Synthesis and Characterization of Se-Zno Photocatalyst with Improved Photodegradation and Antibacterial Properties
Sonde, O. I1,2*, Oloyede, A. R4, Oladoyinbo, F. O1, Sanyaolu, N. O5, Ibikunle, A. A5, Afolabi, T. A1, Arotiba, O. A2,3, Dare, E. O1
Affiliation
1 Department of Chemistry, Federal University of Agriculture Abeokuta, Ogun State, Nigeria
2 Department of Applied Chemistry, University of Johannesburg, P.O. Box 17011, Doornfontein 2028, South Africa
3 Centre for Nanomaterials Science Research, University of Johannesburg, P.O. Box 17011, Doornfontein 2028, South Africa
4 Biotechnology Centre, Federal University of Agriculture Abeokuta, Ogun State, Nigeria
5 Department of Chemical Sciences, Olabisi Onabanjo University, Ago-iwoye, Ogun State
Corresponding Author
Sonde, O. I, Department of Chemistry, Federal University of Agriculture Abeokuta, Ogun State, Nigeria, Department of Applied Chemistry, University of Johannesburg, P.O. Box 17011,Doornfontein 2028, South Africa, E-mail: sonde.idowu@gmail.com
Citation
Sonde,O.I., et al. Synthesis and Characterization of Se-Zno Photocatalyst with Improved Photodegradation and Antibacterial Properties. (2018) J Nanotechnol Material Sci 5(1): 30-34.
Copy rights
© 2018 Sonde, O.I. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Photocatalyst; Degradation; Antibacterial; Nanocomposite; Wastewater Pollutant.
Abstract
The removal of toxic organic pollutant from wastewater is an interested area in pollution control. Among organic pollutants are dyes such as methylene blue and orange II that originates from sources such as textile industrial processes. Zinc oxide based photocatalyst has emerged as one of the most fascinating materials in the modern era due to its semiconducting and catalytic properties. Se-ZnO photocatalyst was prepared using reduction process. The synthesized Se-ZnO nanocomposite was confirmed by XRD, SEM, EDS and TEM analysis. The photocatalytic degradation assessment of Se-ZnO nanocomposite was investigated by the kinetics of degradation of methylene blue and orange II dyes using simulated solar light. The photocatalytic degradation was monitored with UV spectroscopy at time interval. Antibacterial activities of the synthesized nanocomposites were also investigated on some pathogens: Escherichia coli, Klebsiella pneumonia, Enterobacter cloacae and Pseudomonas aeruginosa using agar well diffusion method. SEM image of Se- ZnO nanocomposite gives a very clear flower-like morphology. TEM image of Se-ZnO nanocomposite showed a more conspicuous pearl-bead-necklace structure. 86% and 25% degradation of methylene blue and orange II dye was achieved respectively within 180 minutes of irradiation. A good inhibition was observed for the growth of E. coli and E.cloacae.
Introduction
Release of colored waste from textile industry into water bodies such as lakes, oceans and rivers has created severe environmental problems as most of these effluents are toxic and hence considered a threat for human survival. Catalyst using semiconductor based nanoparticle is currently used to solve the persistent environmental problem and adequate research is being carried out to investigate the antibacterial applications of the catalyst due to their unique physiological properties caused by Nano sized dimensions and large surface area/volume ratios. In view of this, a growing interest in heterogeneous photocatalysis as an advance oxidation technique has been developed. The uses of semiconductor nanoparticles as photocatalyst and as antibacterial agents to initiate redox reactions have generated special interest. Zinc oxide has emerged an efficient catalyst as far as wastewater treatment is concerned because it generates more number of H2O2 efficiently and its higher rate of mineralization.
The stability of dyes towards light and oxidizing agents also create a problem for their removal by different waste treatment procedures. Therefore, their removal methods are selected with a great deal of care and considerateness[1-3]. Before now, various technologies have been adopted for the removal of contaminants from industrial effluent including, filtration, surface complexation, chemical precipitation, ion exchange, membrane processing, flocculation etc. However, all these methods have some setbacks due to metal selectivity, low adsorption, and high consumption of chemicals and use of expensive equipment[4,5].
The photocatalytic decomposition of organic pollutants in wastewater received considerable research attention[6]. Doping metal ions into Zinc oxide can influence the performance of these photocatalysts[7]. This affects the dynamics of electron: Hole pair recombination and interfacial charge transfer. Selenium is a trace element, very essential for normal health and reproduction. It has received considerable attention because of its remarkable antibacterial applications such as anticancer, antimicrobial, antidiabetic and antioxidant activities[8]. This study will investigate the photodegradation and antibacterial activities of synthesized Se-ZnO nanocomposite.
Figure 1: Chemical structure of (a) methylene blue (b) orange II dyes.
Experiment
Materials
Zinc chloride was purchased from Sigma Aldrich while sulphuric acid, nitric acid, polyethylene glycol, ammonia, selenium metal, methylene blue and orange II dyes were obtained from Merck Chemicals. All chemicals and reagents were purchased from credible suppliers and used without further purification. The purity of such chemicals was ascertained to be acceptable for the intended reactions. De-ionized water was used for all calibration and solution preparations.
Synthesis of Se- ZnO nanocomposite
Zinc chloride (1.36 g) was added to 200 mL of de-ionized water in a beaker while stirring. Selenium metal (16 mg) was then added and the mixture was stirred for hours. Polyethylene glycol (4 mL) and aqueous ammonia (4 mL) was gradually added while stirring, this was left to stir for several hours. The mixture was washed with both de-ionized water and ethanol during filtration and dried in an oven for 2 hours at 80°C. The nanocomposite was obtained after calcinations at 800°C for 3 hours[9].
Characterization
X-ray diffraction (XRD) measurements were performed using RigakuUltima IV diffractometer operated at 40 kV and 40 mA. The Cu Kα radiation (λ = 0.15406 nm) as the source. Scanning electron microscopy (SEM) images were obtained on a Vega 3 XMU instrument and transmission electron microscopy (TEM) analysis was performed on JEOL (JEM-2100 electron microscope).
Photocatalytic Degradation Assessments
The photocatalytic performance of the materials was evaluated by measuring the rate of photo degradation of organic dye (methylene blue and orange II dyes) prepared by dissolving in distilled water. These solutions were used as a test contaminant for evaluating photocatalytic properties of the Se-ZnO nanocomposite under solar simulated irradiation. The material (0.02 g) was suspended in 20 mL of the organic dye solution. This amount was chosen after preliminary tests were carried out on the effect of catalyst amount on the degradation efficiency of the dye. Solar simulator (Oriel, Newport) equipped with an Oriel 500 W Xenon was used for the experiment. The power output of the solar simulator was set to 300 W in order to give an irradiance of 1 000 W/m-2 at about 25°C, using an Air Mass 1.5 Global Spectral Filter. An Oriel PV reference cell system equipped with a 2 cm × 2 cm monocrystalline silicon photovoltaic cell and a Type K thermocouple was used to set the simulator irradiance to 1 sun and to monitor the temperature of the system. Prior to photocatalytic reactions, the suspensions were ultra-sonicated for 10 minutes and then magnetically stirred in the dark for 30 minutes to allow for adsorption equilibrium before visible-light illumination. Aliquots of the suspension (4 mL) were withdrawn at constant time intervals using a 10-mL nomadic disposable syringe and filtered through a 0.22 μm PVDF membrane filter for various periods of irradiation up to 180 minutes. Variations in the concentrations of the dye were monitored on a Cary 60 UV-Vis, Agilent Technologies at λ = 615 nm. The degradation of the dye was estimated using the degradation efficiency equation:
Degradation efficiency (%) = (1 - C)/Co × 100%
Where Co is initial concentration and C is the concentration after “t” minute of light irradiation.
Antibacterial Assessments
Agar Diffusion Method
Each extract was tested in triplicate with 0.1 mL and 5 μg/mL of ciprofloxacin as positive control. These were then left on the bench for 1 hour for proper diffusion of the nanoparticles[10]. Appropriate amounts of the as-prepared nanocomposite were then gently placed on immunized plates and the plates were incubated at 37°C for 24 hours. Zone of inhibition was determined by measuring the clear area of no bacteria growth that formed around Se-ZnO nanocomposite.
Results and Discussion
The preparation of Se-ZnO nanocomposite was followed by characterization using SEM, TEM and XRD. The SEM image of Se- ZnO nanocomposite (Figure 2a) gives a very clear flower-like morphology. The EDS spectra of Se-ZnO nanocomposite (Figure 2b) confirmed the presence of Selenium, Oxygen and Zinc.
Figure 2: (a) SEM image (b) EDS spectra of Se-ZnO nanocomposite.
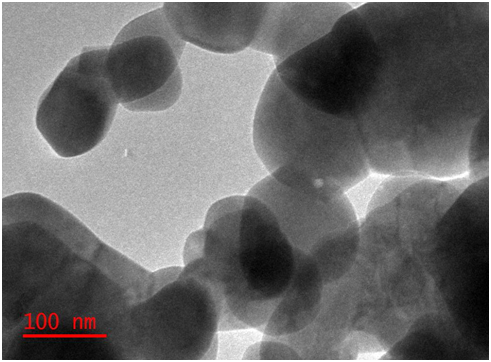
TEM image of Se-ZnO nanocomposites (Figure 3) showed a more conspicuous pearl-bead-necklace structured that appeared twined at a higher magnification because the percentage of dopant is very small, the morphology is been dominated by the Zinc oxide which has the largest percentage.
Figure 3: TEM image of Se-ZnO Nanocomposite.
Figure 4: XRD spectra of Se-ZnO Nanocomposite.
Figure 4 show X-ray diffraction pattern of Se-ZnO nanocomposite with peaks at 31.9, 34.5, 36.4, 47.7, 56.7, 62.9, 66.5, 68.1 and 69.2 which match to the (1 0 0), (0 0 2), (1 0 1), (10 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2) and (2 0 1) crystalline plane of Zinc oxide respectively. This data also confirmed the wurtzite structure of Zinc oxide (JCPDS card No. 36-1451). The peak at 77.13 indicates the presence of selenium in the XRD spectra as shown above.
Photodegradation of Organic Dye
Methylene blue dye removal efficiency of 86% was achieved under UV radiation and Orange II dye removal efficiency of 25% was achieved after 180 minutes of irradiation. The degradation efficiencies were lower compared to the removal of methylene blue dye. This is not surprising since orange II dye is anionic dye (negatively charge), whereas methylene blue dye is cationic dye (positively charge), like charges repel, unlike charges attract (figure 5). The degradations obeyed the Langmuir-Hinshelwood first order kinetics; ln (Co /C) = k(t) Where Co is the initial dye concentration, C is the concentration of the dye at time t and k is the rate constant[11].
Figure 5: Degradation profile using Se-ZnO nanocomposite (a) Methylene blue (b) Orange II dyes.
Plot of ln(Co /Ct) against time “t” for the degradation of organic dye in the presence of Se-ZnO nanocomposite (figure 6 & 7) showed a fairly good linear model fits for Se-Zinc oxide indicating the reaction followed pseudo- first order model. A Plot of Ct/Co against time give the normalization concentration decay time of 20 ppm organic dye degradation using Se-ZnO nanocomposite (figure 8). This shows that degradation occurred with higher rates and as the graphs deviated from linear to exponential decay, degradation decreases and the R-squared value indicated how close the data are to the regression line.
Figure 6: Kinetics of methylene blue degradation using Se-ZnO nanocomposite.
Figure 7: Kinetics of orange II dye degradation using Se-ZnO nanocomposite.
Figure 8: Normalization concentration decay time of 20 ppm (a) methylene blue (b) orange II dyes using Se-ZnO nanocomposite under UV light.
Antibacterial Assessment
A very good inhibition was observed with synthesized Selenium-Zinc oxide nanocomposite for the growth of E. coli and E.cloacae which is close to the standard (ciprofloxacin) as shown in Figure 9 below.
Figure 9: Antibacterial assessment of Se-ZnO nanocomposite.
Conclusion
In photocatalysis using doping method, one need to identify suitable impurity combinations that will enhance optical absorption in the visible region as well as trap electrons to reduce charge carrier recombination. A novel photocatalyst Selenium Doped Zinc oxide nanocomposite can be used in solving the ever increasing environmental problems associated with textile effluents. This catalyst is found to be applicable in that it is economical, easy to handle and potentially viable with excellent antibacterial property for both small and large scale industrial effluent treatment.
Acknowledgement: The authors would like to thank the Centre for Nanomaterials Science Research and Department of Applied Chemistry University of Johannesburg, South Africa for the support during the research work.
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1. Abdullah, A.A. Photocatalytic Degradation of Rhodamine B Dye in Wastewater Using Gelatin/CuS/PVA Nanocomposites under Solar Light Irradiation.(2017) Journal of Biomaterials and Nanobiotechnology 8: 66-82.
- 2. Manohar, R.P., Shrivastava, V.S. (2014).ChemicaSinica 5: 8-17.
Pubmed||Crossref||Others
- 3. Lambert,S.D, Graham, N.J.D., Sollars, C.J., et al. Evaluation of inorganic adsorbents for the removal of problematic textile dyes and pesticides. (1997)Water Sci. Technology 36(2-3): 173.
- 4. Mohd, O.A., Mohammad, M.K., Sajid, A.A., et al. (2015). Journal of Saudi Chemical Society 19: 494–450.
- 5. Sangareswari, M., Sundaram, M.M. (2015) IRJET 2: 526-537.
Pubmed||Crossref||Others
- 6. Helin, N., Qinmin,W., Hongxia, L., et al.
Pubmed||Crossref||Others
- 7. Shengyi, Z., Yuanhao, G., Changle, C. Visible-Light Active and Magnetically Recyclable Nanocomposites for the Degradation of Organic Dye.(2014) Materials 7(5): 4034-4044.
- 8. Zhanglian, H., Ahmad, M. Enhanced photocatalytic activity of Ce-ZnO nanopowders synthesized by combustion method. (2015) Journal of Rare Earths 33(3): 255-262.
- 9. Birringer, M., Block, E., Kotrebai, M., et al. Chemical speciation influences comparative activity of selenium-enriched garlic and yeast in mammary cancer prevention. (2000)J Agric Food Chem48:4452-4452.
- 10. Li, W., Wu, D., Yu, Y., et al. Investigation of a novel ZnO/TiO2–B photocatalyst with enhanced visible photocatalytic activity. (2014) Physica E: Low-Dimensional Systems and Nanostructures 58: 118–123.
- 11. National Committee for Clinical Laboratory Standards (NCCLS), (1990). Man.Clin.Microbiol., 5th Ed.
Pubmed||Crossref||Others
- 12. Kuvarega, A.T., Krause, R.W.M., Mamba, B.B. Nitrogen/palladium-codoped TiO2 for efficient visible light photocatalytic dye degradation. (2011)J. Phys. Chem C 115(45): 22110–22120.