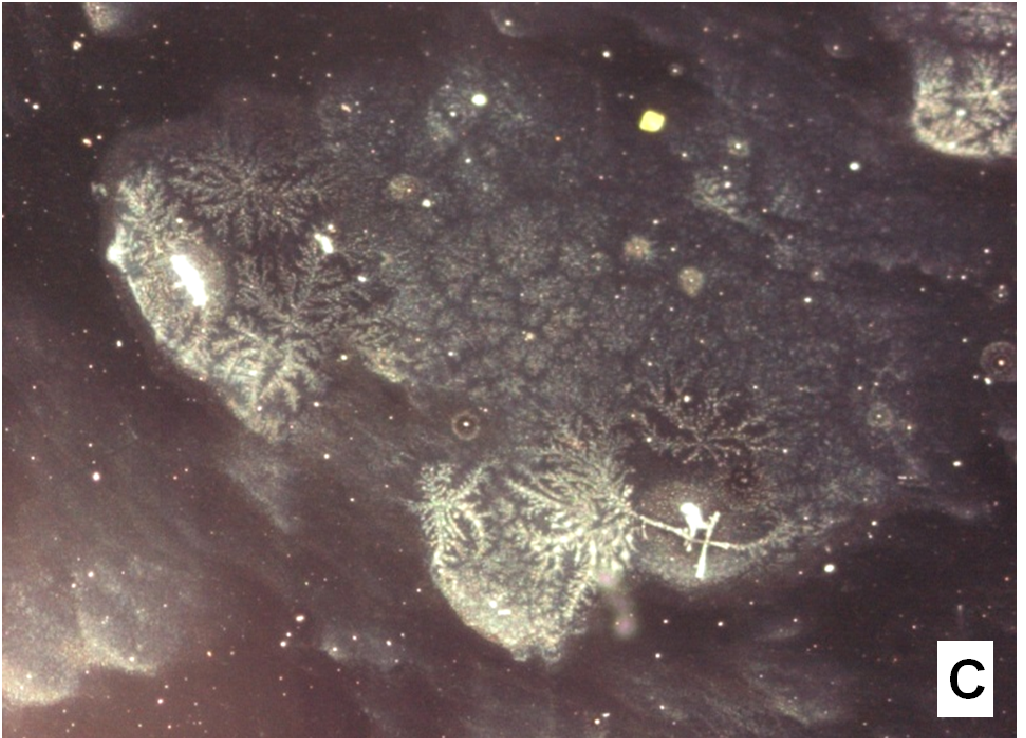
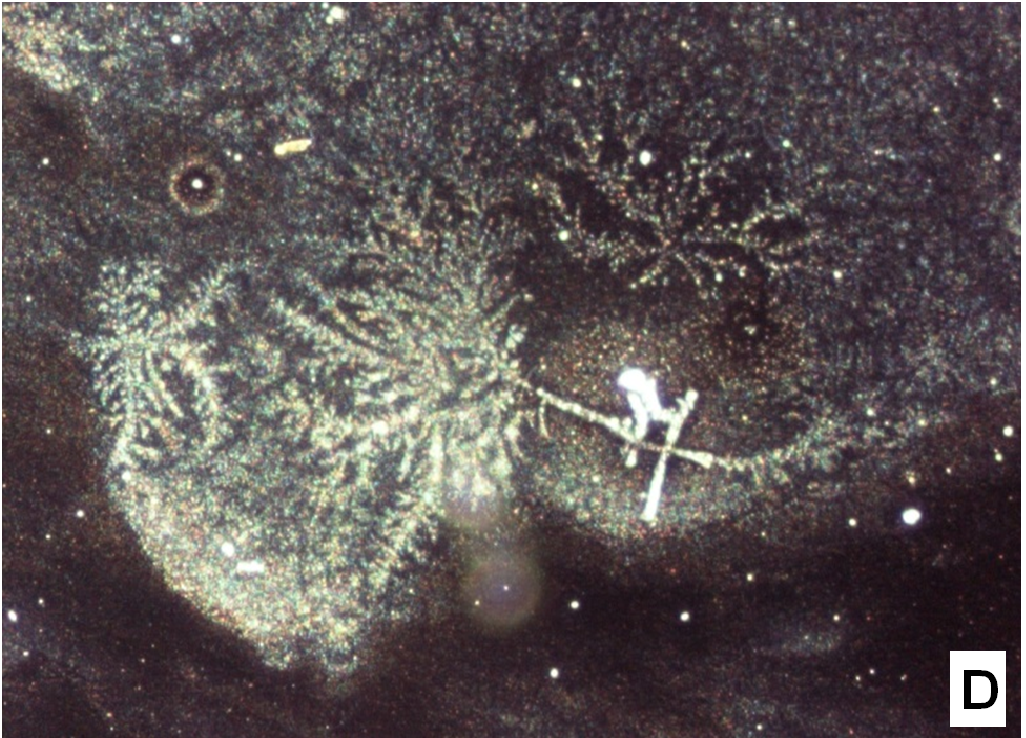
The nifedipine/soluplus co-precipitates were prepared in two different drug/carrier weight ratios (1:9 and 1:1), by dispersing drug and carrier in a common solvent (ethanol). Other formulations were prepared for comparison, containing pure sucrester P1670 and PVP K30 and two soluplus/PVP K30 (3:1 and 1:1 w/w) mixtures, all at 1:9 w/w drug/total carrier weight ratio (Table 1). The co-precipitates were obtained after evaporation of the solvent at a reduced pressure: the release of solvent was found to be complete by thermogravimetry analysis. The mass stored in a dry and dark place was milled and sieved and the particles underwent a series of analyses. SEM images reveal that particles thus obtained have sharp edges typical of well compacted structures (Figure 1A).
Figure 1(A,B,C,D): SEM images
Nifedipine solubility in solid soluplus was reported ranging between 7.3 and 3.55 % w/w, according to the temperature parameter considered, applying the Flory–Huggins theory to predict the solubility[15]. The miscibility limit of nifedipine/soluplus co-precipitates was reported to be 30% w/w drug loading, using different preparation methods (freeze drying, melting and solvent evaporation)[12]. These values agree with what predicted by the Hildebrand’s solubility parameters: the carriers used to prepare co-precipitates and nifedipine display Δδ values well below the critical limit[21,22] (Table 2). These values indicate likely miscibility and make it possible to predict the formation of a homogeneous dispersion of the drug into the carriers; while for the sucrester it could be expected that the drug could be present partly in undissolved/re-precipitated form, being borderline the δ value reported for the sucrester P1670[23].
Table 2: Solubility parameters of all the components of the solid dispersions
| Drug/Carrier System | δ (MPa1/2) | Δδ(MPa1/2) |
| Nifedipine/Soluplus | 24.8/22.1 | 2.7 |
| Nifedipine/PVP K30 | 24.8/22.5 | 2.3 |
| Nifedipine/Sucrester P1670 | 24.8/17.4 | 7.4 |
Thermal analysis
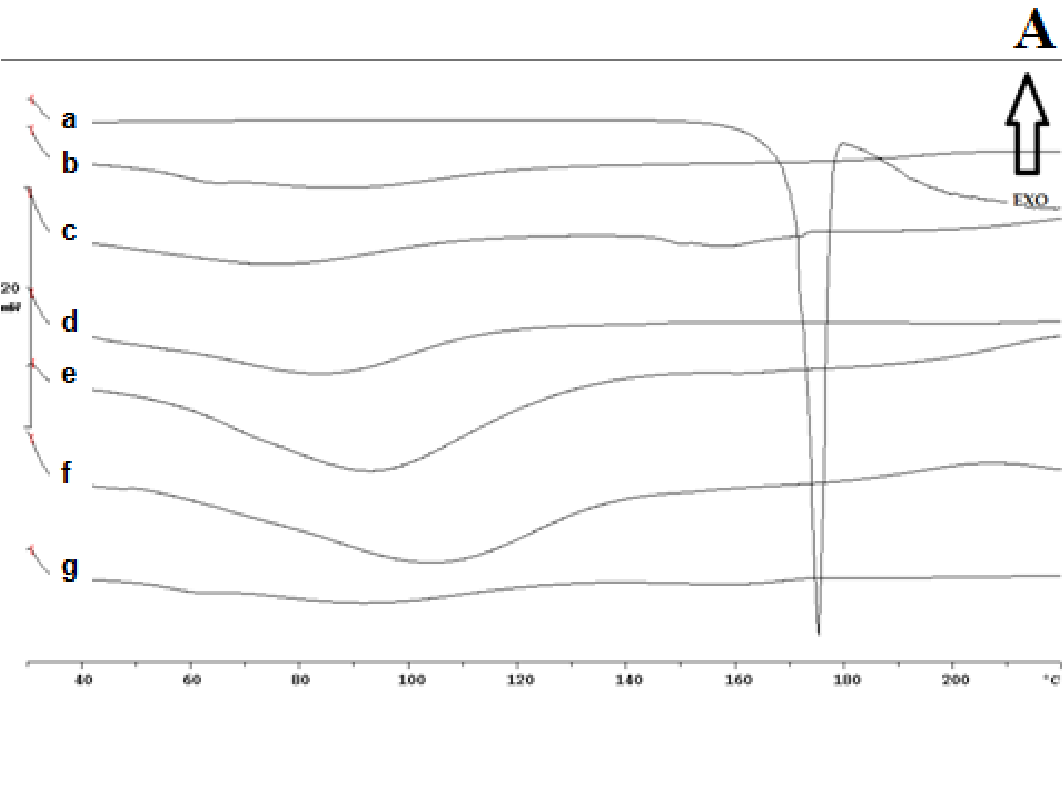
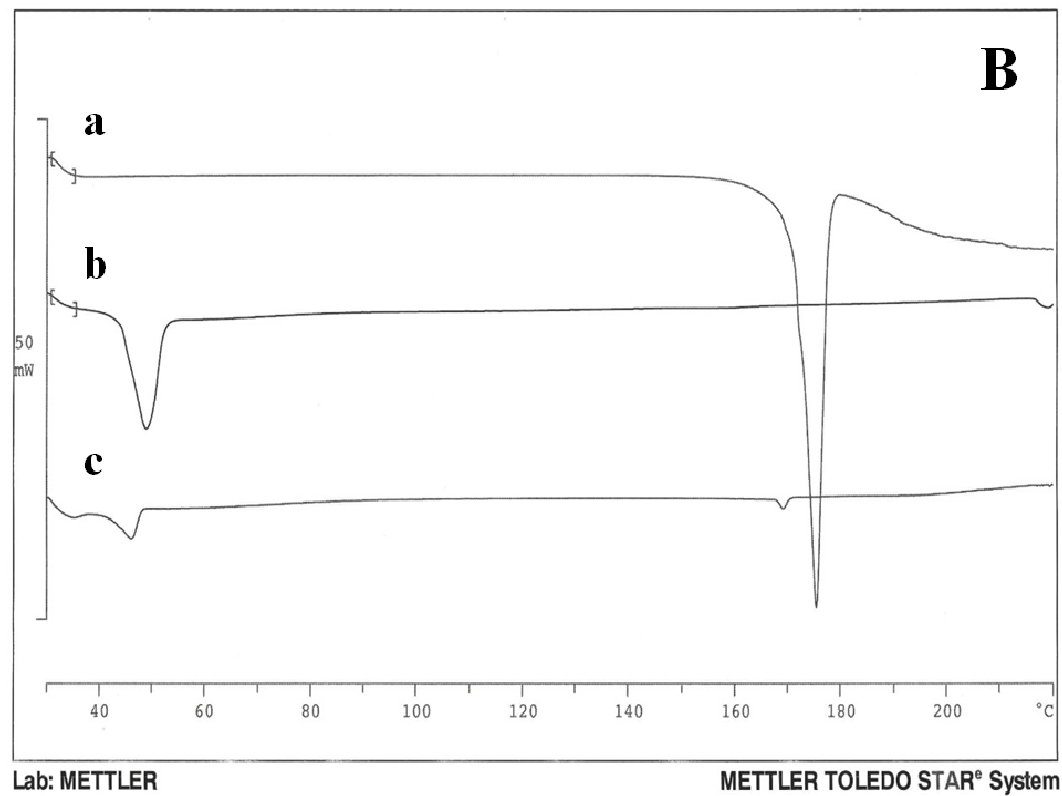
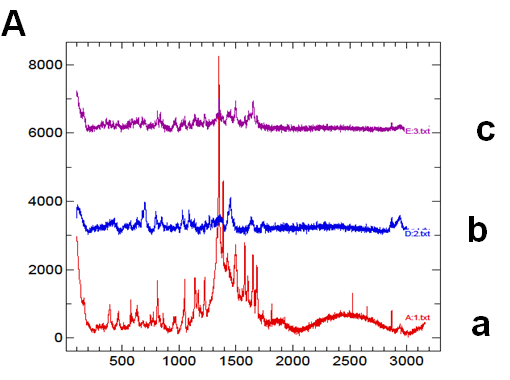
The thermogram of pure nifedipine shows an endothermic peak at 174°C, corresponding to melting point of the stable form; that of soluplus is practically flat and only a large and poorly appreciated endothermic can be observed at about 60°C, related to its softening, soluplus being amorphous in nature. No melting peak is present in the thermogram of PVP, rather a poor deviation of the baseline, possible related to loss of humidity. No melting peak was evident in the DSC thermogram of the 1:9 and 1:1 w/w co-precipitate prepared with soluplus; that containing PVP, alone or in mixture with soluplus also did not show the nifedipine melting endothermic. Similarly the thermogram of the physical mixture of the same composition did not show any melting endothermic (Figure 2A (a-g)).
Figure 2A: Thermograms
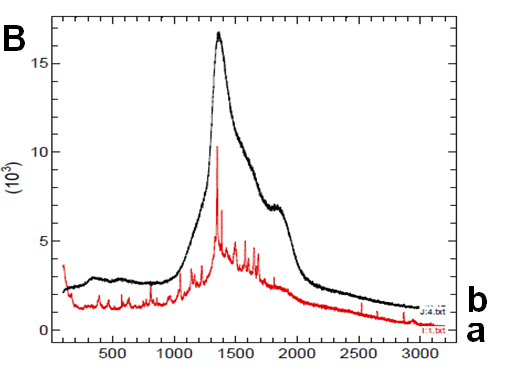
The disappearance of the nifedipine melting peak in the co-precipitate thermograms could be attributable to incorporation of the drug into the softened carrier domains, during heating for recording of the thermogram; or to its possible amorphous state. Lipophilic sucresters display characteristic melting, while the ones with high HLB value, like P1670, only soften during heating. (Figure 2B) shows the thermograms of drug (a), carrier (b) and co-precipitate (c). The sucrester shows a melting endotherm at 51°C; the co-precipitate thermogram has a melting endothermic of reduced surface area shifted to a lower temperature: this could indicate a notable dissolution of nifedipine inside the molten carrier. A small endothermic is also evident at about 170°C that, at the present composition, could represent a small portion of undissolved drug, suggesting the saturation of the system at present composition (Figure 2Bc).
Figure 2B: Thermograms
Thermomicroscopy allowed observation of molten vesicles after heating particles of the co-precipitate, but only at high temperatures, starting from 110°C; at 130°C melting appears complete. The particles of the dispersion richer in nifedipine start to melt above 140°C and melting is complete at about 160°C. The same phenomena were observed also in the presence of PVP, even at different temperatures. HSM photos of the co-precipitate did not allow observation of undissolved or precipitated particles of drug inside the molten vesicles (Figure 3A-F).
Figure 3(A,B,C,D,E,F): HSM photos A) DS N/S 1/9 110°C, B) DS N/S 130°C, C) DS N/S 1: 1 140°, D) DS N/S 1: 1 150°C, E) NIFE/ PVP 1:9 a 180°C, F) DS Sol/PVP 1: 1 a 180°C
The thermogravimetric profiles (not shown) of the co-precipitates suggested in most case the absence of any loss of weight, due to residual solvent used for the preparation; otherwise the samples were kept under reduced pressure until constant weight.
Release of nifedipine from soluplus co-precipitates
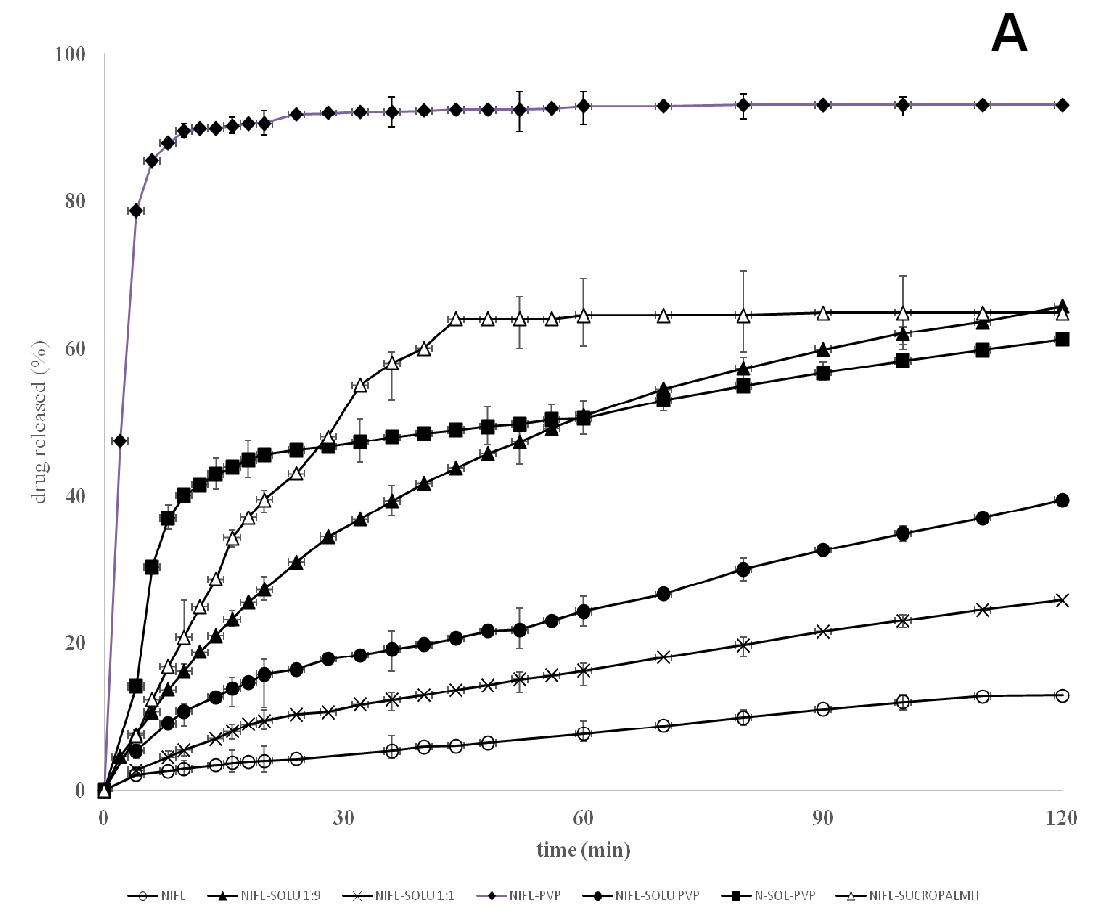
Pure nifedipine poorly dissolves, with less than 15% dissolved after 60 min, under the dissolution conditions used for the tests[24]: the hydrophobic property of the drug molecule prevents its wettability by the dissolution medium. The dissolution profiles concerning the co-precipitates indicate that, as the concentration of polymer in the co-precipitate increases, the release of nifedipine also increases compared to the pure drug. Soluplus is soluble in water and, as it dissolves, nifedipine present in the co-precipitate also acquires solution form. However, after 2 h the released drug reaches 60%, but only 40% in samples containing a lower soluplus percent content and continues very slowly (Figure 4).
Figure 4(A,B): Release profiles
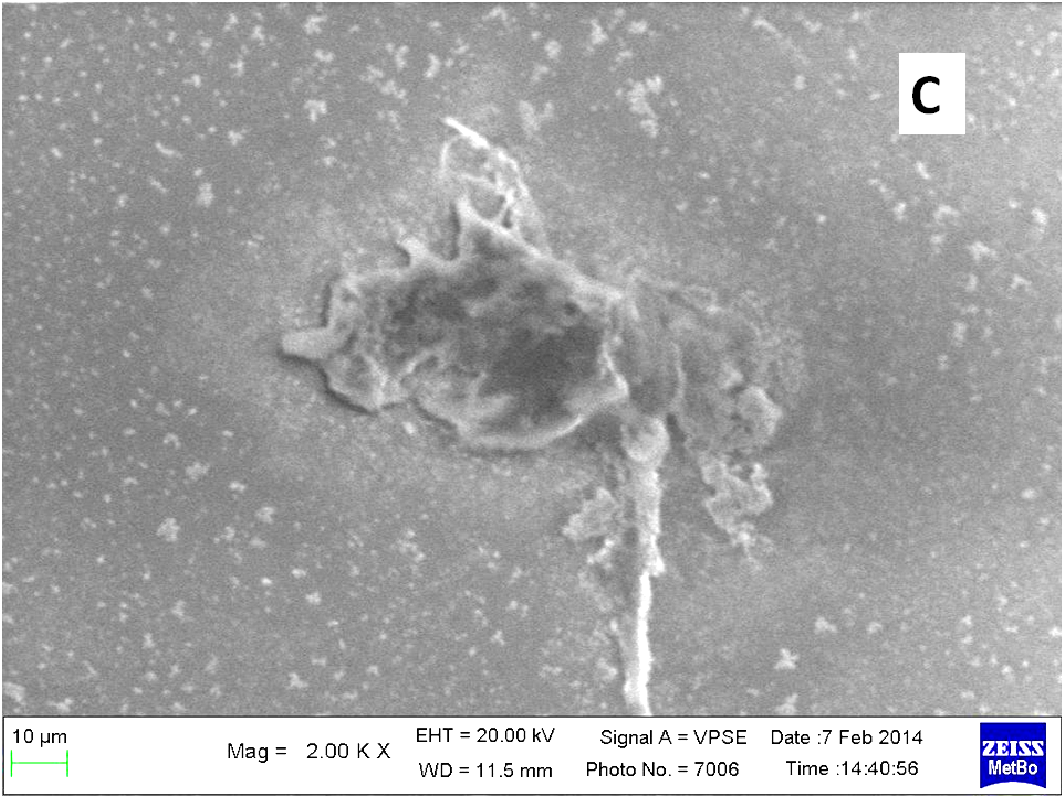
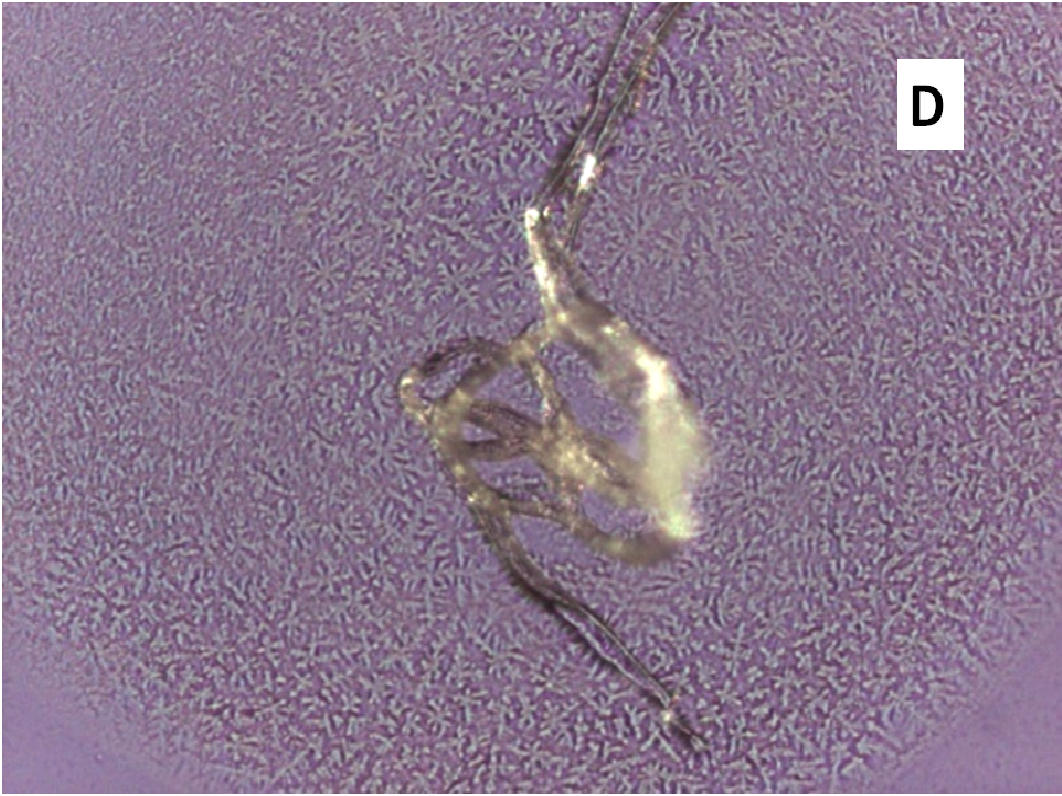
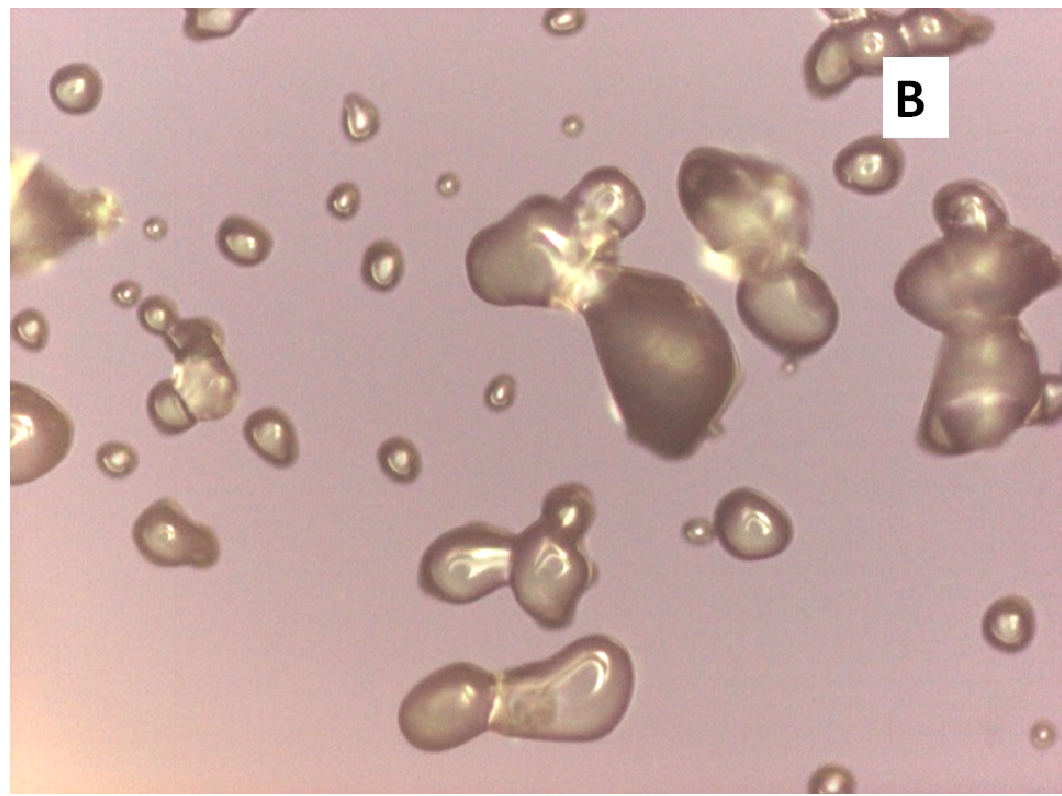
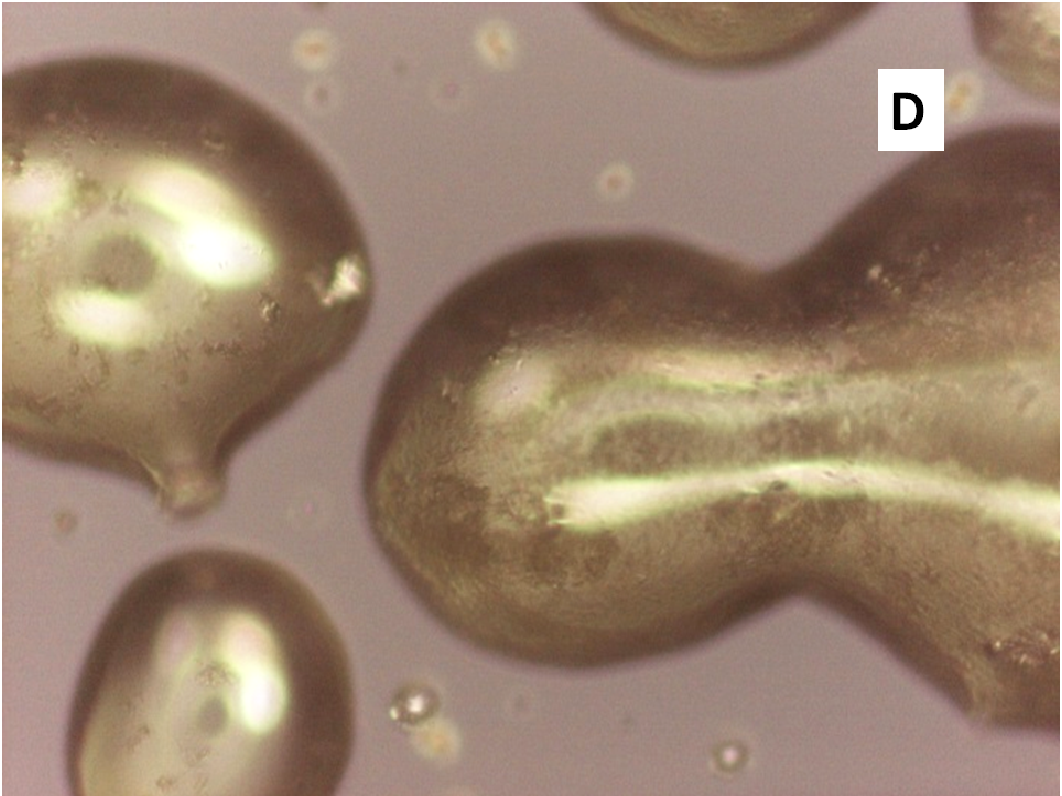
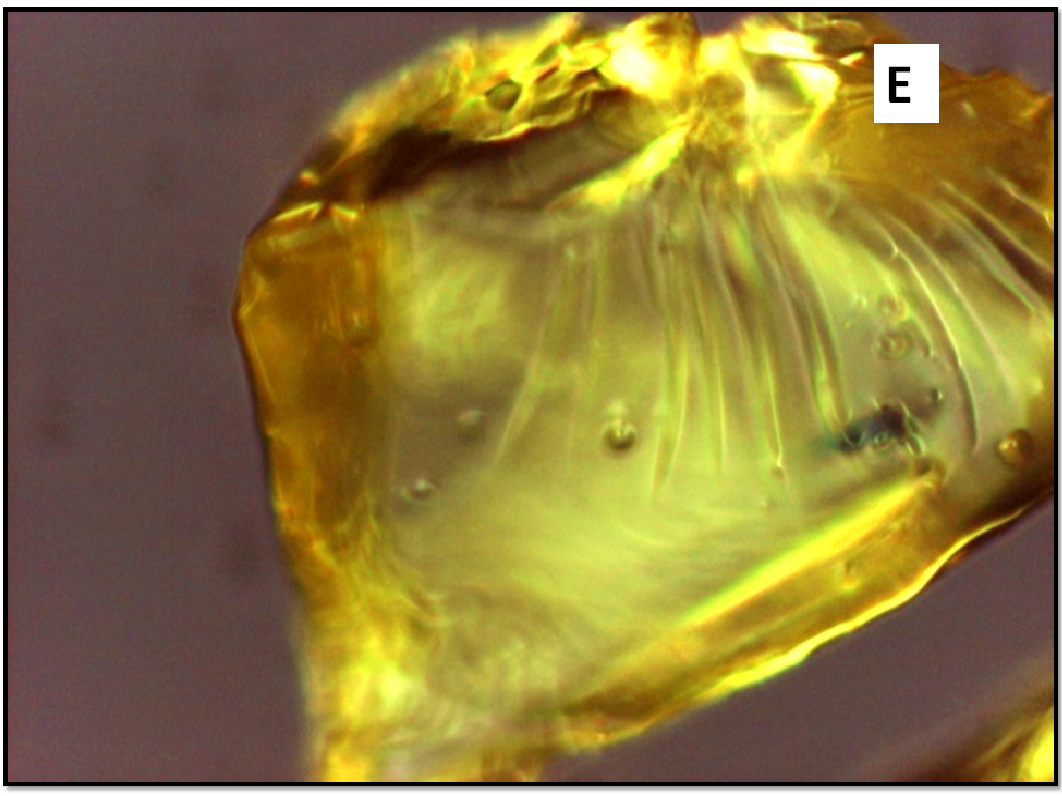
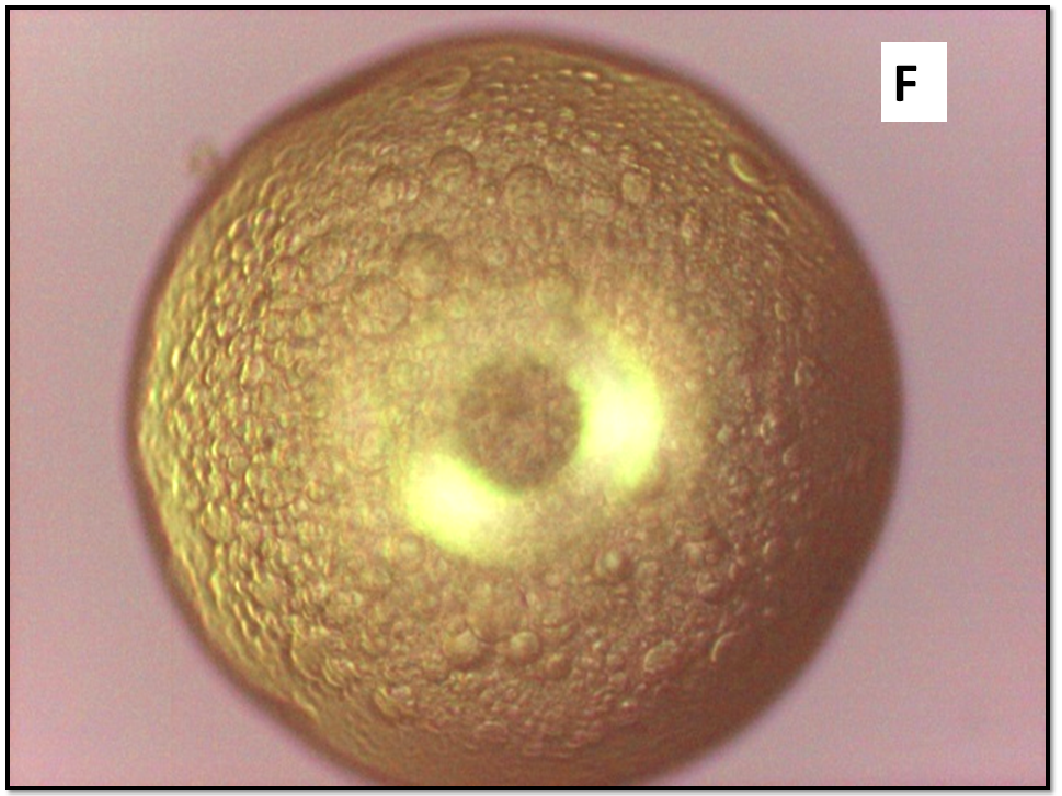
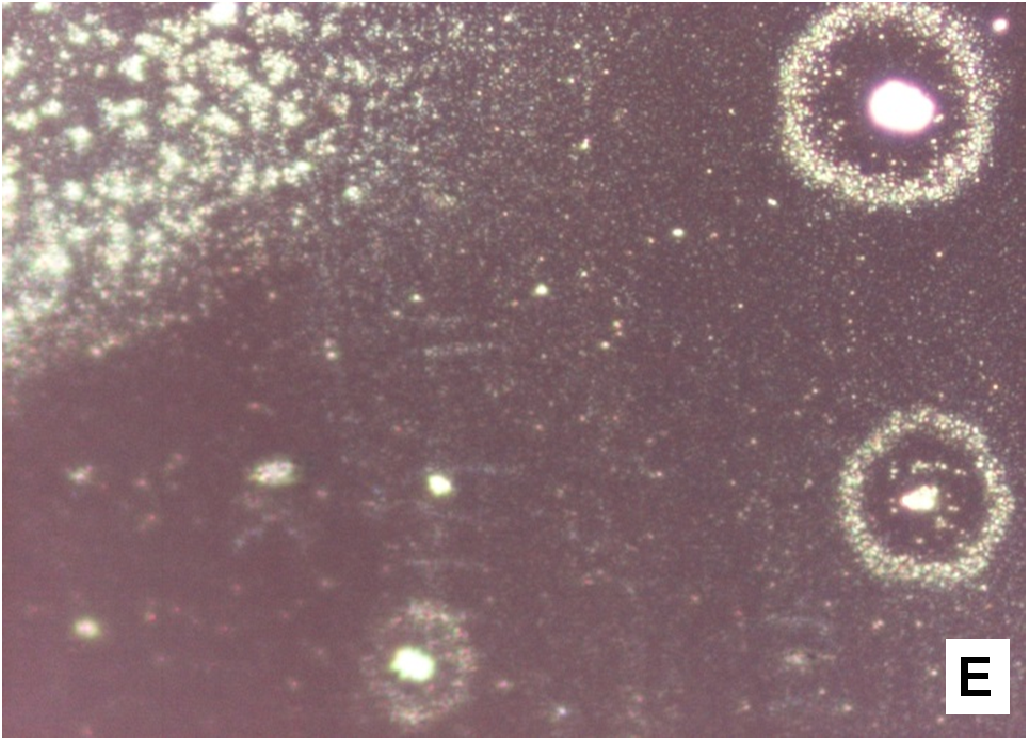
About two hours after the beginning of the dissolution test, particles started appearing in suspension, which offered particular designs and images: at the optical microscope revealed a large variety of flower-like shapes composed in some case of several branches, in the presence of undissolved and gelled solid particles (Figure 5). These last residues show at SEM irregular shapes with rounded edges surrounded by a number of smaller formations (Figure 1B-D) at higher magnification, these last particles appear as agglomerates of tiny particles.
Figure 5(A,B,C,D,E): photos at optical microscope
Release from PVP-based co-precipitates
The association nifedipine/PVP K-90 was reported to markedly enhance the dissolution rate of nifedipine, that was supposed due to an increase of nifedipine solubility in the presence of PVP[25]. In the present case, PVP K30, employed to prepare co-precipitate at 1:9 weight ratio, produced similarly a very prompt release (Figure 4), also two soluplus/PVP mixtures were tested at 3:1 and 1:1 ratios as carrier. The soluplus/PVP K30 (1:1 w/w)-based co-precipitate, exhibited a significantly fast initial dissolution rate, having a burst release (45%) within the first 10 min, followed by a slow release that showed a parallel trend to the profile of dispersions containing only soluplus. A lower PVP percent did not offer particular differences: after 2 h the release was slightly lower that the system containing only soluplus.
Release from sucrester co-precipitate
The system nifedipine/sucrester releases about 40% in the first 20 min, displaying acceleration compared to that of pure nifedipine; however the release does not further increase significantly, reaching, after 1 h, only about 65% of the starting drug present in the dissolution medium. At the end of the test no particle in suspension was visible, such as those observed in the case of dispersion with soluplus.
Nifedipine/sucrester co-precipitates have been previously examined only at a 1:14 weight ratio in the form of tablet. It was reported that, though sucresters of high HLB show promise as candidates to increase drug dissolution, their use could be restricted for hydrolytic instability[26].
Spectroscopic analysis
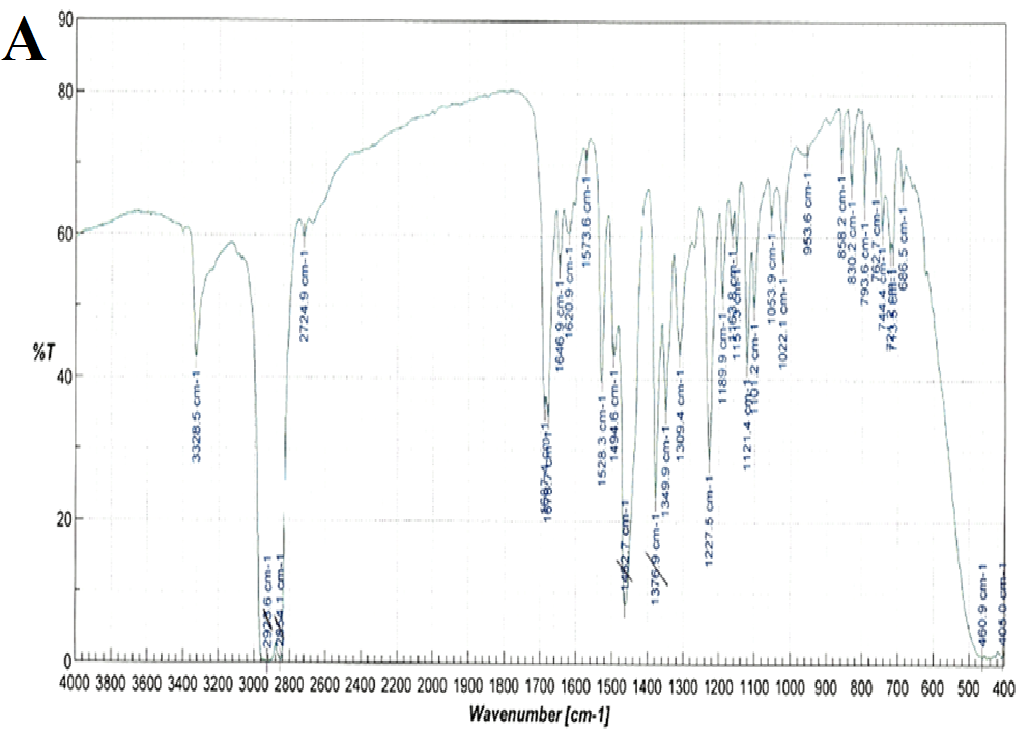
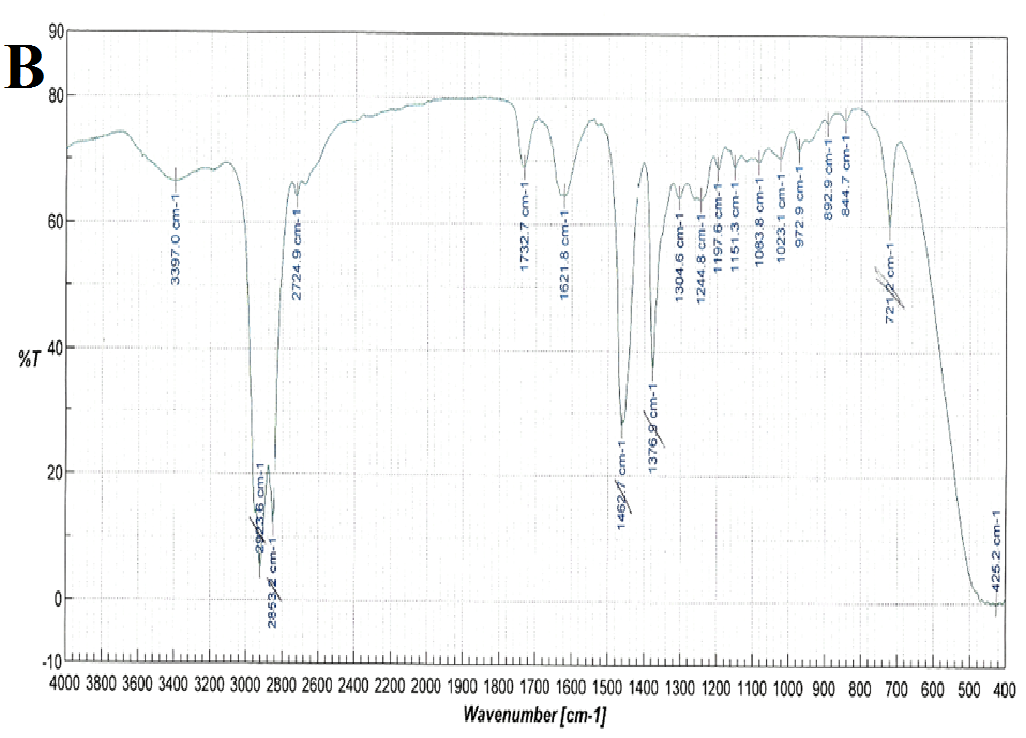
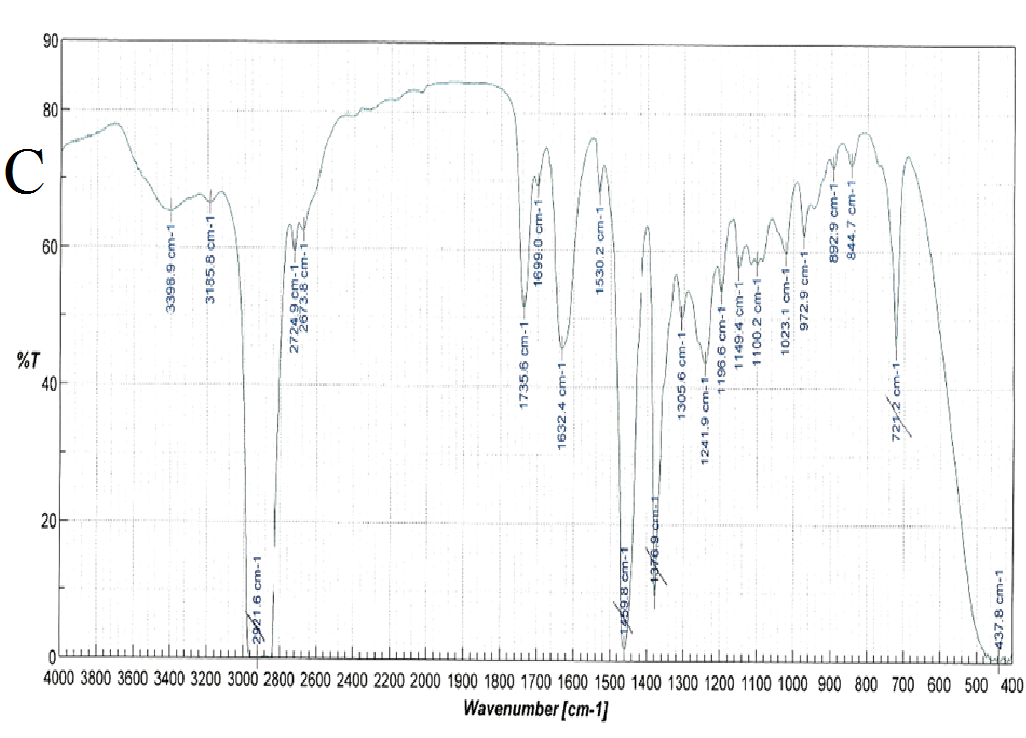
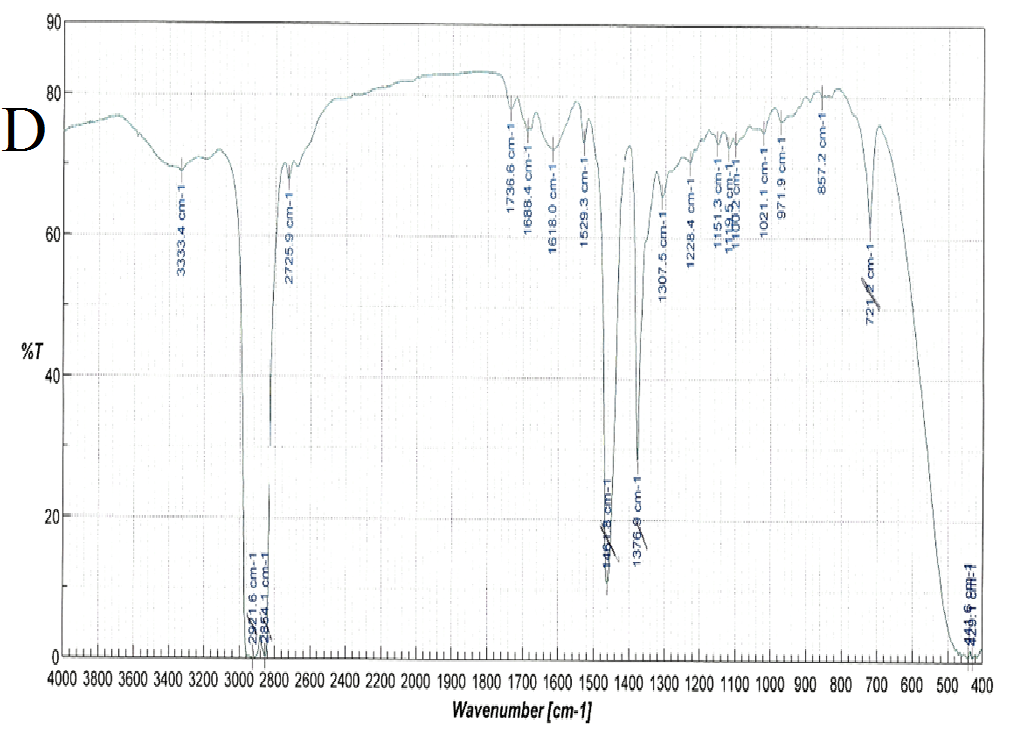
FT-IR spectra were recorded for nifedipine, soluplus and the co-precipitates of two the concentrations considered (Figure 6): attention was paid to the portion of the spectra (1300-1800 cm-1), where the stretching peaks of the most distinctive functional groups of the drug (C = O ester: 1687-1679 cm-1 and NO2: 1528 cm-1), and polymer (1733, 1622, 1463 cm-1 attributable to the stretching of the C = O and C - O - C groups) do not overlap. These band positions and assignments correspond to those reported[27] for α-nifedipine. The same peaks were observed in all the samples examined (especially that containing higher percentages of the drug).
Figure 6(A,B,C,D): FT-IR Spectra
Comparison of these peaks in the case of the co-precipitates results a shift corresponding to the nitro group of nifedipine, from 1528.3 for pure nifedipine to 1530.2 for the SD 1:9 and 1529.3 for the SD 1:1 suggesting also the influence of the composition on this shift. This result agrees with what previously reported[12].
Also the ester carbonyl stretching peak for nifedipine was observed to shift, but, since also the carbonyl band of the vinilcaprolactam and vinyl acetate parts of soluplus are found in the same range, this fact prevents the precision of the shift measurement. The three peaks selected for soluplus were found at 1735, 1632 and 1458 cm-1; and 1737, 1629 and 1642 cm-1 for 1:9 and 1:1 systems respectively.
The NH function of the nifedipine molecule was considered as source of interaction, since capable of forming hydrogen bonds with a carbonyl function of soluplus[14]. In the spectra of the co-precipitate this peak appears round and its position difficult to assess with precision: however it was reported that a broadened NH stretching peak can be considered as a mark of the formation of a co-precipitate of nifedipine[14].
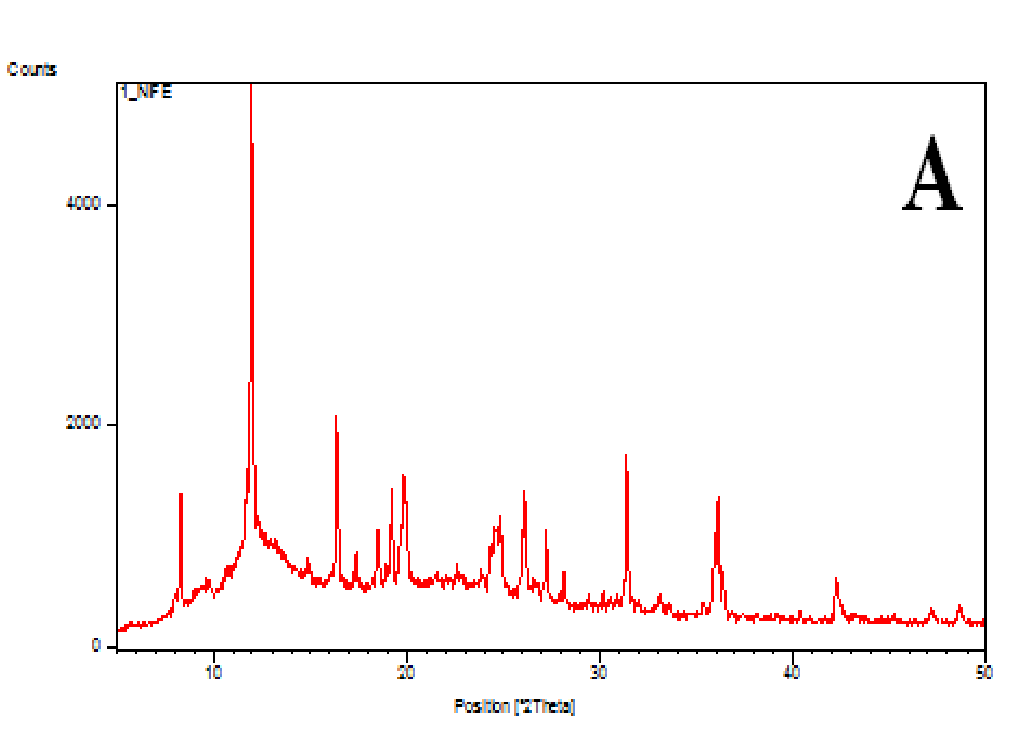
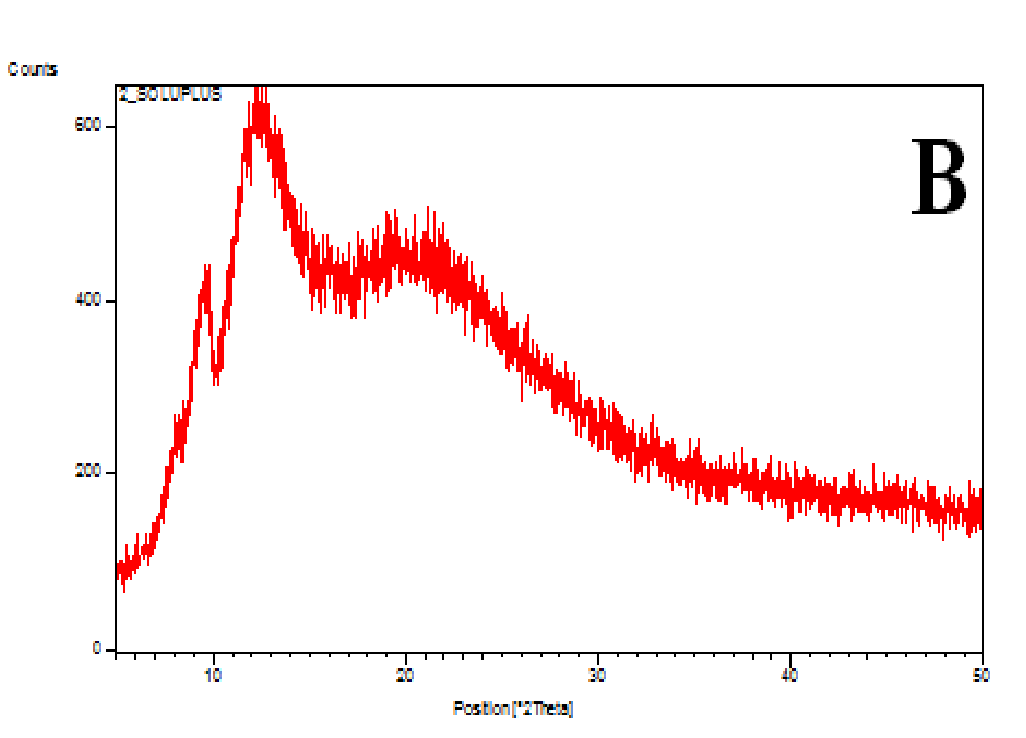
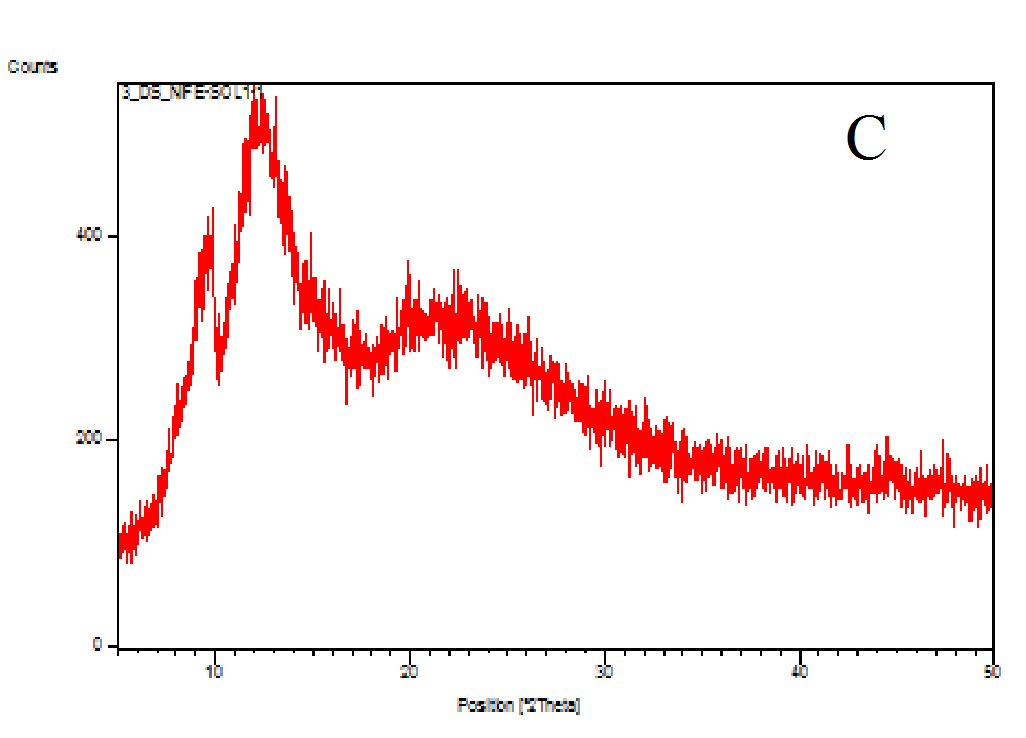
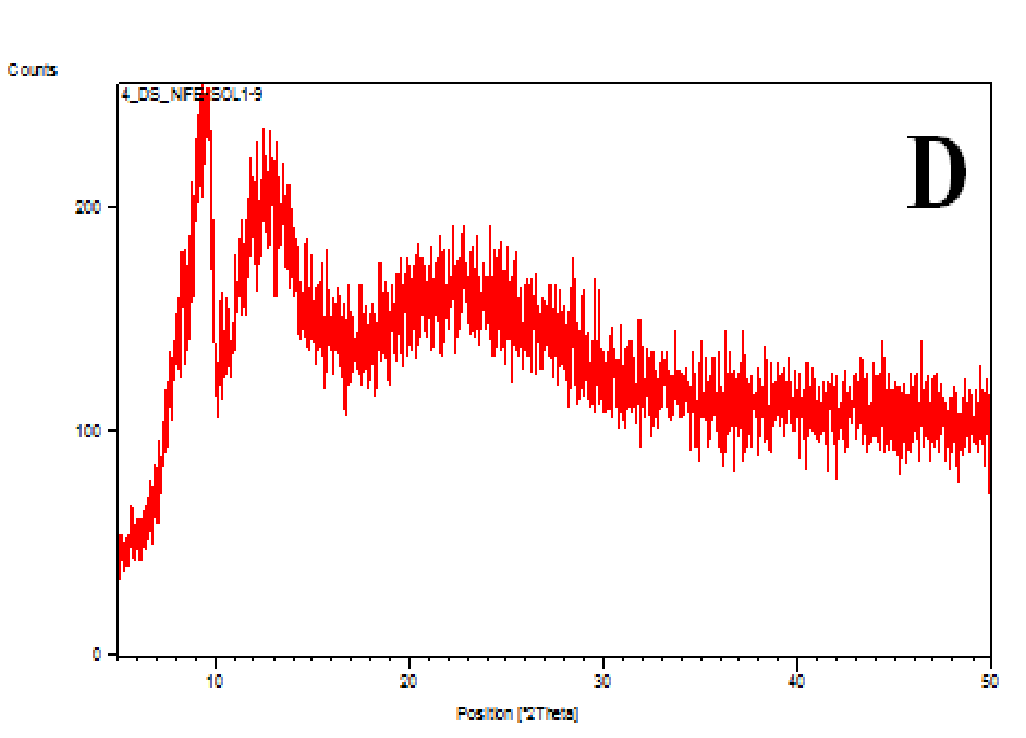
The XRD patterns of the nifedipine, carriers and dispersions were tested (Figure 7A-D). Nifedipine raw material is crystalline as demonstrated by sharp and intense diffraction peaks (Figure 7A). Soluplus (Figure 7B) as well as PVP K-30 (whose spectrum not shown) are amorphous powders, having no crystalline structure. The two co-precipitates did not show the characteristic peaks of nifedipine (Figures 7C and D): the number of peaks and peak height of soluplus were reduced after the formation of the co-precipitate: the peak profile practically overlaps that of the carrier.
Figure 7(A,B,C,D): Diffractograms
Micro-Raman spectra were directly registered on the starting solid particles (Figure 8A) and on the dissolution residue (Figure 8B). Nifedipine representative peaks are difficulty evident at the particle surface of the co-precipitate and the spectrum profile recalls rather that of the carrier: this could be related to the low drug concentration, but also to the possibility that the drug molecule is masked by the carrier. The high intensity peak in the spectrum of the particle (shown in the photo of Figure 1B), though not perfectly resolved (Figure 8B), suggests the presence of nifedipine and that the residue in the photo (Figure 1B) represents a modification of a starting particle (Figure 1A).
Figure 8(A,B): Micro Raman spectra
Discussion
Soluplus represents a development for polyethylene-glycol-based surfactants: PEGs offer a fully hydrophilic backbone, while poloxamers display only a partially hydrophilic structure, alternating poly-oxyethylene with poly-oxypropylene chains with solubilising ability: both types of polymers show a low melting point. On the contrary soluplus was designed as a graft co-polymers composed of poly-oxyethylene glycol, poly-vinyl caprolactam and poly-vinyl acetate portions that make this polymer more lipophilic, better solubiliser of poorly soluble molecules and matrix former for co-precipitates, both with the solvent method and hot melt extrusion. Soluplus in fact does not show a sharp melting point, rather it progressively softens at increasing temperatures; moreover its relatively high Tg (about 70°C) suggests stability for the possible amorphous forms embedded inside the final matrix. All these properties explain the successful use of soluplus as drug release enhancer for a range of drugs[11,28-31] even taking into account possible variants[32,34]. The gelling tendency of soluplus, which could impact on release performances of formulations where it was inserted, was rarely reported. On contact with the dissolution medium, soluplus is known[16] to enter the solution as single polymer chains, which then organize in uni-chain polymer micelles. At increasing concentration of soluplus uni-chain micelles assemble into multi-chain ones, having a colloidal texture and increasing size. This aspect is more relevant when temperature increases: at 40°C, that represents a LCST value for soluplus, the chains loose the hydration crown and progressively associate, decreasing their solubility and forming a cloudy suspension that could precipitate. This behaviour is responsible of the gel forming property of soluplus.
According to the lack of the nifedipine melting point in the thermograms (Figure 2) of the co-precipitate, which indicates that the drug is present in an amorphous rather a crystalline form, as confirmed also by the diffuse and broad X-ray diffraction patterns (Figure 7), it was expected a rapid release of nifedipine from the soluplus co-precipitate. On the contrary examination of the release profiles (Figure 4) reveals that soluplus does not offer the same results as those above reported.
The formation of a precipitate in the presence of soluplus or nifedipine however was not previously reported: during the release test with soluplus/nifedipine co-precipitate we observed that the medium turned cloudy and turbid and unusual formations of flower-like aggregates were clearly visible, at optical microscope, when extracted from the medium and dried (Figure 5).
The formation of precipitates could have been the result of a rapid supersaturation. The phase solubility study shows an increase in nifedipine apparent solubility as the soluplus concentration increased, passing from 2.34 mg/100 ml at 1% p/v to 17.0 mg/100 ml at 10% p/v (at 25°C). However in our case the apparent solubility values were obtained in conditions of equilibrium and did not allow supposing that the dissolution medium could reach supersaturation[35]. Moreover, the amount of the co-precipitate (100 mg) used for each dissolution test (at all the drug/carrier ratios) does not make it possible to reach such high concentrations as to favour supersaturating of nifedipine.
We rather suppose that this phenomenon is caused by the occurrence of important interactions between soluplus and nifedipine.
Schematically nifedipine molecule can be represented by a di-hydropyridine ring substituted in 2-6 and 3-5 by two methyl and two acetate groups and carrying in the 4 position a 2-nitro phenyl ring: the molecule presents several rotational degrees of freedom, responsible of a number of polymorphs, and a number of functional groups acting as H-bond donor/acceptor, such as carbonyl, nitro and NH groups. An intermolecular bond between one of the ester carbonyl and NH group of an adjacent nifedipine molecule is present in the solid state[27]. Similarly soluplus, among other claimed advantages, offers a variety of functional groups, such as the hydroxy groups at the end of the poly-oxyethylene chains, the carbonyl groups of the vinyl acetate moiety and the amide function of the caprolactam portion.
The association between these two multi-functional systems was reported to originate a strong and efficient mutual interaction when studied by computational approach and FT-IR[14] and Raman spectroscopy[12], since these interactions cause the functional group shift or broadening of peaks compared with the spectra for the pure drug and polymer.
In the present systems interaction between drug and carrier was suggested by the shift observed for the nitro group peak of nifedipine in the co-precipitate with soluplus: this result was supported by two recent papers[12,13] that document important and specific interaction between this drug and this polymer, even though it was also reported[13] that no distinctive shift of soluplus and nifedipine bands could be observed in a similar system.
These interactions were investigated in the solid state[12,14], but they may operate also in solution for systems containing nifedipine and soluplus. In polymer micelles the soluplus polycaprolactam moieties create hydrophobic pockets to accommodate nifedipine molecules; polyethylene glycol groups located on the external surface of colloidal micelle usually provides to micelle solubility. But, close to the gelling temperature and due to the possible multiple interactions nifedipine/soluplus above described, the hydrophilicity of the system could decrease and increase the propensity of the aggregates in solution to precipitate or to jellify before the dissolution of the dispersion particles. This could explain the different shapes of the formations settled on the vessel walls of different shape and size (Figures 1 & 5). The larger formations could represent undissolved and in-situ gelled particles of the co-precipitate: these are visible only some time after the beginning of the test, since at 37°C gelation is slower than at 40°C, that is the critical temperature.
Gelation can occur directly on the particles forming a gel-like encapsulation that greatly reduces the dissolution rate, especially for larger-sized particles and was also observed for tablets containing 1:3 carbamazepine/soluplus dispersion[29].
Release of nifedipine may continue across the gel layer at a reduced rate. Outside the gelled particles nifedipine molecules enter a medium rich in soluplus chains and are captured by means of hydrophobic interactions, evidenced by Raman spectroscopy[12], between nifedipine and soluplus: aromatic hydrocarbon of nifedipine could form weak van der Waals interactions with the cyclic amide presenting in the polyvinyl caprolactam segment of soluplus. The system becomes denser and this interaction reduces hydrophilicity of soluplus chains similarly to the phenomena occurring at LCST, causing the formation of precipitate. Soluplus possesses branched chains of different structure providing a number of contiguous sites for an efficient interaction with nifedipine. The shape of the original gelled particle (regular or branched), from which nifedipine escapes, is responsible of the images that can refer to coral atolls (Figure 5E) or to branched or fractal flowers (Figure 5D).
To support these ideas we offer some additional experimental findings: SEM photos of the larger formations extracted from the medium offer evidences of the gelation of dissolving particles, whose rounded edges and shapes differ from those of the starting particles; they appear covered by foreign material precipitated from the medium or with the external surface (Figure 1A) modified at the contact with the medium (Figure 1B-D). Micro Raman spectra recorded on one of these particles confirm that they represent residues of the original particles modified by gelation. From the comparison of the distinctive peaks, the spectrum (Figure 2B) reveals that this residue contains nifedipine, supporting the idea that gelation of the polymer wraps the co-precipitate particles and slows appearance of nifedipine in solution.
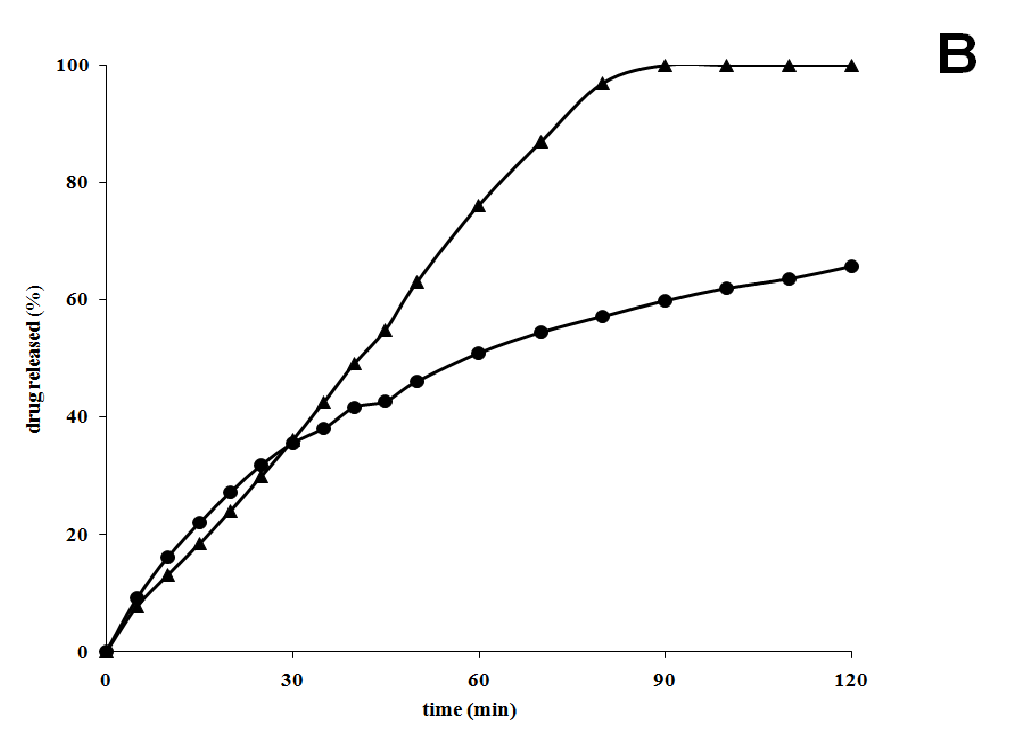
In solution at 37°C, the rate of the formation of these structures is reduced with respect to the reference value 40°C; particulates thus appear slowly some time after the beginning of the release test. As evidence of the temperature role in the release mode, we repeated the test at a lower temperature (25°C) with the same formulations: the release was complete in less than one hour, without any problem related to formation of colloidal structures, and thus also more rapid than at 37°C. At the beginning, the release profiles at the two temperatures overlap: the effect of the colloidal formations, that slows the release at 37°C, starts after 20 min and appears to modify also the release mechanism from an apparent zero order (Figure 4B). At a temperature far from the critical one the colloidal phenomena do not affect the release of the drug and soluplus behaves like an enhancer of the nifedipine release from the co-precipitate.
PVP was selected as a carrier for comparison. Differently to soluplus, PVP does not form gels and enters the solution as single straight chains. Due to chain structures, the carbonyl group of PVP is regarded as favourable site for accepting H-bonds; while proton acceptor groups in soluplus are the ether, the carbonyls of acetate and amide moieties. These two polymers are widely recognized to originate a network of H bonds with a drug in a solid dispersion, allowing for high drug amorphisation degree and crystallization inhibiting effect. A recent paper, using the pKBHX scale, evaluated that PVP interacts with cilnidipine via hydrogen bond more strongly than soluplus[36]: since cilnidipine possesses the same functional groups of nifedipine, the same should apply to nifedipine. It is therefore expected that pure PVP displays a greater dragging towards nifedipine, thus favouring a rapid release from the co-precipitate (Figure 4A).
On the contrary the release of nifedipine from soluplus/PVP (3:1 w/w) co-precipitates did not display important differences respect to pure soluplus as carrier. The retarding action of the gel formation of soluplus almost overcomes the enhancing effect to release of PVP (Figure 4) however, no strange formation was observed during the release tests. At 1:1 soluplus/PVP weight ratio, the release profile can be interpreted as if the drug is distributed almost equally between the two carriers: the nifedipine portion associated to PVP is rapidly released; the release profile of the portion associated to soluplus parallels that of pure soluplus. The absence of particulate formation can be attributed to different causes: a disturbing effect of the added PVP on soluplus gelation, the reduced soluplus concentration in the medium, the different behaviour in solution; but also to the molecular structure of PVP, that offers only the pyrrolidone carbonyl to possible interaction with nifedipine[36,37].
Sucrester P1670, the palmitate ester of sucrose, prepared by acylation of sucrose by palmitic acid, is a non-ionic surfactant (HLB 16 and CMC 5.6 mg/l) exhibiting interesting surface-active properties. It was chosen for comparison in the release of nifedipine from co-precipitate for its ability to form a gel at about 37°C, similarly to soluplus[38]. Due to this property this sucrester does not drive the notable improvement of nifedipine release, differently to what reported[39-41].
No micro-precipitate was observed, since the different molecular structures for soluplus and sucrester prevent interactions with nifedipine, responsible of decreasing hydrophilicity of the system and of the precipitate formation: the gelling properties of the carrier alone account for the reduction of the release extent, but not for the formation of micro-particulate.
Conclusion
The nifedipine/soluplus association appears rather remarkable in view of the particular chain structure of the polymer and the molecular structure of nifedipine. The gelling ability of the polymer associated to the network of the possible interactions between nifedipine and soluplus functional groups could explain the slow release of nifedipine from the co-precipitates with soluplus and is responsible of the formation of the micro-structures observed in suspension during the release.
These particulates could not be observed in the absence of one of the two operative parameters, such as in the case of co-precipitates using PVP K30 and sucrester P1670 as carriers.
References
- 1. Sardari, F., Jouyban, A. Solubility of nifedipine in ethanol + water and propylene glycol + water mixtures at 293.2 to 313.2 K. (2013) Ind Eng Chem Res 52(40): 14353-14358.
- 2. Law, S.L., Lo, W.Y., Lin, F.M., et al. Dissolution and absorption of nifedipine in polyethylene glycol co-precipitate containing phosphatidylcholine. (1992) Int J Pharm 84:161-166.
- 3. Zajc, N., Obreza, A., Beleb, M., et al. Physical properties and dissolution behaviour of nifedipine/mannitol co-precipitates prepared by hot melt method. (2005) Int J Pharm 291(1-2): 51-58.
- 4. Rajebahadur, M., Zia, H., Nues, A., et al. Mechanistic study of solubility enhancement of nifedipine using vitamin E TPGS or solutol HS-15. (2006) Drug Deliv 13(3): 201-206.
- 5. Bley, H., Fussnegger, B., Bodmeier, R. Characterization and stability of co-precipitates based on PEG/polymer blends. (2010) Int J Pharm 390(2): 165-173.
- 6. Datta, A., Ghosh, N.S., Ghosh, S., et al. Development, characterization and solubility study of co-precipitate of nifedipine hydrochloride by solvent evaporation method using poloxamer 407. (2011) Int J Appl Biol Pharm Technol 2(1): 1-7.
- 7. Kataria, M.K., Bhandari, A. Formulation and evaluation of solid dispersion for dissolution enhancement of nifedipine. (2014) World J Pharm Sci 2(3): 224-236.
- 8. Aparna, K., Meenakshi, B., Monika, S. Improvement of dissolution rate and solubility of nifedipine by formulation of co-precipitates. (2010) T Ph Res 4: 38-50.
- 9. Cilurzo, F., Minghetti, P., Casiraghi, A., et al. Characterization of nifedipine solid dispersions. (2002) Int J Pharm 242(1-2): 313-317.
- 10. Vippagunta, S.R., Maul, K.A., Tallavajhala, S., et al. Solid-state characterization of nifedipine solid dispersions.(2002) Int J Pharm 236(1-2): 111-123.
- 11. Alam, A.A., Al Masum, M.A., Islam, R.B., et al. Formulation of co-precipitate and surface co-precipitate of nifedipine: A comparative study. (2013) African J Pharm Pharmacol 7(25): 1707-1718.
- 12. Keratichewanun, S., Yoshihashi, Y., Sutanthavibul, N., et al. An investigation of nifedipine miscibility in co-precipitates using raman spectroscopy. (2015) Pharm Res 32(7): 2458-2473.
- 13. Soulairol, I., Tarlier, N., Bataille, B., et al. Spray-dried co-precipitates of nifedipine and vinylcaprolactam/vinylacetate/PEG6000 for compacted oral formulations. (2015) Int J Pharm 481(1-2): 140-147.
- 14. Li, X., Jiang, C., Pan, L., et al. Effects of preparing techniques and aging on dissolution behavior of the co-precipitates of NF/Soluplus/Kollidon SR: identification and classification by a combined analysis by FT-IR spectroscopy and computational approaches. (2015) Drug Dev Ind Pharm 41(1): 2-14.
- 15. Altamimi, M.A., Neau, S.H. Use of the Flory-Huggins theory to predict the solubility of nifedipine and sulfamethoxazole in the triblock, graft copolymer Soluplus. (2015) Drug Dev Ind Pharm 42(3): 446-455.
- 16. BASF. Soluplus - technical information. The BASF Chemical Company-Pharma Ingredients & Services, Limburgerhof. (2010)
- 17. Hardung, H., Djuric, D., Ali, S. Combining HME & solubilization: Soluplus -The solid solution. (2010) Drug Deliv Tech 10: 20–27.
- 18. Lee, D.H., Yeom, D.W., Song, Y.S., et al. Improved oral absorption of dutasteride via Soluplus®-based supersaturable self-emulsifying drug delivery system (S-SEDDS). (2015) Int J Pharm 478(1): 341-347.
- 19. Linn, M., Collnot, E.M., Djuric, D., et al. Soluplus® as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. (2012) Eur J Pharm Sci 45(3): 336-343.
- 20. Burger A, Koller KT. Polymorphism and pseudopolymorphism on nifedipine. (1996) Sci Pharm 64: 293-301.
- 21. Greenhalgh, D.J., Williams, A.C., Timmins, P., et al. Solubility parameters as predictors of miscibility in solid dispersions. (1999) J Pharm Sci 88(11): 1182-1190.
- 22. Liu, J., Cao, F., Zhang, C., et al. Use of polymer combinations in the preparation of solid dispersions of a thermally unstable drug by hot-melt extrusion. (2013) Acta Pharm Sinica B 3(4): 263-272.
- 23. De Schaefer, G.R., De Ruiz Holgado, M.E.F, Arancibia, E.L. Solubility parameters, hydrophile-lipophile balance and solubility in sucrose derivatives surfactants obtained by GLC. (2002) J Arg Chem Soc 90: 55-63.
- 24. Hecq, J., Deleers, M., Fanara, D., et al. Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. (2005) Int J Pharm 299(1-2): 167-177.
- 25. Mahale, A.M., Sreenivas, S.A. Enhancement of dissolution profile of nifedipine by co-precipitate technique. 2011 J Pharm Cosmetol 1:1-8.
- 26. Ntawukulilyayo, J.D., Bouckaert, S., Remon, J.P. Enhancement of dissolution rate of nifedipine using sucrose ester coprecipitates. (1993) Int J Pharm 93(1-3): 209-214.
- 27. Chan, K.L.A., Fleming, O.S., Kazarian, S.G., et al. Polymorphism and devitrification of nifedipine under controlled humidity: a combined FT-Raman, IR and Raman microscopic investigation. (2004) J Raman Spectrosc 35(5): 353-359.
- 28. Rajeswari, K.R., Abbulu, K., Sudhakar, M., et al. Studies on dissolution enhancement of lovastatin using soluplus by solid dispersion technique. (2012) Int J Pharm Pharm Sci 4(3): 124-128.
- 29. Sambath, L., Kottaimuthu, A., Ashis Kumar, M, et al. Physicochemical characterization and in-vitro dissolution behavior of gliclazide – soluplus co-precipitates. (2013) Int J Pharm Pharm Sci 5(2): 204-210.
- 30. Shamma, R.N., Basha, M. A novel polymeric solubilizer for optimization of carvedilol solid dispersions: Formulation design and effect of method of preparation. (2013) Powder Technol 237: 406-414.
- 31. Lian, X., Dong, J., Zhang, J., et al. Soluplus® based 9-nitrocamptothecin co-precipitate for peroral administration: Preparation, characterization, in vitro and in vivo evaluation. (2014) Int J Pharm 477(1-2): 399-407.
- 32. Dong, Z., Chatterji, A., Sandhu, H., et al. Evaluation of solid state properties of co-precipitates prepared by hot-melt extrusion and solvent co-precipitation. (2008) Int J Pharm 355(1-2): 141-149.
- 33. Hughey, J.R., Keen, J.M., Miller, D.A., et al. The use of inorganic salts to improve the dissolution characteristics of tablets containing Soluplus° -based co-precipitates. (2013) Eur J Pharm Sci 48(4-5): 758-766.
- 34. Foppoli, A., Petroni, C., Palugan, L., et al. Development of Soluplus®-based co-precipitates containing a disintegration adjuvant. (2014) IX Word Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology.
- 35. Yu, H., Xia, D., Zhu, Q., et al. Supersaturated polymeric micelles for oral cyclosporine: A delivery. (2013) Eur J Pharm Biopharm 85(3 Pt B): 1325-1336.
- 36. Chen, C., Xie, X., Li, Y., et al. Influence of different polymers on crystallization tendency and dissolution behavior of cilnidipine in solid dispersions. (2014) Drug Dev Ind Pharm 40(4): 441-451.
- 37. Aso, Y., Yoshioka, S. Molecular mobility of nifedipine-PVP and phenobarbital-PVP co-precipitates as measured by 13C-NMR spin-lattice relaxation time. (2006) J Pharm Sci 95(2): 318-325.
- 38. Szűts, A., Makai, Z., Rajkob, R., et al. Study of the effects of drugs on the structures of sucrose esters and the effects of solid-state interactions on drug release. (2008) J Pharm Biomed Anal 48(4): 1136–1142.
- 39. Ntawukulilyayo, J.D., Bouckaert, S., Remon, J.P. Enhancement of dissolution rate of nifedipine using sucrose ester coprecipitates. (1993) Int J Pharm; 93(1-3): 209-214.
- 40. Cespi, M., Casettari, L., Palmieri, G.F., et al. Rheological characterization of polyvinylcaprolactam–polyvinyl acetate–polyethylene glycol graft copolymer (Soluplus®) water dispersions. (2014) Colloid Polym Sci 292(1): 235-241.
- 41. Hughey, J.R., Keen, J.M., Miller, D.A., et al. The use of inorganic salts to improve the dissolution characteristics of tablets containing Soluplus®-based solid dispersions. (2013) Eur J Pharm Sci 48(4-5): 758-766.