The Feasibility of Walnut and Extra Virgin Olive Oil Supplementation in Older Adults
Mara Z. Vitolins1*, Caroline S. Blackwell1, Jeff D. Williamson2, Capri G. Foy1, Sharon Wilmoth1, Kaycee M. Sink2, Lindsay M. Reynolds1, Robert P. Byington1, David M. Reboussin1
Affiliation
- 1Wake Forest School of Medicine, Division of Public Health Sciences, Medical Center Boulevard, Winston-Salem, NC, 27157 USA
- 2Wake Forest Baptist Medical Center, Department of Internal Medicine, Section on Gerontology and Geriatric Medicine, Medical Center Boulevard, Winston-Salem, NC, 27157 USA
Corresponding Author
Mara Z. Vitolins, Department of Epidemiology and Prevention, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, United States of America, Tel: 00-1-(336) 716-2886; Fax: 00-1-(336) 713-4300; E-mail: mvitolin@wakehealth.edu
Citation
Vitolins, M.Z., et al. The Feasibility of Walnut and Extra Virgin Olive Oil Supplementation in Older Adults. (2017) J Food Nutr Sci 4(1): 49- 54.
Copy rights
© 2017 Vitolins, M.Z. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Abstract
Researchers in Spain provided randomized, controlled trial evidence that adding extra virgin olive oil (EVOO) and nuts to diets of older adults lowered cardiovascular disease risk. Supplementing these foods may represent a simple and straightforward approach to favourable dietary change with potential for dissemination to the broader public. This was an 8-week feasibility trial in which all participants were asked to supplement their ad libitum diets with both walnuts and EVOO to determine their interest in participating and to assess retention and adherence once enrolled. Inclusion criteria were broad: Adults ≥ 55 years old treated for hypertension with medication; exclusions included walnuts/EVOO allergies, homebound or diagnosis of dementia. Recruitment was assessed as number of weeks to accrue 25 participants. Adherence was assessed by participant self-report using a daily diary. Blood pressure (BP), body weight, and HDL cholesterol were measured to estimate the variability of outcomes. Results: Twenty- seven participants were recruited in 2 ½ weeks; 26 of the 27 participants remained in the study for a retention rate of 96% (95% CI: 78% - 100%). Of 216 possible diaries, 185 were returned (86%). On average, weight increased over 8 weeks by 0.8 pounds. Mean systolic BP dropped by 0.25 mmHg while mean diastolic BP decreased by 1.0 mmHg. Mean HDL increased by 1.96 mg/dL. A full scale walnut/EVOO trial in older adults with hypertension seems realistic given our high rates of recruitment, retention, and adherence, coupled with minimal weight gain and favorable trends in BP and HDL
Introduction
Cardiovascular disease continues to be common, expensive, and deadly, especially in the aging population, and low cost interventions that require minimal or no medical monitoring and cause limited side effects are needed. Researchers with the Prevention with Mediterranean Diet (PREDIMED) trial in Spain reported that adding extra virgin olive oil (EVOO) or nuts to the diets of older adults substantially lowered cardiovascular disease (CVD) risk factors and events[1,2]. PREDIMED was a randomized, controlled clinical trial of 7,447 participants (55 to 80 years old) at high risk of cardiovascular disease. Participants were randomized to one of three diets: 1) Mediterranean diet supplemented EVOO; 2) Mediterranean diet supplemented with nuts, or 3) a low fat control diet. The primary endpoint was a composite of cardiovascular events (myocardial infarction, stroke and cardiovascular death)[3]. An interim analysis prompted stopping the trial after a median follow-up of 4.8 years[2]. A primary event occurred in 288 participants: 96 in the Mediterranean diet with EVOO, 83 in the Mediterranean diet with nuts, and 109 in the control diet. Multivariable-adjusted hazard ratios were 0.70 (95% CI 0.54 - 0.92) for the Mediterranean diet plus EVOO and 0.72 (95% CI 0.54 - 0.96) for the Mediterranean diet plus nuts. No serious diet-related adverse effects or weight gain were reported during the trial.(2) The PREDIMED investigators also reported that, despite intensive dietary counseling, diets in the 3 arms changed little from baseline over the course of the study except for the increased nut and EVOO consumption in the Mediterranean diet arms[2].
Similarly, a number of Mediterranean diet intervention trials in US adults have produced mixed results with regards to adherence[2-14]. These studies targeted multiple, simultaneous dietary modifications to move participants to a Mediterranean-like diet pattern. In all studies, participants were provided nutrition counseling with a registered dietitian (one-on-one, telephone counseling, group sessions) and were frequently contacted or seen by study staff though the longest intervention period of these studies was 16 weeks. Efforts to move US adults toward a more Mediterranean diet pattern to improve health outcomes are vital, but perhaps smaller modifications that lead to better dietary patterns gradually should also be considered. Previous interventions to treat obesity and prevent weight gain have demonstrated that small, incremental changes to lifestyle behaviors can be effective, sustainable, and can lead to broader changes over time[15-17].
With the success of PREDIMED showing that nuts and EVOO can impact CVD outcomes even in the absence of major changes in diet pattern, a similar approach could have a tremendous public health impact in the U.S. It is therefore essential to assess whether adults 55 and older who live in the U.S. are interested in participating in a trial where they are asked to consume walnuts and EVOO daily. Additionally, it is important to assess their willingness to attend study related-data collection visits.
We conducted an eight-week feasibility study in adults being treated for hypertension. PREDIMED participants diagnosed with hypertension were shown to benefit from the supplemental foods and in order to develop a low-cost and pragmatic approach to identifying participants at high risk for CVD and other chronic diseases, we used current treatment for hypertension as a proxy[18]. We assessed adherence to consuming both walnuts and EVOO and collected data to estimate the variability of blood pressure, weight, and other measures for use in determining sample size for a larger trial.
Materials and Methods
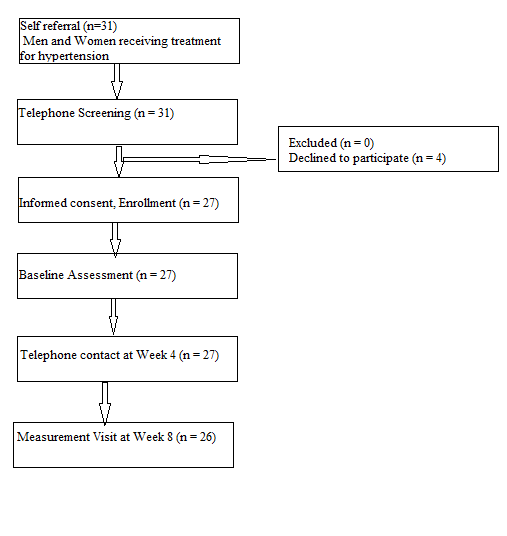
This was a feasibility pilot study (Figure 1). Men and women residing in and around Winston-Salem/Forsyth County, North Carolina were recruited to participate in this study. Inclusion criteria were intentionally broad and minimal: Men and women ≥ 55 years of age being treated for hypertension (defined by being prescribed at least one anti-hypertension medication); willingness to comply with study visits as outlined in the protocol; able to read and speak English; and ability to understand and willingness to sign a written informed consent document. Exclusion criteria included: Allergies or hypersensitivities to olive oil or walnuts; plans to move from the study area during the next eight weeks; dementia that was medically documented or suspected; and homebound for medical reasons. This study was reviewed and approved by the Institution Review Board (IRB) of Wake Forest University Health Sciences (IRB00028877) in August 2014 and participants were required to sign an informed consent document prior to participation.
Figure 1: CONSORT diagram for participants in an 8-week pilot study to determine the feasibility of walnut and extra virgin olive supplementation.
Intervention foods: walnut and extra virgin olive oil
Walnuts, compared to other tree nuts, have been reported to have the highest level of both total and free polyphenols (antioxidants) compared to 9 other types of nuts including almonds and hazelnuts[19]. Therefore, we selected to provide only walnuts rather than a mix of walnuts, hazelnuts and almonds as used in the PREDIMED trial. The walnuts were provided by the California Walnut Growers Association in 1-ounce (29 g) single serving packets. All participants were asked to consume one packet each day. The EVOO was provided by The North American Olive Oil Association in three, 34-ounce bottles. All participants were asked to consume at least two tablespoons (30 ml) per day. Participants also received a measuring spoon for the EVOO and guidance on how to incorporate the walnuts and EVOO into their daily meals/snacks including the provision of recipes. To avoid weight gain, participants were encouraged to substitute these foods for other foods in their diets rather than to simply add them to their diets. Participants received the entire eight-week supply of walnuts and EVOO at the baseline visit.
Study measures
At the baseline and eight-week assessment visits, participants provided a fasting (8 hour) blood sample and completed study questionnaires on demographics, personal medical history, current medications, and a 14-point Mediterranean Diet Adherence Screener developed and validated in PREDIMED[20]. Two first-phase systolic and fifth-phase diastolic Korotkoff blood pressures were obtained from the right arm of the subject using an automated blood pressure monitor (OMRON HEM 907-XL) after resting 5 minutes and sitting upright. These measures were then averaged. Body weight was measured using a professional digital scale (Tanita WB-100A) with participants wearing lightweight, indoor clothing with no shoes. Height was measured in centimeters using a SECA 213 stadiometer attached to a beam scale. At the end of the baseline visit, the participants were scheduled for a telephone contact at 4 weeks.
Feasibility measures
Recruitment was assessed by number of weeks to accrue the 25 study participants and we anticipated it would take approximately eight weeks to complete enrollment. Low cost approaches to recruit participants were utilized: study flyers were posted at local medical clinics and businesses and flyers were mailed to participants from previous trials. Successful retention was pre-defined as no more than two participants lost to follow-up at the eight-week visit. We also assessed adherence to consuming both walnuts and EVOO measured by participant self-report using a simple daily diary. The participants recorded the following information: Intake of walnuts (Yes, No); Intake of olive oil (Yes, No). Side effects were monitored by telephone contact and participant report. Participants were encouraged to contact study staff if they felt they were experiencing side effects related to the study foods.
Laboratory measures
HDL cholesterol concentrations were measured using the heparin manganese precipitation procedure described in the Manual of Laboratory Operations of the Lipid Research Clinics Program. These measurements were performed in a local Lab-Corp facility in compliance with the CDC NHLBI Lipid Standardization Program.
Statistical analysis
This single-arm, feasibility study was designed to obtain estimates of accrual, retention, and adherence, as well as estimates of the variability of outcomes that might be used in a subsequent, randomized trial. We planned to recruit 25 participants over eight weeks, a sample size that would ensure the probability of successful retention (≥ 23 of 25 retained) would be < 10% if the true probability of retention was 80% or less. The study was not powered to test the changes in the outcome measures over time. Descriptive statistics (means, standard deviations, frequencies, etc.) are used to summarize pre-intervention participant characteristics and the outcome measures mentioned above. Exact binomial confidence intervals are provided for the estimated proportions and asymptotic confidence intervals are provided for outcome means at each visit and for their change over time.
Results
Recruitment began on October 3, 2014 and ended less than two weeks later when 27 participants (2 more than targeted to recruit) were accrued on October 16, 2014. Table 1 shows the characteristics of the community members who were enrolled. Sixty- three percent of the study participants were female. Participant ages ranged from 57 to 87 with a median of 67 years. More than half of the participants were white (85%) and married (59%). The majority (85%) had some college education, 41% worked full or part-time, and 33% lived alone. Slightly over half (56%) of the participants had smoked previously, but only one participant currently smoked. All participants had hypertension. Other common comorbidities included diabetes (44%), previous CVD (41%), and a history of kidney/bladder infections (37%). All participants were taking at least one medication.
Table 1: Participant Characteristics (N = 27).
| Characteristic | N (%) |
|---|---|
| Age | |
| Median (Range) | 67 (57 – 87) |
| ≥ 60 | 22 (81) |
| Gender | |
| Male | 10 (37) |
| Female | 17 (63) |
| Race | |
| African American/Black | 4 (15) |
| White | 23 (85) |
| Ethnicity | |
| Non-Hispanic | 27 (100) |
| Marital Status | |
| Single | 1 (4) |
| Married/Married-like | 16 (59) |
| Separated/Divorced | 4 (15) |
| Widowed | 6 (22) |
| Education | |
| High School | 4 (15) |
| > High School, < Bachelors | 14 (52) |
| Bachelors | 4 (15) |
| Masters | 4 (15) |
| Other | 1 ( 4) |
| Smoking Status | |
| Ever | 15 (56) |
| Current | 1 ( 4) |
| Working Full/Part-Time | 11 (41) |
| People in Home | |
| 1 | 9 (33) |
| 2 | 16 (59) |
| 3 | 1 ( 4) |
| 4 | 1 ( 4) |
Retention and Adherence
Twenty-six of the 27 participants remained in the study the entire eight weeks and completed the final study visit for a retention rate of 96% (95% CI: 78% - 100%). One-hundred eighty-five of the 216 possible weekly diaries were returned (86%). Participants, on average, consumed walnuts 6.3 days per week and EVOO 5.9 days per week. The lowest average walnut consumption for an individual was 4.4 (63%) days per week. The lowest average EVOO consumption for an individual was 2.0 (29%) days per week. Across the 185 weekly diaries, walnuts were consumed on 5+ days 89% of the time while EVOO was consumed on 5+ days 84% of the time.
Side effects
Six participants experienced side effects from consuming the study products, including feeling bloated, mild stomach upset, and loss of appetite. None of the side effects were serious and all resolved without medical intervention.
Outcomes
Table 2 provides a summary of selected outcome variables at baseline and 8 weeks post-treatment. There were slight increases in weight and HDL and a slight decrease in blood pressure over the study period. On average, weight increased by 0.8 pounds. Weight change ranged from a loss of 6.8 pounds to a gain of 8.6 pounds. While 9 participants had a weight gain of 1% or more, 5 others lost 1% or more. Mean SBP dropped by 0.25 mmHg while mean DBP decreased by 1.0 mmHg. Mean HDL increased by 1.96 mg/dL. Twelve participants increased their HDL by 1 mg/dL or more while 4 decreased by 1 mg/dL or more. After 8 weeks of intervention, the Mediterranean Diet Screener score on average increased by 1.15 points (baseline mean score= 5.56 and mean score at 8 weeks = 6.69) compared to baseline scores, representing an increase of more than 20% in adherence to the Mediterranean diet.
Table 2: Baseline and post-treatment summary of selected outcome variables.
| Baseline | 8 Weeks | Difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Mean | 95% CI | N | Mean | 95% CI | N | Mean | 95%CI |
| SBP | 27 | 139.7 | 133.2, 146.2 | 26 | 139.5 | 133.3, 145.8 | 26 | -0.25 | -7.26, 6.76 |
| DBP | 27 | 78.6 | 74.5, 82.8 | 26 | 77.5 | 74.0, 81.1 | 26 | -1.00 | -4.09, 2.09 |
| Weight (lbs) | 27 | 189.2 | 168.3, 210.2 | 26 | 191.0 | 169.4, 212.6 | 26 | 0.82 | -0.62, 2.25 |
| BMI | 27 | 30.8 | 28.0, 33.6 | 26 | 31.0 | 28.1, 33.8 | 26 | 0.01 | -0.27, 0.30 |
| HDL (mg/dL) | 27 | 50.4 | 43.3, 57.5 | 25 | 52.8 | 43.5, 62.1 | 25 | 1.96 | -1.36, 5.28 |
Discussion
In this feasibility pilot study, we found that adults 55 years of age and older are interested in consuming EVOO and walnuts. We planned to accrue 25 participants in eight weeks, but using one clinical site, we accrued 27 participants in 2½ weeks with very little effort using low cost recruitment approaches. Weekly adherence to walnut and EVOO consumption was very good (90% of the days for walnuts and 84% for EVOO). Retention was excellent as 96% of participants enrolled remained in the study and completed the eight-week clinic visit.
The PREDIMED investigators reported that with the exception of adding nuts and EVOO to their diets, changes in dietary intake were minimal[2,21]. While disappointing that dietary counselling did not result in significant diet modifications, it is not surprising that consumption of these foods reduced CVD risk. Tree nuts, particularly walnuts, and EVOO are exceptional sources of cardio-protective nutrients. Walnut consumption has been found to decrease LDL cholesterol and total cholesterol levels, oxidative stress, and improve vascular reactivity and endothelial function[22-27]. EVOO contains a polyphenol, hydroxytyrosol, which has been reported to protect cells in the circulatory system from damage by reactive oxygen molecules[28-30]. The monounsaturated fat content of EVOO has been linked to decreased blood pressure[31,32]. While these foods have been tested separately, coupling them together could result in even greater benefits on CVD health.
As in PREDIMED, participants reported only minor side effects, mainly stomach upset, bloating and loss of appetite, and one participant discontinued consuming the study products as a result of a pre-existing condition. PREDIMED investigators did not report weight gain in their participants. However, in this pilot study one participant contacted study staff regarding weight gain and was provided additional counselling for how to replace foods in the diet. Nine participants had a weight gain of one percent or more at the 8-week clinic visit. Several possible explanations for this weight gain include that participants were provided both walnuts and EVOO and therefore more calories (146 calories-walnuts; 240 calories-EVOO) than what PREDIMED participants were provided. Additionally, as the pilot study was initiated in October, the eight-week intervention period included the Halloween and Thanks giving holidays. This is a time of the year when weight gain is not uncommon in adults. Interestingly, five participants lost one pound or more during the study. Nonetheless, weight gain could potentially offset beneficial health effects provided by the EVOO and walnuts although this has not fully been investigated. We note that the biomarker data is trending in the right direction even with the weight gain in some participants. Certainly the trend in HDL levels is encouraging and this was found after only 8 weeks of consumption of the two foods. The small reductions in blood pressure, specifically DBP, also mirror those seen in PREDIMED[2].
The change in Mediterranean Diet Screener scores achieved in this study are quite similar in magnitude to those reported by PREDIMED investigators and achieved over a much shorter intervention period[2]. The PREDIMED intervention groups increased scores from approximately 8.7 to 10.6 points on average at one year (combined for both intervention groups), a 22% increase in adherence to the Mediterranean diet. However, this Screener includes foods that are not common to the typical US diet (sofrito, wild game); therefore, for a larger trial, a Mediterranean Diet Screener modified for participants living in the US will be needed.
Evidenced-based, straightforward, simple but meaningful approaches to initiate favorable changes to the diets of Americans are desperately needed. Walnuts and EVOO are generally recognized as safe and can be consumed by free living adults without requiring medical monitoring. In this pilot study we found that older adults were interested, as noted by the rapid recruitment of participants, in consuming walnuts and EVOO, were willing to consume these foods regularly and that they were willing to return for clinic visits. These findings support that additional investigations to test the combined benefits of these two foods to improve cardiovascular biomarkers and perhaps, as in PREDIMED, to reduce cardiovascular events appear warranted.
Acknowledge:
The authors would like to thank the participants in this pilot study for their time and contribution to participate in this trial. Walnuts were donated by the California Walnut Growers Association and Extra Virgin Olive Oil was donated by the North American Olive Oil Association. Neither group had any role in the design of this trial; in the collection, analysis, or interpretation of data; in the writing of this report; or in the decision to submit this manuscript for publication.
Conflict of interest:
The authors declare no conflict of interest
Funding and Authorship:
No funding is declared. MV conceived and designed the study and wrote the manuscript. DR analyzed the data and wrote the manuscript. CB, JW, CF, SW, KS, LR, and RB researched data and reviewed/edited the manuscript. All authors read and approved the final manuscript.
References
- 1. Underlying Cause of Death 1999-2013 on CDC WONDER Online Database, released 2015. Data are from the Multiple Cause of Death Files, 1999-2013, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Centers for Disease Control and Prevention. 15 A.D. Feb 19
- 2. Estruch, R., Ros, E., Salas-Salvado, J., et al. Primary prevention of cardiovascular disease with a Mediterranean diet. (2013) N Engl J Med 368(14): S1279-S1290.
Pubmed || Crossref || Others - 3. Martinez-Gonzalez, M.A., Corella, D., Salas-Salvado, J., et al. Cohort profile: design and methods of the PREDIMED study. (2012) Int J Epidemiol 41(2): S377-S385.
Pubmed || Crossref || Others - 4. Sidahmed, E., Cornellier, M.L., Ren, J., et al. Development of exchange lists for Mediterranean and Healthy Eating diets: implementation in an intervention trial. (2014) J Hum Nutr Diet 27(5): S413-S425.
Pubmed || Crossref || Others - 5. Djuric, Z., Ruffin, M.T., Rapai, M.E., et al. A Mediterranean dietary intervention in persons at high risk of colon cancer: recruitment and retention to an intensive study requiring biopsies. (2012) Contemp Clin Trials 33(5): S881-S888.
Pubmed || Crossref || Others - 6. Djuric, Z., Ren, J., Blythe, J., et al. A Mediterranean dietary intervention in healthy American women changes plasma carotenoids and fatty acids in distinct clusters. (2009) Nutr Res 29(3): S156-S163.
Pubmed || Crossref - 7. Stendell-Hollis, N.R., Thompson, P.A., West, J.L., et al. A comparison of Mediterranean-style and MyPyramid diets on weight loss and inflammatory biomarkers in postpartum breastfeeding women. (2013) J Womens Health (Larchmt) 22(1): S48-S57.
Pubmed || Crossref || Others - 8. Jones, J.L., Park, Y., Lee, J., et al. A Mediterranean-style, low-glycemic-load diet reduces the expression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in mononuclear cells and plasma insulin in women with metabolic syndrome. (2011) Nutr Res 31(9): S659-S664.
Pubmed || Crossref || Others - 9. Tuttle, K.R., Shuler, L.A., Packard, D.P., et al. Comparison of low-fat versus Mediterranean-style dietary intervention after first myocardial infarction (from The Heart Institute of Spokane Diet Intervention and Evaluation Trial). (2008) Am J Cardiol 101(11): S1523-S1530.
Pubmed || Crossref || Others - 10. Toobert, D.J., Glasgow, R.E., Strycker, L.A., et al. Long-term effects of the Mediterranean lifestyle program: a randomized clinical trial for postmenopausal women with type 2 diabetes. (2007) Int J Behav Nutr Phys Act 4(1): S1.
Pubmed || Others - 11. Toobert, D.J., Strycker, L.A., Glasgow, R.E., et al. Effects of the mediterranean lifestyle program on multiple risk behaviors and psychosocial outcomes among women at risk for heart disease. (2005) Ann Behav Med 29(2): S128-S137.
Pubmed || Crossref || Others - 12. Toobert, D.J., Glasgow, R.E., Strycker, L.A., et al. Biologic and quality-of-life outcomes from the Mediterranean Lifestyle Program: a randomized clinical trial. (2003) Diabetes Care 26(8): S2288-S2293. https://doi.org/10.2337/diacare.26.8.2288
Pubmed || Crossref || Others - 13. McManus, K., Antinoro, L., Sacks, F. A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. (2001) Int J Obes Relat Metab Disord 25(10): S1503-S1511.
Pubmed || Crossref || Others - 14. Bihuniak, J.D., Ramos, A., Huedo-Medina, T., et al. Adherence to a Mediterranean-Style Diet and Its Influence on Cardiovascular Risk Factors in Postmenopausal Women. (2016) J Acad Nutr Diet 116(11): S1767-S1775.
Pubmed || Crossref || Others - 15. Hill, J.O., Wyatt, H.R., Reed, G.W., et al. Obesity and the environment: where do we go from here? (2003) Science 299(5608): S853-S855.
Pubmed || Crossref || Others - 16. Rodearmel, S.J., Wyatt, H.R., Stroebele, N., et al. Small changes in dietary sugar and physical activity as an approach to preventing excessive weight gain: the America on the Move family study. (2007) Pediatrics 120(4): S869-S879.
Pubmed || Others - 17. Ross, R., Hill, J.O., Latimer, A., et al. Evaluating a small change approach to preventing long term weight gain in overweight and obese adults--Study rationale, design, and methods. (2016) Contemp Clin Trials 47: S275-S281.
Pubmed || Crossref || Others - 18. Medina-Remon, A., Tresserra-Rimbau, A., Pons, A., et al. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The PREDIMED randomized trial. (2015) Nutr Metab Cardiovasc Dis 25(1): S60-S67
Pubmed || Crossref || Others - 19. Vinson, J.A., Cai, Y. Nuts, especially walnuts, have both antioxidant quantity and efficacy and exhibit significant potential health benefits. (2012) Food Funct 3(2): S134-S140.
Pubmed || Crossref || Others - 20. Schroder, H., Fito, M., Estruch, R., et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. (2011) J Nutr 141(6): S1140-S1145.
Pubmed || Crossref || Others - 21. Zazpe, I., Sanchez-Tainta, A., Estruch, R., et al. A large randomized individual and group intervention conducted by registered dietitians increased adherence to Mediterranean-type diets: the PREDIMED study. (2008) J Am Diet Assoc 108(7): S1134-S1144.
Pubmed || Crossref || Others - 22. Munoz, S., Merlos, M., Zambon, D., et al. Walnut-enriched diet increases the association of LDL from hypercholesterolemic men with human HepG2 cells. (2001) J Lipid Res 42(12): S2069-S2076.
Pubmed || Others - 23. Rajaram, S., Haddad, E.H., Mejia, A., et al. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. (2009) Am J Clin Nutr 89(5): S1657-S1663.
Pubmed || Crossref || Others - 24. Banel, DK, Hu, FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. (2009) Am J Clin Nutr 90(1): S56-S63.
Pubmed || Crossref || Others - 25. Berryman, C.E., Grieger, J.A., West, S.G., et al. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. (2013) J Nutr 143(6): S788-S794.
Pubmed || Crossref || Others - 26. Zhao, G., Etherton, T.D., Martin, K.R., et al. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. (2007) Am J Clin Nutr 85(2): S385-S391.
Pubmed || Others - 27. Ros, E., Nunez, I., Perez-Heras, A., et al. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. (2004) Circulation 109(13): S1609-S1614.
Pubmed || Others - 28. Rafehi, H., Ververis, K., Karagiannis, T.C. Mechanisms of action of phenolic compounds in olives. (2012) J Diet Suppl 9(2): S96-S109.
Pubmed || Crossref || Others - 29. Salvini, S., Sera, F., Caruso, D., et al. Daily consumption of a high-phenol extra-virgin olive oil reduces oxidative DNA damage in postmenopausal women. (2006) Br J Nutr 95(4): S742-S751.
Pubmed || Crossref || Others - 30. Bogani, P., Galli, C., Villa, M., et al. Postprandial anti-inflammatory and antioxidant effects of extra virgin olive oil. (2007) Atherosclerosis 190(1): S181-S186.
Pubmed || Crossref || Others - 31. Teres, S., Barcelo-Coblijn, G., Benet, M., et al. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. (2008) Proc Natl Acad Sci U S A 105(37): S13811-S13816.
Pubmed || Crossref || Others - 32. Storniolo, C.E., Casillas, R., Bullo, M., et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. (2017) Eur J Nutr 56(1): S89-S97.
Pubmed || Others