Synthesis, Characterization and Antimicrobial Studies of Schiff Base Ligand Derived from Cefadroxil and Benzaldehyde
Bristi Sarker, S.M. Moazzem Hossen
Affiliation
Department of Pharmacy, University of Science and Technology Chittagong, Bangladesh
Corresponding Author
Farhana Hoque, Department of Pharmacy, University of Science and Technology Chittagong (USTC), Chittagong-4202, Bangladesh, Tel: +88-01712298492; E-mail: farhanahoquebd@yahoo.com
Citation
Hoque. F., et al. Synthesis, Characterization and Antimicrobial Studies of Schiff Base Ligand Derived from Cefadroxil and Benzaldehyde. (2016) J Pharm Pharmaceutics 3(2): 101- 104.
Copy rights
© 2016 Hoque. F. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Schiff base; Cefadroxil; Benzaldehyde; TLC; Antibacterial; Antifungal; MIC
Abstract
Infectious diseases have been a major health problem for decades in the under developed countries and the emergence of multi drug resistant strains further complicates the situation. In the ongoing quest to discover more effective remedies, several antibiotics have been procured or synthesized. Advanced research into synthetic chemistry has pointed out several organic compounds with antimicrobial potential. Schiff bases are one of such compounds. Cefadroxil is a bactericidal antibiotic. This research aims at discussing the synthesis of a Schiff base out of a fairly common antibiotic cefadroxil and benzaldehyde. This ligand was synthesized and characterized by elemental analysis and some other physico-chemical techniques. This synthesized compound was characterized by different spectroscopic techniques. It was also subjected to melting point determination, TLC (thin layer chromatography), Chemical tests and solubility tests. The Schiff base was screened for antibacterial and antifungal activity in-vitro by disc diffusion method. MIC study for the most susceptible organism was also performed.
Introduction
Diseases caused by various microorganisms have been a major health problem for decades, especially in the developing and underdeveloped countries. Moreover, the tendency of microorganisms to develop resistance to antibiotics further necessitates the urge to develop newer drugs.
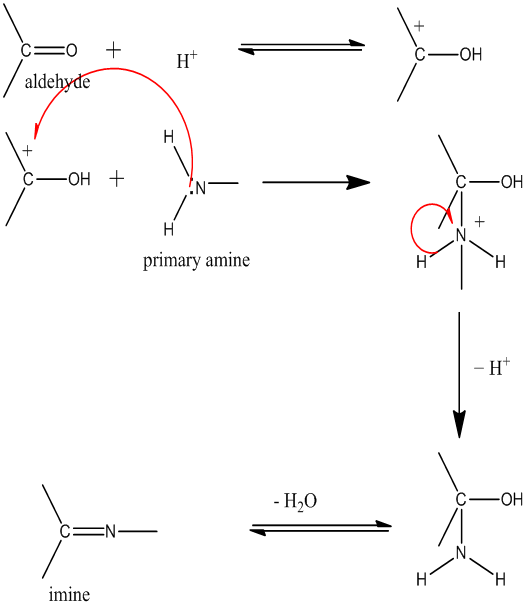
Schiff bases are the compounds containing the azomethine group (-C = N- group)[1].
They are usually formed by the condensation of an active carbonyl compound.
Schiff bases were first reported by Hugo Schiff in 1864 (Schiff, 1864). Structurally a Schiff base is a nitrogen analogue of a carbonyl compound in which the carbonyl group is replaced by an imine or azomethine group. The imine group in these compounds is proved to be responsible for various biological activities, antibacterial and antifungal being prominent[2]. The general formula of a Schiff base is RHC = N-R1, where R and R1 are alkyl, aryl, cycloalkyl or heterocyclic[3]. The Schiff base complexes with many transition metal ions have attracted considerable interest because of their growing importance as model molecules for biological systems such as oxygen carriers[4-7]. Schiff bases are common ligands in coordination chemistry. The imine nitrogen is basic and exhibits pi-acceptor properties. The ligands are typically derived from alkyl diamines and aromatic aldehydes[8].
Cefadroxil (formerly trademarked as Duricef) is a broad-spectrum antibiotic of the cephalosporin type, effective in Gram-positive and Gram-negative bacterial infections. It is used to treat bacterial infections such as urinary-tract infections, skin infections, and chest or throat infections. It is suitable for adults and older children, and can be taken during pregnancy. Some people who are allergic to penicillin antibiotics may not be able to take cefadroxil, so make sure your doctor knows if you have ever had an allergic reaction to any other antibiotic. Cefadroxil is an orally active cephalosporin in clinical practice, belongs to the group of β-lactam antibiotics[9]. It is chemically designated as 5-thia-l-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[amino(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-, monohydrate. Its antibacterial activity is dependent on the presence of the β -lactam functionality that can be hydrolyzed under aqueous conditions[10].
Considering the above facts, a schiff base was synthesized out of cefadroxil and benzaldehyde. The antimicrobial properties of this schiff base evaluated in hope to establish it as an effective therapeutic agent.
Materials and Methods
Chemicals and reagents
All chemicals were of analytical grade and used as received. All solvents were purified using conventional methods.
Physical measurement
Infrared spectrum was registered on a Shimadzu Infrared Spectrophotometer using potassium bromide disc, within the range of 4000 - 500 cm-1 at the University of Chittagong. UV-visible spectrum was recorded on a UV-visible spectrophotometer at the University of Science and Technology Chittagong (USTC). 1HNMR spectrum was registered on a Bruker NMR spectrophotometer using deuterated methanol as solvent, at BCSIR laboratories, Dhaka. Melting point was determined on an electro-thermal melting point apparatus and TLC was performed using conventional TLC plates at the University of Science and Technology Chittagong (USTC).
General method of Schiff base preparation
Primary amine and aldehyde (1:1) were taken in a round bottom flask along with solvent. Then the product was allowed to condense and collected by filtration. This was then re-crystallized for purification. This re-crystallization process was repeated 3 times until pure product was obtained. The product was dried in desiccators over anhydrous silica gel.
Antimicrobial screening
Four pathogenic bacteria viz., Salmonella typhi (Gram negative), Escherichia coli (Gram negative), Staphylococcus aureus (Gram positive), Bacillus subtilis (Gram positive) and two species of fungus Candida albicans and Aspergillus fumigatus were collected from the Department of Microbiology, University of Chittagong. Nutrient agar was used as a culture media for bacteria while potato dextrose agar was employed in antifungal studies. The Schiff base was dissolved in methanol at a concentration of 0.030 mg/ml. The in-vitro antimicrobial activity of the Schiff base was assessed by disc diffusion method. The diameter of the zone of inhibition produced by the compound was compared with the parent drug, cefadroxil.
Preparation of Schiff base ligand
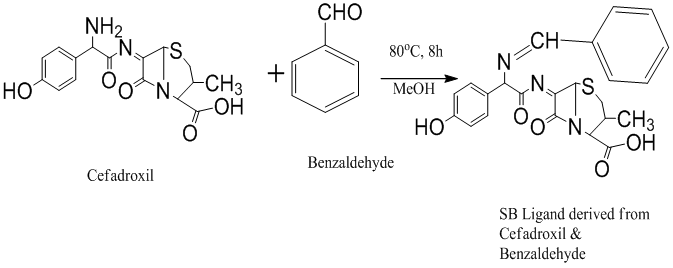
Cefadroxil and benzaldehyde (1:1) were taken in a round bottom flask along with methanol as solvent. These were added dropwise with stirring using a magnetic stirrer. The mixture was refluxed for 8 hours at 80°C. The reaction was monitored by using TLC. After 8 hours, the reaction mixture was cooled in an ice water bath and left for 24 hours in the refrigerator to allow condensation of the product. After the product was condensed, it was filtered off and collected. This was then re-crystallized for purification. This re-crystallization process was repeated 3 times until pure product was obtained. The product was dried in a dessicator over anhydrous silica gel. Yield: 80% (Figure 1).
Figure 1: Reaction of Schiff base ligand (SB) preparation derived from cefadroxil and benzaldehyde.
Results and Discussion
Infrared spectroscopy
The results of infrared spectral analysis are presented in (Table 1). The spectrum displayed characteristic bands for the phenolic OH group at 3318 cm-1, couple bands for aromatic C = C at 1535 cm-1 and 1450 cm-1 and most importantly for an azomethine group C = N at 1624 cm-1.
Table 1: Infrared spectral data of the prepared Schiff base compound N-benzal cefadroxil.
| Assignment (cm-1 ) | |||||
|---|---|---|---|---|---|
| Azomethine C=N | Amide C=O | Ar-C=C | Ar-OH | Ar-H | Ar-OH |
| 1624 (strong) |
1678 (strong) |
1535 and 1450 (strong) |
3425 and 3410 (strong) |
3060 (weak) |
3318 (weak, broad) |
UV-visible spectroscopy
The electronic absorption spectra of the Schiff base was recorded at room temperature using distilled water as solvent. The wavelength maximum was observed at 374 nm. It was different from cefadroxil.
1HNMR spectroscopy
The observed chemical shifts for the different types of protons in the 1HNMR spectrum are presented in (Table 2). The 1HNMR spectrum of the prepared compound indicated a singlet at 8.1 ppm owing to the presence of an azomethine proton (CH = N). Another significant observation was the presence of arayl protons over a range of 6.8 – 7.6 ppm occuring as multiplets. A singlet for a phenolic proton was observed at 4.1 ppm. It has been confirmed through research that azomethines show chemical shifts in the vicinity of 8.0 – 8.7[11].
Table 2: 1HNMR spectral data of the compound N-benzal cefadroxil.
| Chemical shift (in ppm) | Assignment of protons |
|---|---|
| 1.2 | singlet, Methyl proton |
| 6.8 – 7.6 | multiplet, Phenyl protons |
| 8.1* | singlet, -CH=N- proton |
| 4.1 | singlet, Phenolic proton |
| 10.0 | Singlet, Carboxylic acid proton |
Melting point determination
The synthesized Schiff base demonstrated a characteristic melting point which is different from cefadroxil.
Rf value determination
A mixture of the solvents N-hexane and methanol was used at a ratio of 10:4 (n-hexane: methanol). An Rf value of 0.6 was observed for the Schiff base while the parent drug cefadroxil demonstrated a value of 0.1.
Antibacterial activity
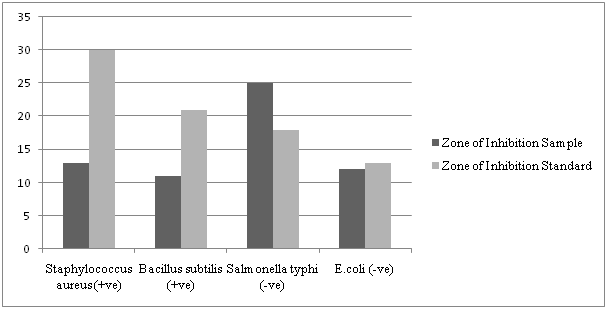
The target compound was screened for antibacterial activity against two species of Gram-positive bacteria (Staphylococcus aureus, Bacillus subtilis) and two species of Gram-negative bacteria (Salmonella typhi, Escherichia coli). Results of the disc diffusion studies are depicted in (Table 3). It was observed that the Schiff base was more active against Gram-negative strains than Gram-positive. However, in most cases, the parent drug was more active than the prototype. Diameters of the zone of inhibition (in mm) for the prototype compound in case of Staphylococcus aureus, Bacillus subtilis, Salmonella typhi and Escherichia coli were found to be 13, 11, 25 and 12 mm respectively. Results for the same selection of bacteria in case of the standard drug cefadroxil were 30, 21, 18 and 13 mm respectively (Figure 2). While the compound appeared to be more active on Salmonella typhi than its precursor, results on Staphylococcus aureus can be deemed satisfactory and the small variations for E.coli can be neglected.
Figure 2: (Column chart): Comparison of zone of inhibition of the Schiff base with the standard.
Table 3: Results of antibacterial activity of the compound N-benzal cefadroxil.
| Species | Zone of Inhibition in 30 mg/ml | |
|---|---|---|
| Sample(SB product) (mm) | Standard(cefadroxil) (mm) | |
| Staphylococcus aureus (+ve) | 13 | 30 |
| Bacillus subtilis (+ve) | 11 | 21 |
| Salmonella typhi (-ve) | 25 | 18 |
| E.coli (-ve) | 12 | 13 |
Antibacterial MIC
MIC was determined only for Salmonella typhi in nutrient agar medium, using the same disc diffusion technique. The synthesized Schiff base was dissolved in methanol at concentrations 0.030, 0.015, 0.0075, 0.0037 and 0.0018 mg/ml respectively. It was observed that 0.0037 mg/ml had been that least effective concentration. Details of this study are given in (Table 4).
Table 4: Results of MIC for Salmonella typhi. (for prepared Schiff base ligand).
| Concentration (mg/ml) | Diameter (mm) |
|---|---|
| 0.030 | 23 |
| 0.015 | 13 |
| 0.0075 | 8 |
| 0.0037 | 6 |
| 0.0018 | 0 |
The MIC assay for Salmonella typhi indicated a concentration of 0.0037 mg/ml to be the least effective concentration
Antifungal activity
Antifungal studies were carried out by disc diffusion technique on potato dextrose agar against Candida albicans and Aspergillus fumigatus. Unfortunately the compound showed no activity, so the results were not compared.
In most cases, the synthesized Schiff base ligand demonstrated less activity than its precursor, but occasionally activity was good, having shown better activity against Salmonella typhi (Figure 3).
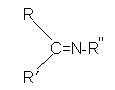
Figure 3: Imine compound Schiff base ligand (SB) derived from primary amine and aldehyde.
Conclusion
Here we developed a convenient and facile method for the synthesis of Schiff base ligand N-benzal cefadroxil by using condensation reaction method. Cefadroxil was used as starting material. This ligand has been characterized on the basis of IR, 1H-NMR and UV spectral data. The products were purified by re-crystallization process. They were confirmed by checking melting point, TLC, Rf value, colour, several spectral data, etc. Some identification tests were performed for several functional groups.
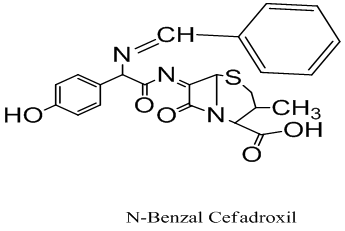
(E)-7-((2-((Z)-benzylideneamino)-2-(4-hydroxyphenyl)acetyl)imino)- 3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0] octane-2-carboxylic acid
Antimicrobial activities against E. coli, S. typhi, P. aureginosa, S. aureus, C. albicans and A. fumigatus were determined. This Ligand was found to be more active than standard against the bacteria Salmonella typhi. It also showed effective antibacterial activity against the other three bacteria. But it was failed to show any visible signs of activity against both the species of fungus Candida albicans and Aspergillus fumigates compared to standard nistatin. So the prepared compound can be used against the diseases which are caused by these testedbacteria. It can be used as starting material in different types of reaction (eg. complexation reaction).
Acknowledge:
Financial support by the University of Science and Technology Chittagong (USTC), Chittagong is gratefully acknowledged. I am also grateful to the Department of Pharmacy, USTC; Bangladesh Council of Scientific and Industrial Research (BCSIR) and University of Chittagong to undergo this research work.
References
- 1. IUPAC, Compendium of Chemical Terminology. (1997) the Gold Book.
- 2. Przybylski, P., Huczyński, A., Pyta, K., et al. Biological Properties of Schiff Bases and Azo Derivatives of Phenols. (2009) Curr Org Chem 13: 124–148.
- 3. Arulmurugan, S., Kavitha, P.H., Venkatraman, R.P. Biological activites of Schiff Base and its complexes: A Review. (2010) Rasayan. J Chem 3(3): 385-410.
- 4. Soliman, A.A., Mohamed, G.G. Study of the ternary complexes of copper with salicylidene-2-aminothiophenol and some amino acids in the solid state. (2004) Thermochimica Acta 421(1-2): 151-159.
- 5. Syamal, A. Syntheses of new oxovanadium (IV) complexes with some new Schiff bases derived from salicylaldehyde or substitiuted salicyaldehyde and 3-aminothiophenol. (1978) Transition Metal Chemistry 3(1): 297-299.
- 6. Salam, M.A., Chowdhury, D.A. Some mixed-ligand complexes of titanium(IV) with dibasic tetradentate bis-Schiff bases of 1,2-diaminoethane as the primary ligands (2000) J Sci Indus Res 35(1-4): 123-127
- 7. Salam, M.A., Chowdhury, D.A., Hossain, M.N., et al. Some mixed-ligand complexes of titanium(IV) containing diamine Schiff bases as the primary ligands and some bidentates as the secondary ligands. (2003) J Sci Indus Res 38(1-2): 41-48.
- 8. Hernández-Molina, R., Mederos, A. Acyclic and Macrocyclic Schiff base ligands. (2003) Comprehensive Coordination Chemistry II: 411-446.
- 9. Shantier, S.W., Gadkariem, E.A., Ibrahim, K.E., et al. Spectrophotmetric determination of cefadroxil in bulk and dosage form using sodium hydroxide. (2010) E-J Chem 8(3): 1314-1322.
- 10. Baertschi, S.W., Dorman, D.E., Occolowitz, J.L., et al. Isolation and structure elucidation of a novel product of the acidic degradation of cefaclor. (1993) J Pharm Sci 82(6): 622-626.
- 11. Tai, D.N., Thanh, N.D., Nam, P.D., et al. 1H- and 13C-NMR spectra of some azomethines of 5-Amino-2-Phenylindole series. (2007) J Chem 45(5): 642- 647.