Serum Albumin level can predict Cardiorenal Syndrome type -1
HITEN PATEL
Corresponding Author
Hiten R Patel, New York Medical College, Saint Joseph Regional Medical Center Main Street, Paterson, New Jersey–(U.S.A), Tel: (917) 627-1437/(973)754-2028/Fax:(973)754-4349; E-mail: hiten0409@yahoo.com
Citation
Hiten R.P., et al. Serum Albumin Level Can Predict Cardiorenal Syndrome Type-1. (2017) J Heart Cardiol 3(2): 40- 45.
Copy rights
© 2017 Hiten R.P. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License
Keywords
Cardiorenal Syndrome type1; Acute Decompensated Heart Failure; Hypoalbuminemia; Acute Kidney Injury; Serum Albumin
Abstract
Background: Cardiorenal Syndrome Type1 (CRS1), defined as increase in serum Creatinine (Cr) of ≥ 0.3 mg/dl from the baseline, occurs in approximately 25 - 40% of patients admitted with Acute Decompensated Heart Failure (ADHF) and presents a myriad of challenges in diagnosis and management. Hypoalbuminemia is independently associated with ADHF and acute kidney injury.
Aim: To study if serum albumin level can predict the CRS1.
Method: Retrospective chart review including the renal function and hospital course of all ADHF patients were performed. Patients were excluded if there was insufficient laboratory data, admission serum creatinine > 2.5 mg/dl and recent use of radio contrast and if the reason of admission was deemed to be other than ADHF.
Results: Over two years, 201 patients (mean age_68.1, 48% male) were enrolled. Despite the standard dose of furosemide about 43% patients developed CRS-1. S. Alb cut-off level of 3.4 g/dl was determined from ROC curve; patients with S.Alb < 3.4 g/dl (group1, n = 90) were compared with patients with higher S.Alb (group 2, n = 111). Group 1 had more elder patients and worse renal function on admission (P = 0.02 and P = 0.012 respectively). Group 1 was found to be at a higher risk of developing CRS1 during hospitalization (OR = 4.48; 95% CI 2.46 to 8.14; P = < 0.0001; adjusted OR for age = 2.7; 95% CI 1.2 to 6.9; P = 0.03; adjusted OR for < 30 ml/min creatinine clearance on admission = 1.7; 95% CI 1.1 to 4.0; P = 0.04) and also had significantly longer hospital stay (13.3 ± 17.5 versus 7.2 ± 5.3; P = 0.003).
Conclusion: Hypoalbuminemia (S.Alb < 3.4g/dl) predicts CRS1 and a complicated hospital course in patients with ADHF.
Introduction
Background
Cardiorenal Syndrome Type1 (CRS1) is characterized by the development of Acute Kidney Injury (AKI) in a patient with Acute Decompensated Heart Failure (ADHF). CRS1 occurs in approximately 25 - 40% of patients admitted with ADHF and presents a myriad of challenges in diagnosis and management[1,2]. Hypoalbuminemia is detected in up to 28% of ADHF patients and is a proven independent mortality predictor in patients with ADHF[3-5]. Hypoalbuminemia is a significant independent predictor for AKI and increased mortality following AKI in patients of surgery, intensive care unit and other hospital settings[6]. Although hypoalbuminemia is independently associated with ADHF and AKI, its role in predicting CRS1 has not been published.
Method
The investigators performed a retrospective cohort study from electronic medical records of adult patients admitted to a 464-bed teaching tertiary-care medical centre in Brooklyn, New York (USA) with a primary diagnosis of ADHF. Data collection was done from July 2011 to July 2013 and the Institutional Review Board approved the study. There was no contact or attempt to contact any of the subjects of the study. Once medical records were identified by admission diagnosis code, baseline characteristics including patient’s demographic profile, comorbidities, left ventricle ejection fraction estimated by echocardiography and medication information were gathered during the same admission. Causes of heart failure were extracted from cardiology consultations and medical records. Patients were excluded if there was insufficient laboratory data, lack of echocardiographic results, admission serum creatinine (SCr) > 2.5 mg/dl, any recent use of radio contrast and if the reason of admission was deemed to be other than ADHF. CRS1 was defined as an increase in SCr of ≥ 0.3 mg/dl from the baseline SCr on admission.
Statistical analysis
For normally distributed continuous variables, data are expressed as means and Standard Deviations (SDs); for categorical variables, data are expressed as frequency (percentage). Groupwise comparisons for normally distributed variables were made using Student’s t test and categorical data were compared using Fisher’s exact test. Decision levels (cut-offs) to dichotomize interval variables were determined by ROC curves. The Odds Ratio (OR), its standard error and 95% confidence interval are calculated according to Altman, 1991. Logistic regression was used to adjust ORs for differences in baseline characteristics if the P value for the difference between the groups was less than 0.1. Pearson correlation coefficient (r) was used to measure the statistical association between two non-normally distributed variables. Kaplan-Meier survival analysis was done using Med Calc Software Version 17.9.7. For the present study, α was set at 0.05; thus, P < 0.05 (two-sided) was considered to be statistically significant. Data were analyzed using SPSS Version 13.0 statistical software (SPSS Incorporated, Chicago, Illinois) except for logistic regression, which was performed using an online routine (available at www.statpages.org/logistic.html; accessed on January 8, 2015).
Results
From our sample of 201 patients (mean age_68.1, SD_15.8), 48% were male and majority was African American. The mean serum albumin level on admission was 3.5 g/dl with SD_0.4. The mean admission Creatinine Clearance (CrCl) was 57.3 ml/min with SD_28.7. Average daily dose of intravenous furosemide for first 3 days was 66.4 mg. Despite this standard dose of furosemide about 43% patients developed CRS1. Of note, patients with CRS1 did not receive a higher dose of diuretics compared to the ones who did not develop CRS1 (P = 0.40).
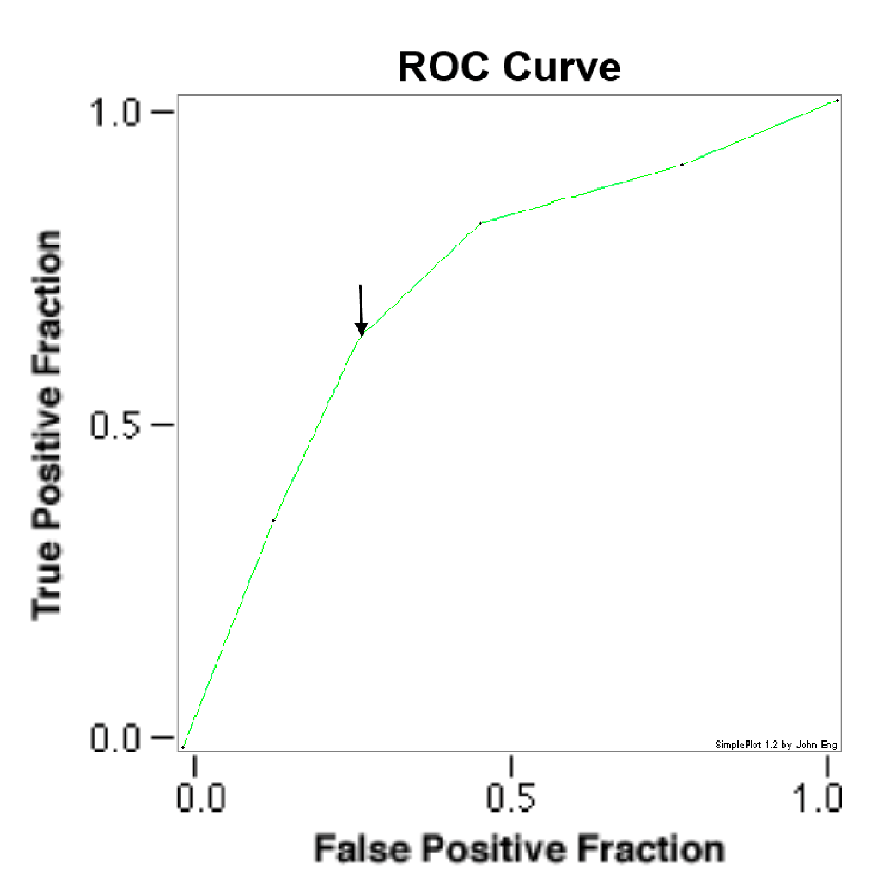
To establish a cut-off level of Serum Albumin, an ROC curve was constructed (Figure 1). From this curve, a cut-off value of 3.4 g/dl was determined (AUC_0.7152 ± 0.0369, P = 0.0439) and the patients were dichotomized to group 1 (serum albumin less than 3.4 g/dl) and group 2 (serum albumin greater than 3.4g/dl). Their baseline characteristics are mentioned in Table 1. Group 1 (90 patients; mean age 71.0 ± 14 years, 44% men) was not significantly different from group 2 (111 patients; mean age 66.0 ± 16 years, 53% men) in their baseline characteristics, except for their age which was statistically significant with a P = 0.02.
Figure 1: ROC curve for serum albumin and CRS 1. 2nd point on curve (black arrow) corresponds to serum albumin level of 3.4 g/dl with AUC_0.7152 ± 0.0369, P = 0.0439. ROC receiver operating characteristic; AUC Area under curve.
Table 1: Baseline Characteristics.
| Group 1 (Albumin < 3.4 g/dl) |
Group 2 (Albumin > 3.4 g/dl) |
P value | |
|---|---|---|---|
| N = 90 | N = 111 | ||
| Age, years, mean ± SD | 71 ± 14 | 66 ± 16 | 0.02 |
| Sex (Male) | 44 | 52 | 0.33 |
| Race | |||
| African American | 82 | 74 | 0.16 |
| Hispanic | 10 | 20 | 0.055 |
| White | 8 | 4 | 0.19 |
| Asian | 0 | 2 | 0.2 |
| DM | 54 | 45 | 0.18 |
| HTN | 83 | 76 | 0.2 |
| CAD | 50 | 52 | 0.97 |
| BMI, kg/m², mean ± SD | 29 ± 5 | 28 ± 6 | 0.2 |
| Medications | |||
| ACEi/ARB | 94 | 88 | 0.16 |
| Beta-blockers | 90 | 84 | 0.197 |
| Spironolactone | 24 | 22 | 0.77 |
| Digoxin | 27 | 29 | 0.72 |
| HF Etiology | |||
| Ischemic | 47 | 50 | 0.68 |
| Hypertension | 12 | 8 | 0.33 |
| Valvular | 2 | 5 | 0.37 |
| Idiopathic | 39 | 37 | 0.78 |
Data presented as % (percentage) unless otherwise specified. ACEi Angiotensin-converting enzyme inhibitor; ARB Angiotensin receptor blocker; BMI Body mass index; CAD Coronary artery disease; DM Diabetes Mellitus; HF Heart Failure; HTN Hypertension
When other potential clinical predictors of worsening renal function[7,8] during ADHF hospitalization were compared (Table 2), it was clear that group 1 had more elder patients (mean age 66.0 ± 16 years versus 71.0 ± 14 years; P = 0.02) and also more number of patients with lower i.e.< 30 ml/min CrCl on admission (30% versus 16%; P = 0.012).
Table 2: Other Predictors of Worsening Renal Function.
| Group 1 (Albumin < 3.4 g/dl) |
Group 2 (Albumin > 3.4 g/dl) |
P value | |
|---|---|---|---|
| N = 90 | N = 111 | ||
| Age (years) | 71 ± 14 | 66 ± 16 | 0.02 |
| Admission SBP(mmHg) | 145.8 ± 33.7 | 141.5 ± 29.6 | 0.35 |
| < 100 mmHg, % 6 3 | 6 | 3 | 0.3 |
| LVEF (percent) | 39.7 ± 17.3 | 35.5 ± 17.4 | 0.1 |
| < 40 percent, % | 31 | 22 | 0.12 |
| Admission CrCl (ml/min) | 50.8 ± 30.2 | 59.4 ± 32 | 0.0535 |
| < 30 ml/min, % | 30 | 16 | 0.012 |
| > 30 ml/min, % | 31 | 21 | 0.23 |
| Serum Sodium levels < 130 (meq/L), % | 38 | 31 | 0.29 |
| Avg. daily furosemide dose for first 3 days (mg/day) | 70.4 ± 20.7 | 64.8 ± 24.4 | 0.085 |
| BNP | 1216 ± 67 | 1116 ± 67 | 0.32 |
Data presented as mean ± SD unless otherwise specified. CrCl Creatinine Clearance; LVEF Left Ventricular Ejection Fraction; SBP Systolic Blood Pressure; BNP Brain Natriuretic Peptide.
Group 1 (serum albumin < 3.4 g/dl) was found to be at a higher risk of developing CRS-1 during hospitalization (OR = 4.48; 95% CI 2.46 to 8.14; P = < 0.0001). This was independent of age (adjusted OR = 2.7; 95% CI 1.2 to 6.9; P = 0.03) and lower i.e. < 30 ml/min CrClon admission (adjusted OR = 1.7; 95% CI 1.1 to 4.0; P = 0.04).
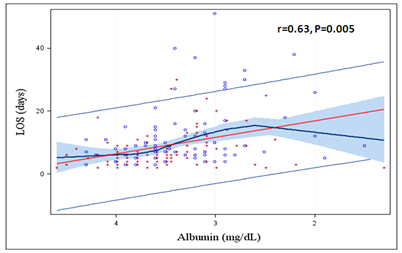
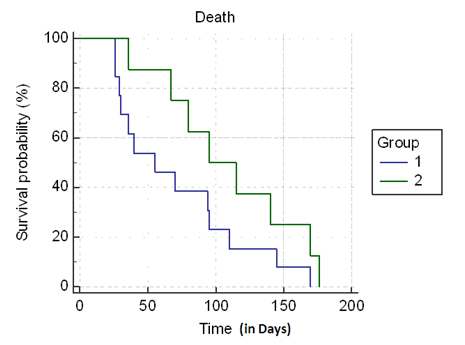
Patients who developed CRS1 had significantly longer hospital stay (13.3 ± 17.5 versus 7.2 ± 5.3; P = 0.003) (Table 3). Also, lower serum albumin had statistically significant correlation with higher Length Of Stay (LOS) on index hospitalization (r = 0.63, P = 0.005) (Figure 2). Patients with CSR1 had worse clinical outcomes with higher in hospital mortality (n = 7 versus 2; P = 0.04) and a trend towards higher 6-months mortality (n = 13 versus n = 8; P = 0.09) (Table 3). Later result might be skewed because mortality data was not available for all patients. Figure 3 shows the Kaplan-Meier survival analysis.
Table 3: Prognosis.
| CRS1(N = 87) | No CRS1 (N = 114) | P value | |
|---|---|---|---|
| Length of stay in days | 13.3±17.5 | 7.2±5.3 | 0.003 |
| In hospital Mortality (n) | 7 | 2 | 0.04 |
| 6-month Mortality* (n) | 13 | 8 | 0.09 |
Data presented as mean ± SD unless otherwise specified. *Mortality data were not available for about 50% of the study population.
Figure 2: Correlation between serum albumin levels and Length Of Stay (LOS) for Acute Decompensated Heart Failure. Lower the serum albumin level, higher the LOS on index hospitalization.
Figure 3: Kaplan-Meier survival curves for patients admitted with Acute Decompensated Heart Failure by Cardiorenal Syndrome 1 (CRS1). Group 1 -CRS1 (blue line), Group 2 - No CRS1 (green line).
Discussion
Hypoalbuminemia has been associated with poor outcome in chronic systolic heart failure[9] and in patients with heart failure with preserved ejection fraction[10]. It has also been associated with lower survival rate and a higher rate of cardiovascular death in patients with ADHF[3-5]. Possible pathophysiologic mechanism(s) for hypoalbuminemia in patients with heart failure include malnutrition from decreased nutritional intake, increased catabolic activity and decreased hepatic synthesis, while hemodilution, hyper metabolic activity, chronic inflammation, proteinuria, protein-losing enteropathy and other mechanisms may also be causative[9].
Hypoalbuminemia is a significant independent predictor for AKI and of death following AKI development in clinical studies of patients in surgical, intensive care unit and other hospital settings[6]. Although hypoalbuminemia is independently associated to baseline higher concentration of blood urea nitrogen and SCr, its prevalence and role in predicting CRS1 has not been published[11,12].
Our patient population is similar to nationally described cohort in age, sex, admission BP groups, length of stay and LVEF. It differs in race/ethnicity group and admission renal insufficiency[13]. The mean admission CrCl was 57.3 ml/min with SD_28.7. Previous studies[14] have shown that a furosemide dose of more than 160 mg/day was associated with worse hospital outcome and impaired renal function. In our cohort, CRS1 developed in 43% of patients despite a low average dose of furosemide (66.4 mg/day). Factors such as increased sympathetic activity from pulmonary congestion and renal venous congestion[15] may have played a role. Also, patients with CRS1 did not receive a higher dose of diuretics compared to the ones who did not develop CRS1 (P = 0.40).
In our study, we demonstrated that a serum albumin level < 3.4 g/dl is associated with an increased risk of CRS1 in patients admitted with ADHF. Uthamalingam S. et al. also described their cohort of patients with ADHF using similar serum albumin cut off of 3.4 g/dl(4). In their cohort, patients with hypoalbuminemia were more likely to have class III/IV heart failure symptoms, lower extremity edema, prior history of chronic obstructive pulmonary disease, previous heart failure and higher concentrations for blood urea nitrogen, SCr and B-type natriuretic peptide, but lower serum sodium, hemoglobin and cholesterol levels and were less likely to be taking ACEIs and/or ARBs. There were no differences between two albumin groups in both LVEF ≤ 40% and > 40% parameters. In our study, almost 45% of patients had hypoalbuminemia and they were more likely to be elder and had worse renal function on admission. Hyponatremia, LVEF < 40% and medication use was similar in both albumin groups.
The present study did not look specifically into the dosages of antihypertensive medications, whether the patients received the target dose for heart failure or whether the dosages were titrated during admission. However, the use of these medications has not been shown to be an independent risk factor for CRS1[12,16-19]. Butler et al[20] classified the doses of different ACE inhibitors used in the heart failure therapy of 382 patients into low, medium and high doses, but did not find any relationship between renal insufficiency and ACE inhibitors at any dose. In ADHF, the cardiovascular system becomes dependent on tachycardia to maintain cardiac output and acute administration of beta-blockers during this stage may lead to cardiogenic shock and worsening renal function. It is unlikely that beta-blockers affected our results because hypotension was an infrequent phenomenon in both groups.
Pathophysiology of CRS1 is multi factorial but the salient feature is inadequate renal perfusion until proven otherwise, which should prompt clinicians to consider the diagnosis of a low cardiac output state. More importantly and more commonly, it’s the marked increase in Central Venous Pressure (CVP) leading to kidney congestion that causes CRS1 by decreasing the pressure gradient in renal glomeruli and ultimately decreasing the filtration rate. This can be identified with through physical examination, ancillary signs, imaging, and laboratory findings. Hypoalbuminemia can lead to decreased renal perfusion due to reduced intravascular on cotic pressure; and it has also been shown to be associated with systemic venous hypertension and severe tricuspid regurgitation[4,21]. Our study showed that hypoalbuminemia could predict CRS1 but weather it causes CRS1 remains yet to be proved.
In CRS1, early diagnosis of AKI is the key but it remains a challenge[22]. The pursuit of diagnosing AKI early has lead to discovery of novel AKI biomarkers. Neutrophil gelatinase-associated lipocalin[23-25] and Kidney injury molecule-1[26,27] seems to be earliest marker of ischemic or nephrotoxic injury while Cystatin C[28,29] is a better predictor of glomerular filtration. But these biomarkers are still in investigational phase. We defined worsening renal function as rise in SCr by 0.3 mg/dl as was used in previous studies[30,31]. These studies showed that the risk of poor outcome persists regardless of whether AKI was transient or sustained[30,31] and that even small acute changes in SCr (0.3 mg/dL) can modify the risk of death[32]. Unfortunately to date, the treatment of acute heart failure is not focused on the preservation of renal function. The best treatment for CRS1 is prevention, so if we have a test that could predict worsening renal function, then maybe we can prevent it by proper allocation of our resources and by being aggressive in treating such patients. Our study shows that a simple test like serum albumin can predict the worsening renal function in patients with ADHF. This can help in risk stratification of ADHF patients and guide appropriate selection of the fluid management strategy including, furosemide drip, addition of thiazide diuretics or spironolactone, ultra filtration and furosemide with hypertonic saline. BNP in combination with bioimpedance vector analysis[33], CVP or non-invasively measured LVEDP[34] can be used to better define the patient’s hydration status. Further clinical trials will be needed to see if earlier identification of at-risk groups using serum albumin level and the use of specific treatment algorithms will prevent CRS1 and improve prognosis in ADHF patients.
The mean LOS of our patients was longer than the mean LOS of patients in American hospitals in recent years (5.3 days)[35,36]. Our overall high-risk cohort could partly explain this; most of them were elderly and majority being African American. It is important to note that the prevalence of comorbidities of patients hospitalized for ADHF has increased over time[36]. Also, a northeast teaching hospital in an urban area like our center has been associated with longer LOS[36-40]. Patients with CRS1 have a more complicated hospital course in ADHF patients[12,17,35,41] so our finding that patients with hypoalbuminemia stayed longer is not surprising. For the same reason, the in hospital mortality of our study population was 4.4% which is slightly higher than the current national average of 3.3% in patients admitted with ADHF.
The small number of patients, its retrospective observational design and the study population that may not be representative of national cohort limits this study. Patients with underlying chronic kidney disease were included but this study shows that CRS1 can develop in patients with normal creatinine levels and can predict adverse outcomes. Also, we were unable to obtain accurate information from the records about the New York Heart Association classification and the amount of weight loss during hospitalization.
Conclusion
Hypoalbuminemia (S.Alb < 3.4 g/dl) predicts CRS1 in patients admitted with ADHF and can aid in their early risk stratification. It also portend a complicated hospital course in patients with ADHF and can have an important impact on appropriate management and better resource utilization in care of these patients.
References
- 1. Ronco, C., Circoira, M., McCullough P.A., et al. Cardiorenal syndrome type 1: Pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. (2012) J Am Coll Cardiol 60(12): 1031-1042.
Pubmed || Crossref || Others - 2. Metra, M., Nodari, S., Parrinello, G., et al. Worsening renal function in patients hospitalized for acute heart failure: Clinical implications and prognostic significance. (2008) Eur J Heart Fail 10(2): 188-195.
Pubmed || Crossref || Others - 3. Kinugasa, Y., Kato, M., Sugihara, S., et al. A simple risk score to predict in-hospital death of elderly patients with acute decompensated heart failure—hypoalbuminemia as an additional prognostic factor. (2009) Circ J 73(12): 2276-2281.
Pubmed || Crossref || Others - 4. Uthamalingam, S., Kandala, J., Daley, M., et al. Serum albumin and mortality in acutely decompensated heart failure. (2010) Am Heart J 160(6): 1149-1155.
Pubmed || Crossref || Others - 5. Arques, S., Pieri. B., Biegle, G. Comparative value of B-type natriuretic peptide and serum albumin concentration in the prediction of in-hospital mortality in elderly patients admitted for acute severe heart failure. (2009) Ann Cardiol Angeiol 58(5): 279-283.
Pubmed || Crossref || Others - 6. Wiedermann, C.J., Wiedermann, W., Joannidis, M. Hypoalbuminemia and acute kidney injury: A meta-analysis of observational clinical studies. (2010) Intensive Care Med 36(10): 1657-1665.
Pubmed || Crossref || Others - 7. Forman D, Butler J, Wang Y, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. (2004) J Am Coll Cardiol 43(1): 61-71.
Pubmed || Crossref || Others - 8. Sarraf, M., Masoumi, A., Schrier, R.W. Cardiorenal syndrome in acute decompensated heart failure. (2009) Clin J Am Soc Nephrol 4(12): 2013-2026.
Pubmed || Crossref || Others - 9. Horwich, T.B., Kalantar-Zadeh, K., MacLellan, R.W., et al. Albumin levels predict survival in patients with systolic heart failure. (2008) Am Heart J 155(5): 883-889.
Pubmed || Crossref || Others - 10. Liu, M., Chan, C.P., Yan, B.P., et al. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. (2012) Eur J Heart Fail 14(1): 39-44.
Pubmed || Crossref || Others - 11. Bellomo, R., Ronco, C., Kellum, J.A., et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. (2004) Crit Care 8(4): 204-212.
Pubmed || Crossref || Others - 12. Ronco, C., Haapio, M., House, A., et al. Cardiorenal Syndrome. J Am Coll Cardiol (2008) 52(19): 1527-1539.
Pubmed || Crossref || Others - 13. Yancy, C.W., Lopatin, M., Stevenson, L.W., et al. Clinical Presentation, Management, and In-Hospital Outcomes of Patients Admitted With Acute Decompensated Heart Failure With Preserved Systolic Function: A Report From the Acute Decompensated Heart Failure National Registry (ADHERE) Database. (2006) J Am Coll Cardiol 47(1): 76-84.
Pubmed || Crossref || Others - 14. Peacock, W.F., Costanzo, M.R., DeMarco, T., et al. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: Insights from the ADHERE registry. Cardiology (2009) 113(1): 12-19.
Pubmed || Crossref || Others - 15. Mullens, W., Abrahams, Z., Gary, S., et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. (2009) J Am Coll Cardiol 53(7): 589-596.
Pubmed || Crossref || Others - 16. McAlister, F.A., Ezekowitz, J., Tonelli, M., et al. Renal insufficiency and heart failure: Prognostic and therapeutic implications from a prospective cohort study. (2004) Circulation 109(8): 1004-1009.
Pubmed || Crossref || Others - 17. Smith, G.L., Lichtman, J.H., Bracken, M.B., et al. Renal impairment and outcomes in heart failure: Systematic review and meta-analysis. (2006) J Am Coll Cardiol 47(10): 1987-1996.
Pubmed || Crossref || Others - 18. Nohria, A., Hasselblad, V., Stebbins, A., et al. Cardiorenal interactions: Insights from the ESCAPE trial. (2008) J Am Coll Cardiol 51(13): 1268-1274.
Pubmed || Crossref || Others - 19. Cowie, M.R., Komajda, M., Murray-Thomas, T., et al. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: Results of the prospective outcomes study in heart failure (POSH). (2006) Eur Heart J 27(10): 1216-1222.
Pubmed || Crossref || Others - 20. utler, J., Forman, D.E., Abraham, W.T., et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. (2004) Am Heart J 147(2): 331-338.
Pubmed || Crossref || Others - 21. Battin, D.L., Ali, S., Shahbaz, U., et al. Hypoalbuminemia and Lymphocytopenia in Patients With Decompensated Biventricular Failure. (2010) Am J Med Sci 339(1): 31-35.
Pubmed || Crossref || Others - 22. Han, W.K., Bonventre, J.V. Biologic markers for the early detection of acute kidney injury. (2004) Curr Opin Crit Care 10(6): 476-482.
Pubmed || Crossref || Others - 23. Ronco, C. NGAL: an emerging biomarker of acute kidney injury. (2008) Int J Artif Organs 31(3): 199-200.
Pubmed || Crossref || Others - 24. Mori, K., Nakao, K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. (2007) Kidney Int 71(10): 967-970.
Pubmed || Crossref || Others - 25. Mishra, J., Ma,Q., Prada, A., et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. (2003) J Am Soc Nephrol 14(10): 2534-2543.
Pubmed || Crossref || Others - 26. Vaidya, V.S., Ramirez, V., Ichimura, T., Bobadilla et al. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. (2006) Am J Physiol Renal Physiol 290(2): 517-529.
Pubmed || Crossref || Others - 27. Ichimura, T., Hung, C.C., Yang, S.A., et al. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. (2004) Am J Physiol Renal Physiol. 286(3): 552-563.
Pubmed || Crossref || Others - 28. Herget-Rosenthal, S., Marggraf, G., Husing, J., et al. Early detection of acute renal failure by serum cystatinC. (2004) Kidney Int 66(3): 1115-1122.
Pubmed || Crossref || Others - 29. Dharnidharka, V.R., Kwon, C., Stevens, G. et al. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. (2002) Am J Kidney Dis 40(2): 221-226.
Pubmed || Crossref || Others - 30. Logeart, D., Tabet, J.Y., Hittinger, L., et al. Transient worsening of renal function during hospitalization for acute heart failure alters outcome. (2008) Int J Cardiol 127(2): 228-232.
Pubmed || Crossref || Others - 31. Latchamsetty, R., Fang, J., Kline-Rogers, E., et al. Prognostic value of transient and sustained increase in in-hospital creatinine on outcomes of patients admitted with acute coronary syndrome. (2007) Am J Cardiol 99(7): 939-942.
Pubmed || Crossref || Others - 32. Jose, P., Skali, H., Anavekar, N., Tomson et al. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. (2006) J Am Soc Nephrol, 17(10): 2886-2891.
Pubmed || Crossref || Others - 33. Di, Somma, S., Vetrone, F., Maisel, A. Bioimpedance Vector Analysis (BIVA) for Diagnosis and Management of Acute Heart Failure. (2014) Current Emergency and Hospital Medicine Reports 2(2): 104-111.
Pubmed || Crossref || Others - 34. Vasudev, R., Rampal, U., Patel, H., et al. Left atrial volume index over late diastolic mitral annular velocity: A new non-invasive parameter for measurement of left ventricular end diastolic pressure. (2017) J Am Coll Cardiol 69(11): 1574-1547.
Pubmed || Crossref || Others - 35. Krantz, M.J., Tanner, J., Horwich, T.B., et al. Influence of hospital length of stay for heart failure on quality of care. (2008) Am J Cardiol 102(12): 1693-1697.
Pubmed || Crossref || Others - 36. Chen, J., Dharmarajan, K., Wang, Y., et al. National Trends in Heart Failure Hospital Stay Rates, 2001 to 2009. (2013) J Am Coll Cardiol 61(10):1078-1088.
Pubmed || Crossref || Others - 37. Taubert, G., Bergmeier, C., Andreson, H., et al. Clinical profile and management of heart failure: Rural community hospital vs. metropolitan heart center. (2001) Eur J Heart Fail 3(5): 611-617.
Pubmed || Crossref || Others - 38. Merlo, J., Ostergren, P.O., Broms, K., et al. Survival after initial hospitalization for heart failure: A multilevel analysis of patients in Swedish acute care hospitals. (2001) J Epidemiol Community Health 55(5): 323-329.
Pubmed || Crossref || Others - 39. Taylor, D.H., Whellan, D.J., Sloan, F.A. Effects of admission to a teaching hospital on the cost and quality of care for Medicare beneficiaries. (1999) N Engl J Med 340(4): 293-299.
Pubmed || Crossref || Others - 40. Alattar, F.T., Imran, N., DeBari, V.A., et al. Fractional excretion of sodium predicts worsening renal function in acute decompensated heart failure. (2010) Exp Clin Cardiol 15(3): 65-69.
Pubmed || Crossref || Others - 41. Pokhrel, N., Maharjan, N., Dhakal, B., et al. Cardiorenal syndrome: A literature review. (2008) Exp Clin Cardiol 13(4): 165-170.
Pubmed || Crossref || Others