Role of Metastable and Spore Hydration to Sterilize Spores by Nitrogen Gas Plasma Exposure and DPA Analysis by HPLC Combined with UV Detection
Affiliation
Chuo University, School of Science, 1-13-27, Kasuga Bunkyo 112-0003, Tokyo
Corresponding Author
Hideharu Shintani, Chuo University, School of Science, 1-13-27, Kasuga Bunkyo 112-0003, Tokyo, Tel: +81425922336, E-mail: shintani@mail.hinocatv.ne.jp
Citation
Shintani, H. Role of Metastable and Spore Hydration to Sterilize Spores by Nitrogen Gas Plasma Exposure and DPA Analysis by HPLC Combined with UV Detection. (2014) J Pharma Sci Drug Des 1(1): 14- 17.
Copy rights
© 2014 Shintani, H. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Nitrogen gas plasma; Radicals; Metastables; Dipicolinic acid; Hydration; Reverse phase HPLC; Ion suppression
Abstract
Many papers have been published so far on gas plasma sterilization. They are mostly conducted by physical researchers, so microbiology and chemical aspects are significantly insufficient. By joining the biologists and chemists, gas plasma sterilization research was outstandingly advanced. The sterilization mechanism by gas plasma was not clarified until now and from the life time and some other reasons metastables or photon can be the most appropriate candidate for sterilization. Spore death is explained from the hydration of dipicolinic acid in the core. By attacking metastables or photons by E=hv energy to spores, it produced pin hole and the interior water and surrounding water penetrate into the core to hydrate DPA. DPA in the surface was collected with water and enriched with SPE (solid phase extraction) column. SPE drain was analyzed with C-18 HPLC column and eluted with an acetonitrile/water (1/4, v/v, pH5) and detected with 235nm. From the coincidence of retention time of the studied target and that of standard substance, spore surface particle can be confirmed DPA. The hydration process can cause within spore, so spore figures are unchanged before and after sterilization.
Introduction
Many papers on gas plasma sterilization have ever been published so far[1-7] and most of papers and books on gas plasma sterilization were conducted by physical researchers[3-7]. Research in sterilization methods and mechanism of sterilization is required to integrate combined knowledge of chemical, engineering and microbiological aspects. The combined effort of these specialists in the area of gas plasma sterilization have contributed to improvement in this area of research[1-2]. The present study aims to clarify our understanding of the mechanisms of sterilization by nitrogen gas plasma.
It is said that hydration of dipicolinic acid (DPA) is the cause of spore death. We find that quite small particle on spore surface by scanning electron microscopy (SEM) and we speculate that small particle may be hydrated dipicolinc acid migrated from the core. In order to clarify this speculation and small particle is DPA or not, they are analyzed by HPLC and MS (mass spectrometry).
Materials and Methods
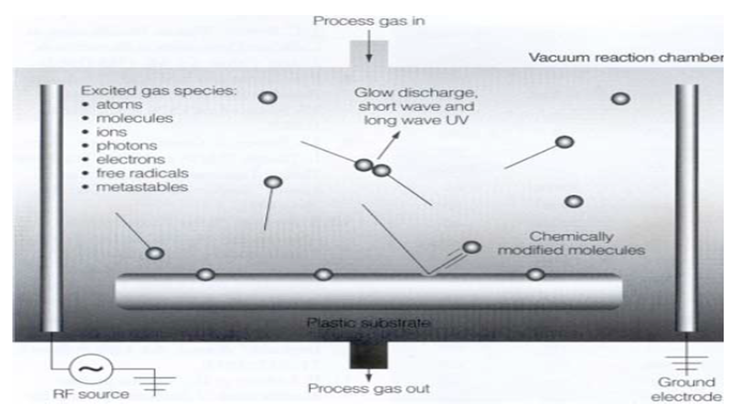
Low pressure nitrogen gas plasma apparatus
The nitrogen gas plasma sterilization chamber used was similar to that described in (Figure 1 in citation 1). The low pressure nitrogen gas plasma apparatus can be used at around 60oC under half atmospheric pressure with 40 to 150 mm gap between the cathode and anode. The sterilization assurance level (SAL) of 100, starting from initial population of 106 CFU (Colony Forming Unit) was achieved in 9min, indicating D value (decimal reduction value, time or does to use one log reduction) is 0.75 min (ISO 11138-1).
Sterilization process of microorganisms
Sterility assurance was confirmed by using the biological indicator (BI). As the BI Geobacillus stearothermophilusATCC 7953[1,2] was inoculated onto a surface modified SUS (stainless steel) by gas plasma and 1x 106 CFU were evenly distributed on the modified SUS surface at nm level to avoid forming clumps[2]. Relatively clump free BI was commercially available from Merck (Tokyo, Japan).
HPLC and automated solid phase extraction (SPE) conditions
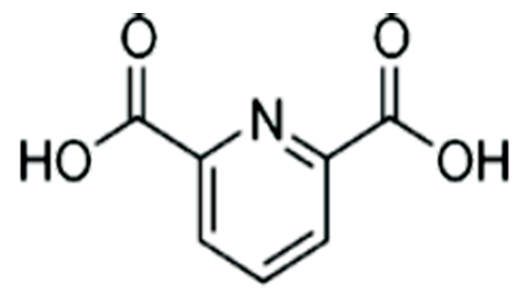
Sterilized spores were extracted with water. Extraction with water was used to distinguish between DPA on the surfaces and within core. As the solubility of DPA into water is only 1%, more than 100 BIs was necessary to collect sterilized spores. The collected water including sterilized spores was adjusted with acetic acid to pH5 to suppress ionization of carboxylic acid of DPA (dipicolic acid, Figure 8) and adhere to C-18 support of SPE. A pH5 adjusted water applied to C18 SPE column in order to adsorb dipicolinic acid to C18 column and eluted with acetonitrile. Acetonitrile was evaporated, condensed and re-dissolved into mobile phase of HPLC (acetonitrile/water, 1/4, v/v, pH5). The 10μL of this solution were applied into HPLC and detected with 235 nm and MS.
Results and Discussion
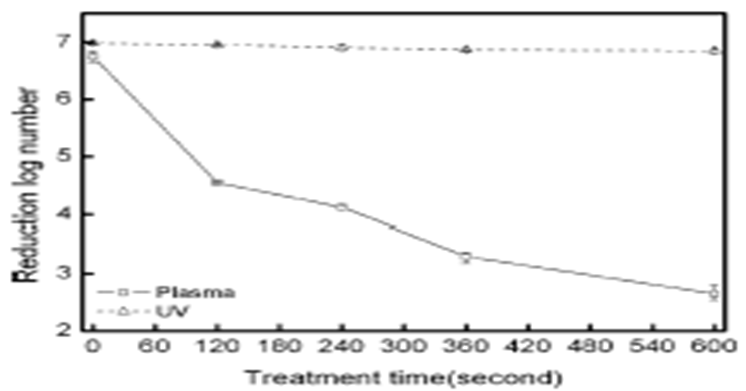
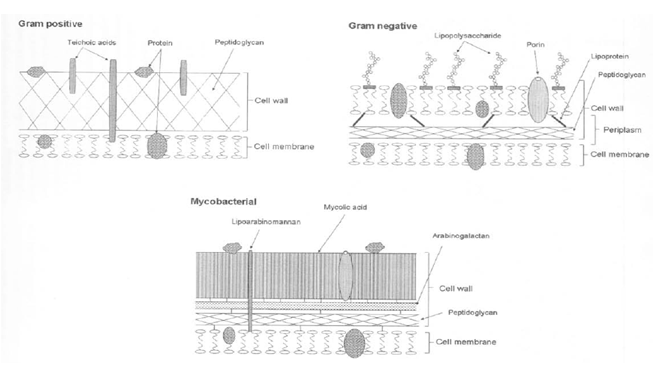
As shown in Figure 1, it has been reported several sorts of sterilization factors to sterilize bacterial spores and microorganism. Among them they are atoms, molecules, positively charged and negatively charged ions, photons, electrons, free radicals and metastables and UV and VUV. Among these UV and VUV contribution was denied by Kong et al[3] (Figure 2). Free radicals, especially OH radical, are an attractive factor due to high oxidation-reduction potential (Table 1), but life period of OH radicals is too short (around a few μs, Table 2) and flight distance during life period of OH radical compared with N2 metastable is too short (Table 3), therefore OH and NO radicals may contribute to sterilization as minor factors, but not as a major factors. Photon has also long life of 1018 years, so flight distance will be infinity. Metastable and photon have an energy due to E = hv and long distance flight, therefore they may be major candidate of the sterilization. From excited state down to ground state, metastables and photons emitted energy as per the equation of E = hv (Figure 5), the produced energy attack the surface layer on the spore or microorganisms and produce pin hole on the spore surface. Ions or charged ones such as electrons in Figure 1 are trapped with the outer membrane of the bacterial or spore outer layers (Figures 3 and 4), so charged ones are considered not to major candidates.
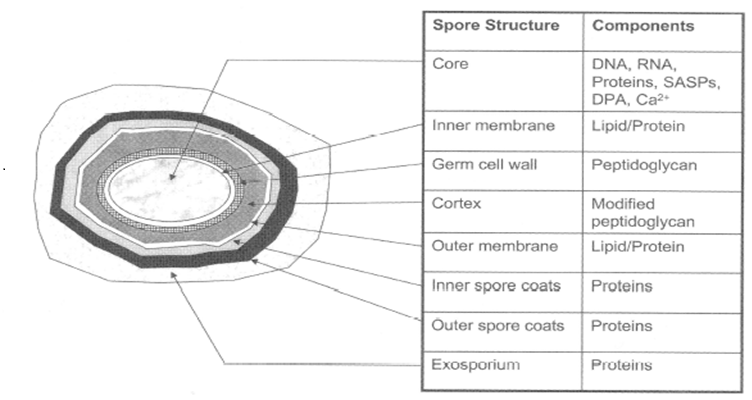
Figure 3: Typical bacterial-endoscope structure DPA is dipicolinic acid and in normal it exists as DPA = Ca (chelating).
Table 1 : Oxidation-reduction potential (V)
| Chemical name | Chemical structure | Oxidation-reduction voltage (V) |
|---|---|---|
| Hydroxy radical | OH. | 2.05 |
| Oxygen atom | O1 | 1.75 |
| Ozone | O3 | 1.52 |
| Hydrogen peroxide | H2O2 | 1.30 |
| Hypochlorous acid | HClO | 1.10 |
| Oxygen molecule | O2 | 0.94 |
Table 2 : Life time of free radicals and metastables
| Chemical name | Life time |
|---|---|
| OH radical | a few s |
| NO radical | a few s |
| Singlet molecular oxygen ¹O2 Oxygen metastable | 7 s |
| Nitrogen metastable | 2 s |
| Photon | 1018 year |
Table 3 : Flight distance
| Species | Flight distance during life period |
|---|---|
| OH radical | 0.003 cm/μs |
| N2 metastable | 144 cm/2s |
| Photon | infinite |
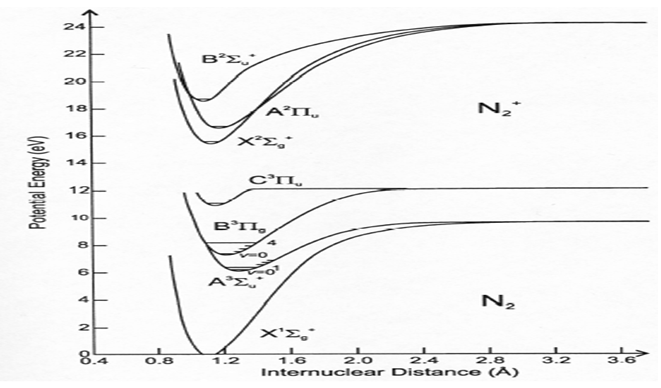
Figure 5: Energy of excited N2 and ground N2 The upper is the excited state and the lower is the ground state.
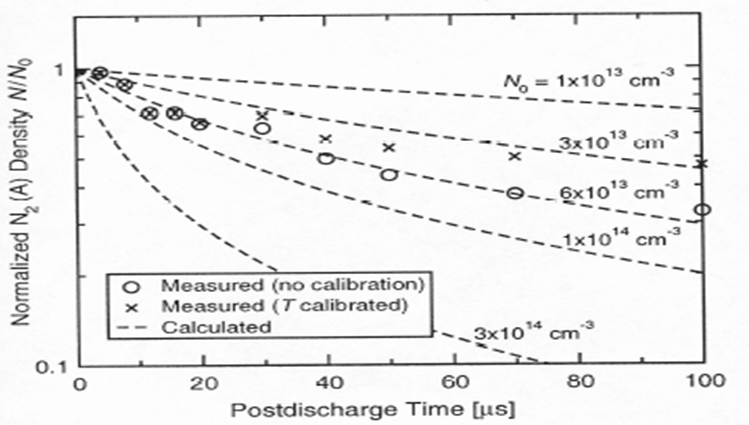
Among metastable, we find that lifetime of singlet Oxygen is 7s and that of nitrogen metastable is 2s (Table 2). As shown in Figure 5, we can observe that the N2 metastable was produced by applying ulsed-amperometric discharge and we measure the lifetime of N2 metastable as a few s (experimentally 2s, Table 2 and Figure 6)[4-6]. During the excited N2 down to the ground state, energy emitted is useful for disruption of spores and bacterial cells. We can therefore hypothesize that N2 or O2 metastables and photon may be the most favorable candidates to inactivate bacterial spores and bacterial vegetative state.
However, we have a problem. Bacterial spore death is considered by causing hydration of DPA (Figures 3 and 8)[8-13], so how can we connect N2 metastable to hydration of DPA. We observed dead spores with SEM (scanning electron microscopy) carefully and found out that dead spores had several white particles on their spore surface. We consider that the white particle may be hydrated dipicolinic acid, so we carefully collected surface white particles with water. This is because if organic solvent is used in place of water, interior DPA may also be recovered together with surface white particle. Use of water as an extraction solvent is for the distinction of DPA in surface and in the core.
HPLC analysis of DPA
Water trapped white particle was adjusted to pH5 with acetic acid and applied to C-18 SPE and thereafter eluted with acetonitrile. SPE was automated type SPE of BenchmateR. The acetonitrile was evaporated and re-dissolved with mobile phase of acetonitrile/water with acetic acid to pH5 (1/4, v/v, pH5). 10μL was injected to HPLC and detected at 235nm. Stationary phase is C-18 (4.6 X 200mm, Shiseido). We confirmed that the interior (within core) DPA was not eluted out and collected surface DPA only was extracted with water. The retention time coincided with that of standard DPA, therefore surface substance is DPA causing spore death. In addition, we confirmed both are identical from the results of MS fragmentation.
The core DPA was hydrated with the penetrated water caused by N2 metastable and photon and core DPA transported from the core to the surface of the spore. Water for hydration is from the interior of spore (Table 4) as well as surrounding spore. We now speculate how the spore died with maintaining spore figure (Figure 7)[7]. The spore surface was attacked by metastables and photon to produce pinhole of the spore surface following the equation of E = hv. The water surrounding the bacterial spore and interior water of the spore (Table 4) may penetrate into the core to hydrate DPA (Figures 3 and 8). The killing process may be caused within the spore, therefore figures of the dead spore were identical to those of the viable spore (Figure 7)[1-3].
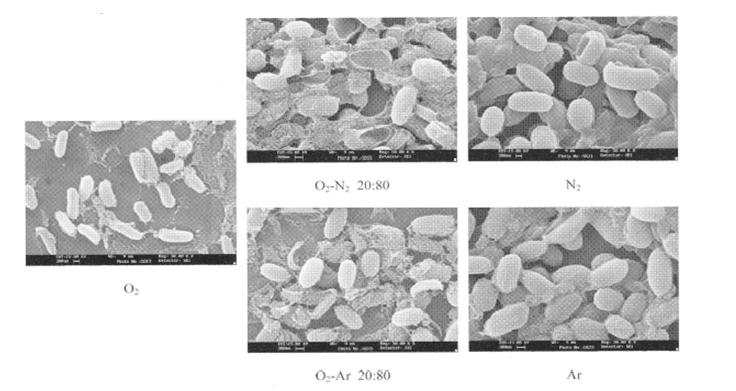
Figure 7: Spore structure after exposure by several gas plasmas From the result, N2 and Ar plasma was unchanged before and after exposure.
Table 4 : Hydrophobicity of several spores
| Strain | Hydrophobicity (%) |
|---|---|
| Bacillus subtilis ATCC 6633 | 94 |
| B. subtilis ATCC 19221 | 95 |
| B. atrophaeus ATCC 9372 | 47 |
| B. cereus T | 95 |
| B. coagulans ATCC 8038 | 49 |
| G. stearothermophilis ATCC 9372 | 53 |
| B. megaterium ATCC 12872 | 88 |
| B. megaterium ATCC 33729 | 30 |
| Clostridium botulinum 213B | 50 |
| C. sporogens ATCC 7955 | 67 |
| C. putrefaciens ATCC 25786 | 78 |
Conclusion
Spore surface pinholes were produced by exposure to the gas plasma with the energy from the N2 metastables and photon. DPA in core was hydrated with interior water and the surrounding water of the spore. The hydrated DPA migrated into the surface layer and remained as white particle. We hypothesize that this is the probable mechanism of spore death by metastables and photon. The spore structures were not changed after death because the killing process was mainly caused within core. This is the mechanism of sterilization of spores by nitrogen gas plasma exposures.
DPA can be analyzed by ion-suppression reversed phase C-18 combined with UV 235 nm and the mobile phase was acetonitrile-water (1/4, v/v) at pH 5 with acetic acid. In addition, we confirmed both are identical from the results of MS fragmentation.
References
- 1. Shintani, H., Shimizu, N., Imanishi, Y., et al. Inactivation of microorganisms and endotoxins by low temperature nitrogen gas plasma exposure. (2007) Biocontrol Sci 12(4): 131-143.
- 2. Sakudo, A., Shintani, H. Sterilization and Disinfection by Plasma, Sterilization Mechanisms, Biological and Medical Applications. (2011) NOVA, Science Publishers, Inc., New York.
- 3. Kong, M.G., et al. Physical mechanisms of inactivation of Bacillus subtilis spores using cold atmospheric plasma. (2006) IEEE Trans Plasma Sci 34(4): 1310-1316.
- 4. Ono Ryo., et al. Laser-induced fluorescence of N2 metastable in N2 pulsed positive corona discharge, 18025006. Plasma Sources Sci. Technol.
- 5. Guerra, V., et al. Role played by the N2 metastable in stationary N2 and N2-O2 discharge. (2001) J. Phys D Applied Phys 34(12): 1745-1755.
- 6. Teramoto Y, et al., Laser-induced fluorescence of metastable N2 in various gases pulsed positive corona discharge. Conf. Presentation- Session 2.2
- 7. Rossi F, et al. Mechanism of sterilization and decontamination of surfaces by low-pressure plasma. (2008) Advanced Plasma Technology (Agostino R et al, eds), Wiley-VCH, pp. 319.
- 8. Setlow, B., Atluri, S., Kitchel, R., et al. Role of dipicolic acid in resistance and stability of spores of Bacillus subtilis with or without DNA-protective α/β type small acid soluble proteins. (2006) J Bacteriol 188(11): 3740-3747.
- 9. Young, S.B., and Setlow, P. Mechanism of killing of Bacillus subtilis spore by hypochlorite and chlorine dioxide. (2003) J Appl Microbiol 95(1): 54-67.
- 10. Yaohua, H. Non-thermal plasma inactivation of Bacillus amyloliquefaciens spore. (2011) "Master's Thesis, University of Tennessee.
- 11. Reineke, K., Schlumbach, K., Baier, D., et al. The release of dipicolinic acid-the rate-limiting step of Bacillus endospore inactivation during the high pressure thermal sterilization process. (2013) Int J Food Microbiol 162(1): 55-63.
- 12. Zhang, P., Kong, L., Wang, G., et al. Monitoring the wet-heat inactivation dynamics of single spores of Bacillus species by using Raman tweezers, differential interference contrast microscopy, and nucleic acid dye fluorescence microscopy. (2011) Appl Environ Microbiol 77(14): 4754-4769.
- 13. Silva, M.P., Pereira, C.A., Junqueira, J.C., and Jorge, A.O.C. Methods of destroying bacterial spores. (2013) Microbial pathogens and strategies for combating them: science, technology and education, A. Mendez-Vilas, ed, Formatex, pp490-496.