The 5 “W” of Micro circulatory Assessment in the Critically Ill Patients
Domizi, R ., Damiani, E ., Scorcella, C ., Pelaia,
Affiliation
Department of Biochemical Science and Public Health, Polytechnic University Marche
Corresponding Author
Abele Donati, Anaesthesia and Intensive Care Unit, AOR Ospedali Riuniti, Via Conca 71, Department of Biochemical Science and Public Health, Polytechnic University Marche, via Tronto 10, 60020 Torrette (Ancona), Italy. Tel: 0039 335 5850328/ 071 5964603; Fax: 071 5964601; E-mail: a.donati@univpm.it
Citation
Donati, A., et al. The 5 “W” of Microcirculatory Assessment in the Critically Ill Patients. (2016) Clin Trials Pathol Case Stud 1(1): 1- 5.
Copy rights
© 2016 Donati, A. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License.
Abstract
Microcirculatory monitoring revealed great impact in predicting the severity of organ dysfunction and the outcome of critically ill patients. Clinicians are trying to introduce this technology in the clinical practice, as a supplement to routine surveillance; unfortunately, despite decades of researches, there are still unsolved questions and practical limitations to this. The purpose of this article is to summarize the state of the art regarding microcirculation through 6 traditional questions (5 “W” and 1 “H”), but also to highlight the open issues and the current restrictions for the use of the device as routine.
Introduction
The microcirculation is a complex network of capillaries, venules and arterioles. All the vessels characterized by a diameter smaller than 100 μm are part of the microcirculation. Several functions have been attributed to it, included oxygen delivery, removal of metabolites and nutrient’s supply to tissues. Microcirculatory perfusion is controlled by myogenic, metabolic and neurohumoral mechanisms and several studies have shown that the endothelial cell works as active determinant of this autoregulation, through cell- to-cell interactions and autocrine and paracrine signaling[1-4].
The regulatory dysfunction of microcirculation is specifically involved in the systemic response to inflammation and infection, and it can lead to organ dysfunction and multiorgan failure. Microcirculatory monitoring have revealed to be a strong predictor of the outcome of critical care patients with severe sepsis and septic shock: presence of microvascular alterations and heterogeneity, failure in improving microcirculatory perfusion with fluid resuscitation and vasoactive drugs have been all related to lower survival and higher severity of organ dysfunction[1,5-7].
Despite years of clinical and experimental studies, there are still unsolved questions about the pathophysiology underlying this deregulation and there is also technical limitation to the bedside use of the technique as complement to macrohemodynamic monitoring. However microcirculatory assessment could be strongly instructive in all those situations where the optimal tissue’s perfusion represents the cornerstone of the treatment. We believe that 5 Ws and 1 H is a perfect formula for getting the complete state of art and to understand the progresses of microcirculatory investigation.
“How”: Technology Underlying Microvascular Monitoring
For a long time microvascular monitoring has been limited to semi-transparent tissues allowing only experimental or ex vivo studies, preventing it to the clinical setting.
Slaaf et al, introduced the first real bedside monitoring with the technology of the Orthogonal Polarization Spectral imaging (OPS)[8]. Therefore, OPS have been experimented in different settings of severe illness and emergency medicine[9-12]. SDF imaging (Sidestream Dark Field- introduced by Goedhart and colleagues) first, and IDF (Incident Dark Field) Illumination then, made further improvements as they obtained higher optical resolution and they increased the percentage of success in acquisition and therefore the possibility to analyze the records collected[13,14].
Cytocam® (Braedius Medical, Huizen, The Netherlands) is the most recent technology introduced in commerce and it is based on IDF illumination; if compared to Microscan® (Micro Vision Medical, Amsterdam, The Netherlands) that is based on SDF imaging, IDF illumination seems to offer advantages for the operator, derived from the introduction of digital signal, the reduction in weight of the device (better handling of the probe and lower pressure artifacts) and the higher optical resolution. Some validation studies are already available in literature and they report superiority of this novel video-microscope on the previous devices[15-17]. Core of the technology for vessel’s detection is an incoming ring light (LEDs light) characterized by a specific wavelength of 530 nm. 530 nm represents the isosbestic point of absorption spectra of the hemoglobin contained in red blood cells (RBC) so that RBC impressed by the light are visualized as flowing granules that indirectly highlight just those vessels that are perfused, hiding vessels that are not perfused at all[10-13].
“How” to Capture Good Quality Videos?
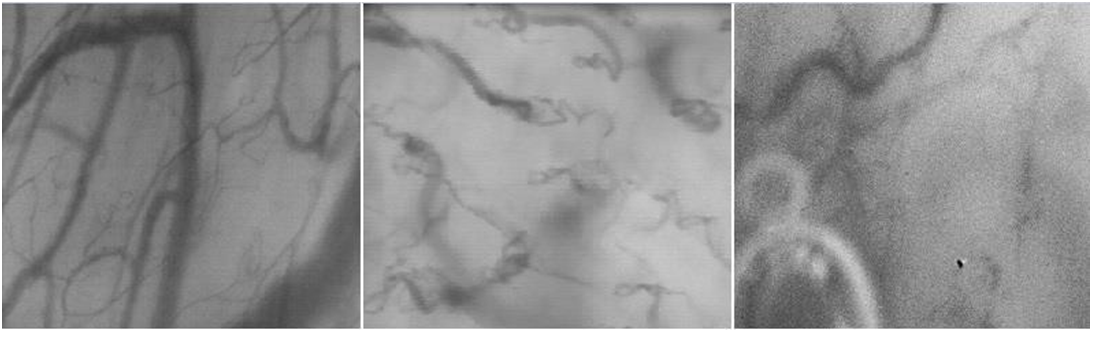
As nicely described by De Backer et al, saliva, fresh blood and contusions are often the main responsibles for an inadequate visualization of vessels; for a good quality of records, all secretions should be carefully removed using tissues or gauzes and high attention should be taken to optimize focus, illumination and contrast (Figure 1)[18]. Other disturbances can derive from pressure’s and motion’s artifacts: pressure artifacts can be focal or global, and they are determined by excessive pressure applied on the mucosa that occludes RBC flow; they can be detected by the observation of large venules, where impaired flow of venules is suggestive of excessive pressure; motion’s artifacts are more frequent in awake patients even more in confused and agitated ones, because they are unable to remain steady for long and to cooperate with the observer. A firm but gentle grip of the probe on patient’s chin usually facilitates stabilization of the image[18,19].
Figure 1: Examples of quality of capture. The image on the left represents a good quality video, while the middle and right ones respectively represent examples of incorrect area of evaluation and of artifacts derived from saliva bubbles and pressure artifacts.
“How” to Analyze Videos?
As Bezemer R et al reported, the off-line analysis is the gold standard for this technology[20]. All capillaries have to be manually detected and drew, then blood flow is individually characterized. Semi-automated software’s are available in commerce. AVA (Automated Vascular Analysis) is the most used software package worldwide, it facilitates the operator in some of the main steps of off-line analysis, but nevertheless it remains time-consuming and partially operator-dependent, so we are far from a real resolution of the limits of this methodology. We should aim to reach a complete automatization of the procedure both to reduce inter-operator dependency and to introduce microcirculatory monitoring in the daily clinical practice in the setting of critical care patients where an evaluation of tissue’s perfusion is undoubtedly important[20].
“Who”: Importance of a Well-trained Observer
As with many other devices introduced into clinical practice, such as Echocardiography and Transcranial Doppler, both video capture and off-line analysis of microcirculation require expertise and skills, and a well-trained observer is fundamental to obtain good-quality images, to avoid artifacts and to give the right meaning to the results.
A well-trained observer can easily recognize and avoid pressure and motion artifacts and he can therefore optimize the acquisition. The operator must also be skilled in the off-line evaluation of the principal parameters. As previously mentioned, inter-operator deviation of the method could be completely deleted just when totally automated software will be available[19,21,22].
Massey MJ, Shapiro NI and colleagues have recently created a score of quality called “Microcirculation Image Quality Score” with the aim to rate image’s quality before off-line analysis and they have introduced 6 different features to control(illumination, duration, focus, content, stability and pressure), to assign the score of optimal, sub-optimal, or unacceptable quality of the record[21]. Further assessment of this score is mandatory, but it is unquestionable that we need a quality control to ensure high relevance studies[22].
What”: Guide to Microcirculatory Parameters
De Backer D, Ince C, et al. published the new roundtable Guidelines in 2007, in order to formulate a consensus statement on microvascular evaluation. These guidelines are still the main guidance for both acquisition and analysis of images[18].
Participants of the round table were some of the principal experts in “microhemodynamic”. All of the experts agreed that a measure of vascular density, of perfusion of the vessels and of heterogeneity of flow should be always performed and registered. They decided that 20 μm in diameter was the right cut-off to divide small vessels (mostly capillaries) from medium and large vessels (20 - 100 μm). Vessels with a diameter smaller than 20 μm are the most physiologically active vessels for oxygen exchange. Medium and large vessels predominantly represent small venules and also rare arterioles[18,19].
Measures of vessel density are TVD (Total Vessel Density) and De Backer Score; PVD (Perfused Vessel Density) instead is an estimate of functional capillary density (FCD). PVD is calculated by multiplying vessel density by the Proportion of Perfused Vessels (PPV). It is one of the most relevant variables, because it identifies and differentiates patterns of bad and good perfusion of small vessels: “no flow” and “intermittent flow” are identified as determinants of a poor oxygenation. Most of these indices are derived from the number of intersections of capillaries with the horizontal and the vertical arbitrary grid-lines and from the measurement of the total capillary length of the surface[19,20].
The Microcirculatory Flow Index (MFI) is a parameter of perfusion. In this case just one horizontal and one vertical line divide the image in 4 equal quadrants, in which the observer reports the predominant type of flow using an ordinal scale (0 for absent flow, 1 as intermittent flow, 2 for sluggish flow and 3 for normal). The average of the 4 quadrants is the final MFI[23,24]. Boerma, et al in their trials found a high intra- and inter-observer reproducibility of MFI[20,25].
Heterogeneity of perfusion is another determinant of oxygen extraction efficacy. It is characterized by alternation of normally perfused areas and poorly perfused ones. When the distance among capillaries increases, those cells that are distant from capillaries find it difficult to exchange gas and metabolites, and a certain degree of cellular distress can appear as result of hypoxia. Therefore high heterogeneity of vascular distribution is generally less tolerated than a homogeneous but sluggish flow[18,19]. 5 spots of sublingual surface should be captured to assess vascular heterogeneity, and at least 3 of the 5 records should be analyzed.
“Where” and “When”: Targets for Microvascular Examination
Sublingual mucosa is the most common and standardized site for evaluation of microcirculation and Boerma’s study demonstrated that it is an excellent mirror of splanchnic vessels[25] Other sites of interest are small intestine (villi), colon (crypts), rectum (crypts), liver (sinuses) and gingival tissue: several studies have been performed, but they mostly involve ex vivo evaluation or experimental trials.
A recent multicentre trial called “Micro SOAP study” showed a high prevalence of microvascular alteration in the critically ill patients. The study consisted in a one-shot evaluation of microcirculation of ICU patients. It involved 36 ICUs worldwide and it tried to correlate specific patterns of microcirculation with specific pathologies; an on-going monocentric study of Donati’s group is trying to characterize patterns of alterations starting from patient’s diagnosis, and also to follow the course of the pathology from the admission throughout all the ICU’s stay[26]. Sepsis and septic shock are the main field of application for microcirculatory assessment[1,27-29].
The new definition of sepsis proposed by Singer M, Angus DC et al, describes sepsis as “a life- threatening organ dysfunction caused by a dysregulated host response to infection”[30].
Decades of studies demonstrated that in the pattern of distributive shocks like septic one, there is an activation of the cytokinesis cascade that causes derangement of the auto-regulatory mechanisms and of endothelial cells function, anomalous production of nitric oxide and altered response of the circulation to vasoactive drugs. When this infection-induced derangement persists, it causes qualitative and quantitative alterations of microcirculation (reduction in total and perfused vessel density and increased heterogeneity of flow) that affect tissue perfusion and organ function[1,4,29].
Further promising fields of application for microvascular monitoring have been studied: hypovolemic and hemorrhagic shock, cardiac failure and diabetes, organ perfusion in anaemia and hemodilution, multiorgan failure and organ failure related to polytrauma[31-34].
The effects on microcirculation have also been reported for several drugs (catecholamine, nitroglycerin, recombinant activated protein C, beta-blockade), fluids (albumin, crystalloids, colloids and blood transfusion), and procedures (normobaric hyperoxia, ischemia-reperfusion syndrome, cardiac resynchronization and cardiac surgery) in the pattern of septic and non-septic patients[35-43].
“Why”: The Rationale behind Microcirculatory Monitoring
Shoemaker’s “supranormal values” theory, River’s Early Goal Directed therapy and all subsequent studies have shown the importance of hemodynamic optimization as treatment’s target for septic and critically ill patients[44-48].
However, the normalization of macrohemodynamic parameters, such as Cardiac Output (CO) and arterial pressure, is not a guarantee for good oxygen delivery and organ perfusion: there is often a mismatch between macro and microhemodinamic[49,50]. Even if there are some surrogates to assess the adequacy of the organ perfusion, as lactate, peripheral temperature and capillary refill, all of them are characterized by well-known limitations: blood lactates are the result of a balance among production, metabolism and excretion, and they do not represent a direct marker of anaerobic metabolism and poor tissue perfusion; the capillary refill time and peripheral temperature suffer from environmental factors, altered thermoregulatory mechanisms and vascular disorders. None of them gives us a true picture of organ perfusion, and moreover none of them is a precocious marker of inadequate treatment: in these cases, the direct visualization of microcirculatory alterations, through direct monitoring at the patient’s bedside, may be an additional resource for clinicians to guide their treatment and to formulate a right prognosis for their patients.
Conclusion
Several years of researches demonstrated that microcirculation is important in the physiopathology of sepsis, septic shock and much other pathology.
Real time monitoring of microcirculation may represent an additional weapon for the diagnosis and a guide to treatment. Unfortunately, the technology is not yet ready to ensure that the instrument is versatile and quick enough to be routinely used in clinical practice. The current limitations to a broad spectrum use of the device are: the necessity of specific expertise of the observer, the time-consuming procedure of analysis and moreover the lack of agreement upon the range of normal values for the main parameters in healthy people. Despite the difficulties, the increasing space given from literature is a clear sign of recognition and legitimation of this technique and a stimulation to find answers to the open questions through high-quality clinical and experimental trials.
References
- 1. Ince, C. The microcirculation is the motor of sepsis. (2005) Crit Care 9(4): S13-S19.
- 2. Klijn, E., Lagrand, W.K., Brugts, J.J., et al. The Microcirculation in Heath and Critical Disease. (2008) Prog Cardiovasc Dis 51(2): 161-170.
- 3. Gutterman, D.D., Chabowski, D.S., Kadlec, A.O., et al. The Human Microcirculation: Regulation of Flow and Beyond. (2016) Circ Res 118(1):157-172.
- 4. Vallet, B. Endothelial cell dysfunction and abnormal tissue perfusion. (2002) Crit Care Med 30(5): S229–S234.
- 5. Segal, S.S. Regulation of blood flow in the microcirculation. (2005) Microcirculation 12(1): 33-45.
- 6. Creteur, J., Preiser, J.C., Dubois, M.J., et al. Microvascular blood flow is altered in patients with sepsis. (2002) Am J Respir Crit Care Med 166(1): 98–104.
- 7. Sakr, Y., Dubois, M.J., Creteur, J., et al. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. (2004) Crit Care Med Sep 32(9): 1825-1831.
- 8. Slaaf, D.W., Tangelder, G.J., Reneman, R.S., et al. A versatile incident illuminator for intravital microscopy. (1987) Int J Microcirc Clin Exp 6(4): 391-397.
- 9. Groner, W., Winkelman, J.W., Harris, A.G., et al. Orthogonal polarization spectral imaging: a new method for study of the microcirculation. (1999) Nat Med 5(10): 1209–1212.
- 10. Harris, A.G., Sinitsina, I., Messmer, K. Validation of OPS imaging for microvascular measurements during isovolumic hemodilution and low hematocrits. (2002) Am J Physiol Heart Circ Physiol 282(4): H1502–H1509.
- 11. Lindert, J., Werner, J., Redlin, M., et al. OPS imaging of human microcirculation: a short technical report. (2002) J Vasc Res 39(4): 368-372
- 12. Cerný, V., Turek, Z., Parízková, R. Orthogonal polarization spectral imaging. (2007) Physiol Res 56(2): 141-147.
- 13. Goedhart, P.T., Khalilzada, M., Bezemer, R., et al. Sidestream Dark Field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. (2007) Opt Express 15(23): 15101–15114.
- 14. Eriksson, S., Nilsson, J., Sturesson, C. Non-invasive imaging of microcirculation: a technology review. (2014) Med Devices (Auckl) 7: 445–452.
- 15. Ince, C., Tibboel, D., Reiss, I.K., et al. Cutaneous microcirculation in preterm neonates: comparison between sidestream dark field (SDF) and incident dark field (IDF) imaging. (2015) J Clin Monit Comput 29(5): 543–548.
- 16. Hutchings, S., Watts, S., Kirkman, E. The Cytocam video microscope. A new method for visualising the microcirculation using Incident Dark Field technology. (2015) Clin Hemorheol Microcirc 62(3): 261-271.
- 17. Gilbert-Kawai, E., Coppel, J., Bountziouka, V., et al. A comparison of the quality of image acquisition between the incident dark field and sidestream dark field video-microscopes. (2016) BMC Med Imaging 16:10.
- 18. Hollenberg, S., Boerma, C., Goedhart, P., et al. How to evaluate the microcirculation: report of a round table conference. (2007) Crit Care 11(5): R101.
- 19. Massey, M.J., Shapiro, N.I. A guide to human in vivo microcirculatory flow image analysis. (2016) Crit Care 20: 35.
- 20. Bezemer, R., Khalilzada, M., Ince, C. Recent Advancements in Microcirculatory Image Acquisition and Analysis. (2008) Yearbook of Intensive Care and Emergency Medicine 677-690.
- 21. Massey, M.J., Larochelle, E., Najarro, G., et al. The microcirculation image quality score: development and preliminary evaluation of a proposed approach to grading quality of image acquisition for bedside videomicroscopy. (2013) J Crit Care 28(6): 913-917.
- 22. Petersen, S.M., Greisen, G., Hyttel-Sorensen, S., et al. Sidestream dark field images of the microcirculation: intra-observer reliability and correlation between two semi-quantitative methods for determining flow. (2014) BMC Med Imaging 14:14.
- 23. Arnold, R.C., Parrillo, J.E., Phillip, D. R., et al. Point-of-care assessment of microvascular blood flow in critically ill patients. (2009) Intensive Care Med 35(10): 1761-1766.
- 24. Pozo, M.O., Kanoore, E.V.S., Ince, C., et al. Comparison of different methods for the calculation of the microvascular flow index. (2012) Crit Care Res Pract 102483.
- 25. Boerma, E.C., Mathura, K.R., Spronk, P.E., et al. Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: a prospective validation study. (2005) Crit Care 9(6): R601–R606.
- 26. Vellinga, N.A., Boerma, E.C., Koopmans, M., et al. Study Design of the Microcirculatory Shock Occurrence in Acutely Ill Patients (microSOAP): an International Multicenter Observational Study of Sublingual Microcirculatory Alterations in Intensive Care Patients. (2012) Crit Care Res Pract: 121752.
- 27. De Backer, D., Durand, A. Monitoring the microcirculation in critically ill patients. (2014) Best Pract Res Clin Anaesthesiol 28(4): 441-451.
- 28. Trzeciak, S., McCoy, J.V., Phillip Dellinger, R., et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. (2008) Intensive Care Med 34(12): 2210–2217.
- 29. Miranda, M.L., Balarini, M.M., Caixeta, D.M., et al. Microcirculatory dysfunction in sepsis: pathophysiology, clinical monitoring, and potential therapies. (2016) Am J Physiol Heart Circ Physiol 00034.
- 30. Singer, M., Deutschman, C.S., Seymour, C.W., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). (2016) JAMA 315(8): 801-810.
- 31. Peters, J., Mack, G.W., Lister, G. The importance of the peripheral circulation in critical illness. (2001) Intensive Care Med 27(9): 1446-1458.
- 32. Boldt, J., Ince, C. The impact of fluid therapy on microcirculation and tissue oxygenation in hypovolemic patients: a review. (2010) Intensive Care Med 36(8): 1299-1308.
- 33. Ferrara, G., Kanoore Edul, V.S., Martins, E., et al. Intestinal and sublingual microcirculation are more severely compromised in hemodilution than in hemorrhage. (2016) J Appl Physiol (1985) 120(10): 1132-1140.
- 34. Hutchings, S., Naumann, D.N., Harris, T., et al. Observational study of the effects of traumatic injury, haemorrhagic shock and resuscitation on the microcirculation: a protocol for the MICROSHOCK study. (2016) BMJ Open 6(3): e010893.
- 35. Houben, A.J., Beljaars, J.H., Hofstra, L., et al. Microvascular abnormalities in chronic heart failure: a cross-sectional analysis. (2003) Microcirculation 10(6): 471-478.
- 36. Hamlin, S.K., Parmley, C.L., Hanneman, S.K. Microcirculatory alterations in shock states. (2014) Crit Care Nurs Clin North Am 26(3): 399-341.
- 37. Erol-Yilmaz, A., Atasever, B., Mathura, K., et al. Cardiac Resynchronization Improves Microcirculation. (2007) J Card Fail 13(2): 95-99.
- 38. Ince, C. To beta block or not to beta block; that is the question. (2015) Crit Care 19(1): 339.
- 39. Morelli, A., Donati, A., Ertmer, C., et al. Microvascular effects of heart rate control with esmolol in patients with septic shock: a pilot study. (2013) Crit Care Med 41(9): 2162-2168.
- 40. Damiani, E., Adrario, E., Luchetti, M.M., et al. Plasma free hemoglobin and microcirculatory response to fresh or old blood transfusions in sepsis. (2015) PLoS One 10(5): e0122655.
- 41. Donati, A., Damiani, E., Luchetti, M., et al. Microcirculatory effects of the transfusion of leukodepleted or non-leukodepleted red blood cells in patients with sepsis: a pilot study. (2014) Crit Care 18(1): R33.
- 42. Milstein, D.M., Helmers, R., Hackmann, S., et al. Sublingual microvascular perfusion is altered during normobaric and hyperbaric hyperoxia. (2016) Microvasc Res 105: 93-102.
- 43. Den Uil, C.A., Lagrand, W.K., Spronk, P.E., et al. Does red blood cell transfusion result in variate microvascular response in sepsis? (2007) Crit Care Med 35(10): 2464-2465.
- 44. Rivers, E., Nguyen, B., Havstad, S., et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. (2001) N Engl J Med 345(19): 1368-1377.
- 45. Dellinger, R.P., Levy, M.M., Rhodes, A., et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. (2013) Intensive Care Med 39(2): 165-228.
- 46. Marik, P.E., Varon, J. Goal-directed therapy for severe sepsis. (2002) N Engl J Med 346(13): 1025-1026.
- 47. Bland, R.D., Shoemaker, W.C., Abraham, E., et al. Hemodynamic and oxygen transport patterns in surviving and nonsurviving postoperative patients. (1985) Crit Care Med 13(2): 85-90.
- 48. Shoemaker, W.C., Appel, P.L., Kram, H.B., et al. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. (1988) Chest 94(6): 1176-1186.
- 49. Donati, A., Domizi, R., Damiani, E., et al. From Macrohemodynamic to the Microcirculation. (2013) Crit Care Res Pract 2013: 892710.
- 50. Ince, C. Hemodynamic coherence and the rationale for monitoring the microcirculation. (2015) Crit Care 19(3): S8