The Pleomorphic Immunoblasts: Infectious Mononucleosis
Affiliation
consultant Histopathologist at A.B. Diagnostics, A-1 Ring Road Rajouri Garden, New Delhi
Corresponding Author
Anubha Bajaj, consultant Histopathologist at A.B. Diagnostics, A-1 Ring Road Rajouri Garden, New Delhi 110027, India, Tel: 00911141446785 / 00911125117399; E-mail: anubha.bajaj@gmail.com
Citation
Bajaj, A. The Pleomorphic Immunoblasts: Infectious Mononucleosis (2019) J Gastrointest Disord Liver Func 4(2): 1- 7.
Copy rights
© 2019 Bajaj, A. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License
Introduction
Preface
Preliminary elucidation of infectious mononucleosis as an “idiopathic adenitis” was achieved in 1880. The uncommon infectious mononucleosis may be engendered by the Epstein Barr virus (EBV), a fact delineated in 1967[1]. Epstein Barr virus (EBV) was initially discerned by Epstein et al in 1964 with Electron Microscopic (EM) exemplification of cultured cells of Burkitt’s lymphoma. A cumulative, pre-illness and post- illness serum assay of diseased subjects may elucidate the primary Epstein Barr viral (EBV) infection with the generation of the syndrome designated as “Infectious Mononucleosis”. The virus may be designated as a human herpes virus 4 (HHV4), one of the categories of eight prevalent human herpes viruses[2]. Paul Bunnell in 1932 described a heterophile antibody test with a competent antibody response towards specific and diverse antigens. Epstein Barr virus (EBV) may induce the majority (> 90%) of infectious mononucleosis in children[2,3].
Disease Pathogenesis: Epstein Barr virus (EBV) may be considered as a subtype of epidemic herpes virus. Viral (EBV) infection may ensue within adolescents and young adults primarily on account of oro-pharyngeal and sexual contact, though viral transmission may be augmented with singular oro-pharyngeal contact per se. Primary viral (EBV) infection may also be transmitted with blood transfusion, Solid Organ Transplant (SOT) or a Haematopoietic Stem Cell Transplant (HSCT). Genetic polymorphisms discerned within the EBV BA MH-1 K fragment lengths and viral nuclear antigens (EBV NA 1, 2 & 3) proteins may be employed to recognize the particular blood donor transmitting the virus (EBV)[3]. The aforementioned risk groups and transplant recipients may subsequently develop infectious mononucleosis. Majority (> 50%) of the children below six years, pre-pubertal and pubertal population may be susceptible to the virus. Pre-adolescents and children may contract the virus (EBV) through obscure means, though parents or siblings may disseminate the viral (EBV) particles intermittently with the oral secretions and inoculate young individuals[3,4]. Adolescents and young children from developed countries may be frequently incriminated The incubation period of infectious mononucleosis varies betwixt 32 days to 49 days[3,6].
Clinical Attributes: Acute phase of infectious mononucleosis may be characterized by a clinical syndrome comprising of sore throat with pharyngitis, cervical lymph node enlargement, fatigue and fever. Anomalous magnitude, quantification and lymph node consistency may comprise of “Lymphadenopathy “. Painful masses of swollen lymph nodes confined to the neck, armpits or groin may constitute as” Lymphadenitis”[4] of global incidence, the disorder demonstrates a lack seasonal predisposition. Pre-adolescents may exhibit ambiguous constitutional symptoms with a consequent delayed discernment of the syndrome. A detailed medical and social history with history of drug ingestion may be necessitated in subjects with lymph node enlargement. The infection may be self limiting and benign. An infection disseminating from the nose, eye, skin or ear to the lymph nodes may induce a lymph node enlargement. An enlarged spleen with palpable inguinal, axillary and cervical lymph nodes may be demonstrated with infectious mononucleosis[3,4]. Virus induced pharyngitis may be prevalent in young children with a decline in advancing age. Characteristic features of clinical diversity may be
• An abrupt onset of sore throat or a swelling of the neck with cervical lymph node enlargement and
• Gradually evolving malaise, myalgia and fatigue.
Constitutional symptoms may be frequent with sore throat (9%), cervical lymph node enlargement (80%), fatigue (7%), upper respiratory catarrh (65%) and headaches (50%), diminished appetite (5%), fever (47%) and myalgia (45%). Clinical symptoms may be elucidated for a period of 10 days though fatigue and cervical lymph node enlargement may continue for 3 weeks. Abdominal pain, hepatomegaly, splenomegaly, nausea, vomiting, palatal petechiae, oedema of the eyelids and peri-orbital region may constitute as adjunctive clinical attributes. Subclinical hepatitis may ensue in an estimated three fourths (75%) of the patients depicting an elevation of alanine aminotransferase with a lack of jaundice and abdominal pain. Generalized rash may not be elucidated, except in association with a transient penicillin hypersensitivity secondary to administration of therapeutic penicillin[3,4]. Disease Characteristics: The commonly employed heterophile antibody test for enunciating the syndrome may be inaccurate in young children, particularly below 4 years of age. Specialized viral (EBV) assessment may be necessitated for the age group to prevent a misdiagnosis. Oro-pharyngeal contact may transmit an abundant amount of infective virus in young children and pre-adolescents[5,6]. The viral particles may be amalgamated from asymptomatic parents or siblings who elucidate minimal quantities of oral Epstein Barr virus and transfer miniature infectious viral inoculums. Parents of juveniles below 6 years of age may accumulate Epstein Barr virus in oral secretions while transmitting minimal infectious inoculums with a median of 4900 viral copies / ml. Acute or convalescent stage of primary Epstein Barr virus infection in young adults may demonstrate a median viral egress of 63100 copies / ml[3,4].
Adolescent infectious mononucleosis may enunciate a cross reactive memory response with CD8+ T lymphocytes. CD8+ T lymphocytes as primed with influenza virus may cross react with the Epstein Barr virus. Adolescents may amplify the influenza virus primed CD8+ T lymphocytes, in contrast to juvenile patients infected with variant influenza viruses. Thus, adolescents may demonstrate an intense reaction to the Epstein Barr virus. However, it is debatable that cross reactive immunity as induced by the CD8+ T lymphocytes may impact the intensity of primary Epstein Barr virus (EBV) infection[3].
Natural killer cells may be crucial for curtailing preliminary viral (EBV) infection. Marked augmentation of CD56 (dim) NKG2A+ immune reactive killer cell immunoglobulin like receptor (KIR) natural killer cells may be elucidated within the peripheral blood of children, contrary to adolescents and adults. This coterie of natural killer cells may alter the outcome of subsequent viral infections. Infectious mononucleosis may be frequent in adolescents and adults as compared to the children on account of the aforementioned aggregates of natural killer cells. As the age of acquiring primary infection is greater in the developed countries, the frequency of the disorder is concomitantly elevated. Majority of the young adults may depict systemic symptoms of infectious mononucleosis following a primary Epstein Barr viral infection[3].
Disease Immunity: With an approximate six week incubation period following a primary Epstein Barr viral infection, viral replication may commence initially within the oral cavity. Epstein Barr virus infects the B lymphocytes and squamous epithelial lining of the tonsils. Viral (EBV) infection localized in the oral cavity may delineate the phenomenon of “switch tropism” which displays a cyclical pattern of viral infection implicating the squamous epithelial cells to B lymphocytes with repetition and reversal. Viral transference may occur from the oral cavity to peripheral blood during the incubation period. Thus, copies of viral genome may be detected in the peripheral blood for a period of two weeks preceding the onset of systemic symptoms[3,4]. Gene Expression Profiling (GEP) demonstrates that two weeks prior to the emergence of constitutional symptoms of infectious mononucleosis, a type I interferon response may be mounted.
Acute phase of infectious mononucleosis may depict an enhanced viral presence within the oral cavity and peripheral blood. Immunoglobulin M (Ig M) may be configured against the Epstein Barr Viral Capsid Antigen (VCA) along with expansion of CD8+ T lymphocytes. The CD8+ T lymphocytes may be critical in curtailing the viral (EBV) infection, especially with the fulminant form of the disorder. The aforementioned modifications may be induced by the defective functioning of T cells such as a competent interaction with and deterioration of virally (EBV) infected B lymphocytes[3].
Acute phase of infectious mononucleosis may be characterized by markedly elevated circulating CD8+ T lymphocytes. Several CD8+ T lymphocytes may be typical for viral (EBV) antigens originating from immediate early and early stages of infective viral lysis, though lymphocytic infiltrate may be predominant with the immediate early phase. Viral antigens of the delayed lytic phase may additionally engender a characteristic CD8+ T lymphocyte response. CD8+ T lymphocytes may react to the latent antigens such as Epstein Barr nuclear antigen 1 and 2 (EBNA2& EBNA3). Thus, it may be surmised that a T lymphocyte reaction may be elicited in the latent and lytic viral antigenic phase. Alternatively, CD4+ T lymphocytes may not be substantially enhanced though the cells may significantly curb the viral (EBV) infection. CD4+ T lymphocytes may identify numerous lytic antigens in concordance with the tetramers of major histocompatibility antigen II (MHCII)[3,6]. CD4+ T lymphocyte may emerge and perpetuate minimally within the peripheral blood during the acute infectious phase of infectious mononucleosis.
Investigative Assay: The immunoglobulin G (IgG) antibody response to diverging viral (EBV) proteins may be adequately deciphered with the line blot method. An estimated six viral (EBV) antigens may be recognized, two of which may be components of the Viral Capsid Antigen (VCA) structural proteins p23 (BLRF2) and p18 (BFRF3). Immunoglobulin G (IgG) antibodies against the viral capsid antigen (EBV VCA) may be discerned within the first week of onset of disease along with a lifelong persistence. Immune response to the p23 component of the viral capsid antigen (VCA) may appear quickly, in contrast to the response to p18 component. Three of the viral (EBV) antigens may arise from the temporal kind of lytic gene products such as the immediate early, early and late antigens. Antibodies directed towards the immediate antigen EA BZLF1 may arise initially and persist. Antibody reaction to the Epstein antigens (EAs) p138 and p54 may be divergent[3]. The antibodies may appear early in the course of viral infection and may be undetectable following convalescence. Antibodies against the latent gene product nuclear antigen (EBNA1) may emerge gradually and may be discerned within three months or later, subsequent to viral infection and onset of illness. Once detected, the particular antibodies may persist lifelong. The delayed EBNA1 antibody reaction may concur with the delayed CD4+ T lymphocyte response to the manifested EBNA1[6,7]. Natural killer cells may be incriminated in complications concordant with viral infection (EBV) secondary to immune deficiencies. Concomitant T lymphocytes and natural killer cells along with their products of cytolysis may be enunciated. Natural killer cells may preferentially decimate the viral (EBV) infected cells during viral transition into the lytic phase. Natural killer (NK) cells may depict a substantial elimination of early infection situated in the oropharynx instead of the peripheral blood during the viraemic phase. Elucidation of natural killer cells in infectious mononucleosis and its emergence in peripheral blood may be debatable. An inverse correlation may be delineated between viral particles in the peripheral blood and the quantifiable natural killer cells. Analogously, the values may be concordant[3]. Specific, potent categories of natural killer cells may be involved in combating the viral infection. Although NKG2C+ NK cells may be impacted by the primary viral (EBV) infection, NKG2A+ NK cells may be amplified in the peripheral blood of infectious mononucleosis. Additionally, NKG2A+ CD54+ NK cells may be identified in the tonsils of viral (EBV) carriers. CD56 [dim] NKG2A+ KIR- NK cells may preferably proliferate as a reaction to virally (EBV) infected cells. The natural killer (NK) cell activation occurring with viral replication may be obstructed by the virus with specific methodologies. The protein product of viral ( EBV) open reading frame BZLF1 may encode a peptide sequence that adheres to the non classical major histocompatibility antigen ( MHC1) molecule human leukocyte antigen (HLA) E which may mobilize and engage the inhibitory receptor NKG2A. Adaptive immune cells may interfere and avert the surveillance initiated by natural killer (NK) cell. Biomarker BILF1 may diminish the MHC class1 molecules such as HLA A and B while the efficacy of HLA C (prohibits NK cells) may be maintained. Transformation of B lymphocytes secondary to viral (EBV) infection may be restricted by the natural killer (NK) cells. CD56 [bright] CD16- NK cells may be primed by dendritic cells on account of the interaction with the virus. The natural killer cells and B lymphocytes when primed by the virus may reduce the B lymphocyte transformation secondary to interaction with interferon gamma[3]. Natural killer cells derived from the tonsils may be efficacious, in contrast to the natural killer cells of the peripheral blood. The CD56 [bright] CD16- natural killer cells may be significantly reduced in the peripheral blood during the course of infectious mononucleosis. Natural killer cells may compartmentalize the Epstein Barr virus as:
• A direct cytolysis of infected cells and
• By intercepting the transformation via interferon gamma.
With the appearance of the convalescent phase (3 - 6 months following the onset of infectious mononucleosis) the quantifiable CD8+ T lymphocytes and natural killer cells may approach normal values[7,8].
Histological Elucidation: A histological sampling may be averted until two weeks following therapeutic incompetency. Subsequent to a two week therapy or persisting magnitude of enlarged cervical lymph nodes beyond four weeks, a surgical biopsy may be mandated with a morphological assessment[3,6].
An augmentation of inter-follicular zone with a polymorphous inflammatory infiltrate may induce a segmental distortion and a partially preserved lymphoid architecture. The inter-follicular cellular infiltrate may characteristically consist of enlarged, round to irregular cells with vesicular nuclei, prominent nucleoli and scanty, basophilic cytoplasm. The cellular morphology may recapitulate the appearance of an immunoblast with commingled miniature to intermediate lymphoid cells, plasma cells, histiocytes and endothelial venules lined with a thick, proliferating endothelium. Frequent clusters and occasional sheets of immunoblasts with foci of mitosis may be exhibited[5]. Pleomorphic Reed Sternberg like cells may appear in a certain subset (45%) of subjects. Zones of geographic necrosis of the lymphoid architecture may appear in an estimated two thirds (60%) of the individuals, generally confined to the expansive inter-follicular regions. In-spite of the morphological attributes suspicious of malignancy such as disrupted lymphoid architecture, clusters and sheets of enlarged cells, Reed Sternberg like cells and zones of necrosis, prominent regions of normal lymph node morphology or architecture of the tonsils (when involved) may be observed in infectious mononucleosis[5]. Zones of normal, uninvolved lymphoid stroma may frequently blend with the atypical lymphoid architecture. Tissue of waldeyer’s ring may depicta preservation of the crypts with epithelial mucosal lining ulceration of the tonsils in an estimated three fourth (73%) instances. Peri-tonsillarabscess may be detected in roughly one third (33%) of the subjects. Reactive lymphoid follicles with germinal centres as well as the sub-capsular, cortical or medullary sinuses may be retained within the lymph node tissue. Follicular hyperplasia may predominate in specific (20%) instances. Lymph node enlargement in infectious mononucleosis or adjunctive viral infections may necessitate an investigation in order to reduce the mortality and augment prognostic outcomes[5].
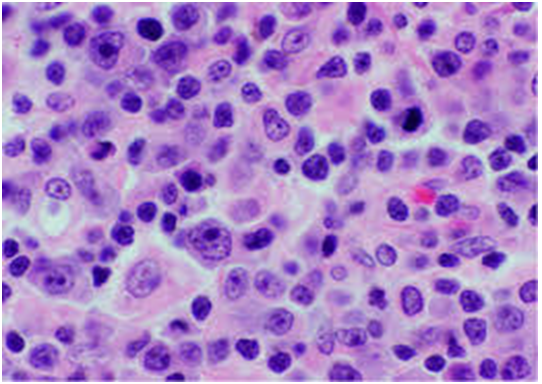
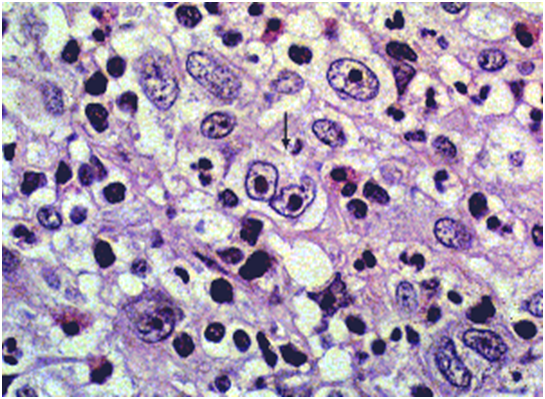
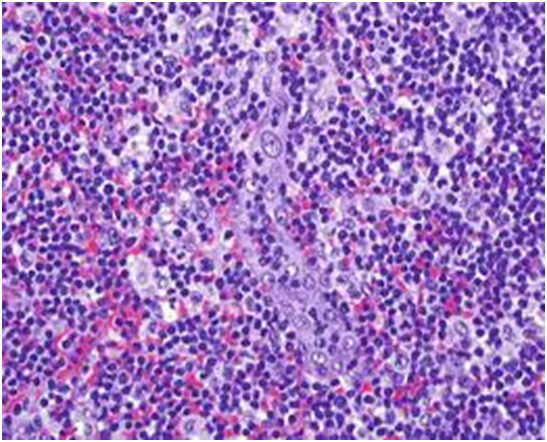
Figure 1: viral (EBV) associated immunoblastic infiltrate[16].
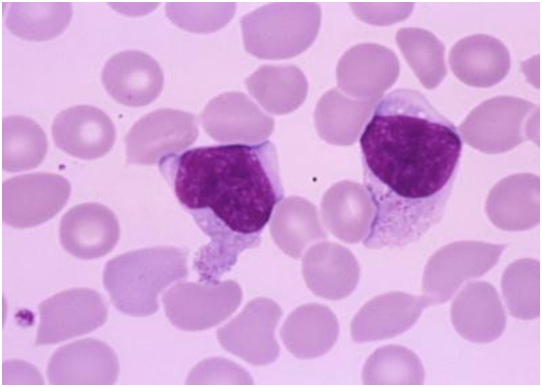
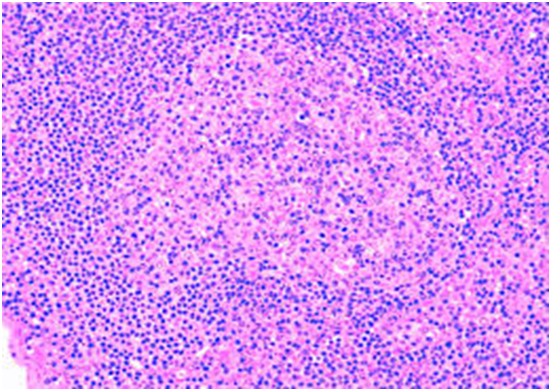
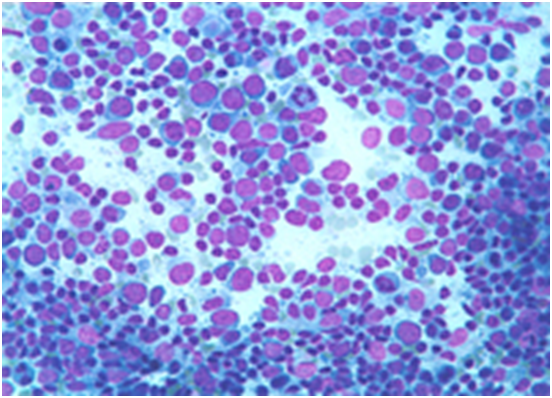
Figure 2: atypical lymphoid cells in peripheral blood[17].
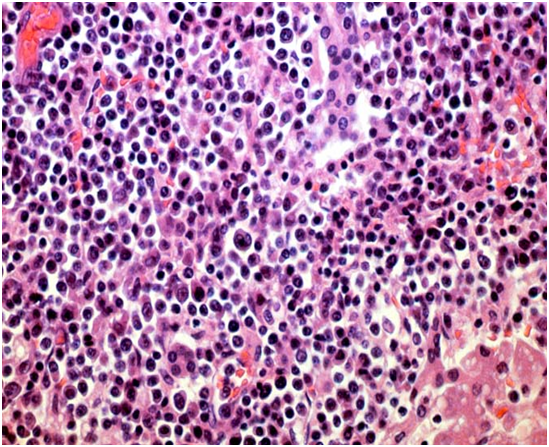
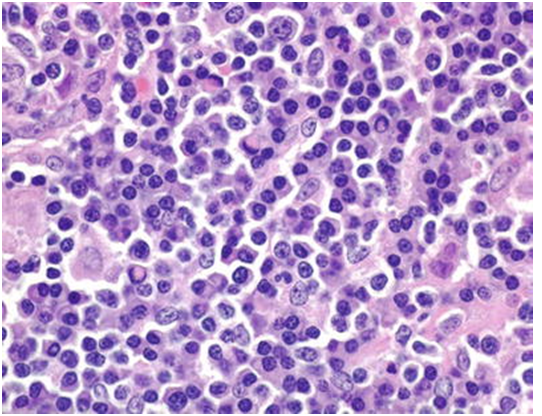
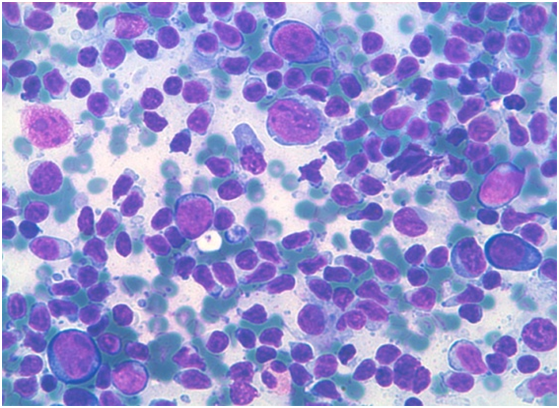
Figure 3: immunoblasts admixed with mature lymphocytes[18].
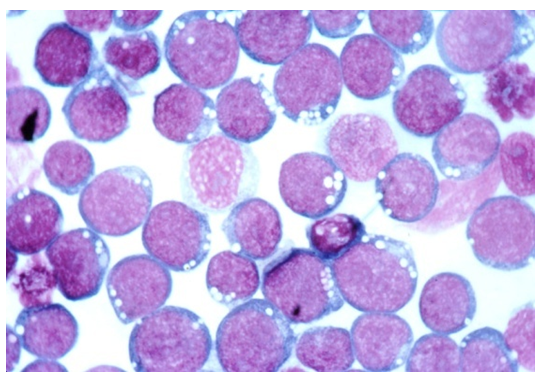
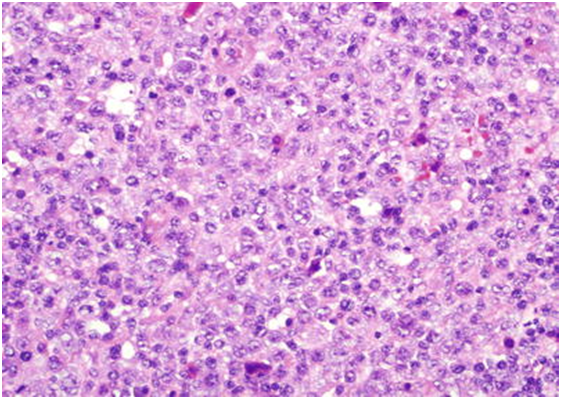
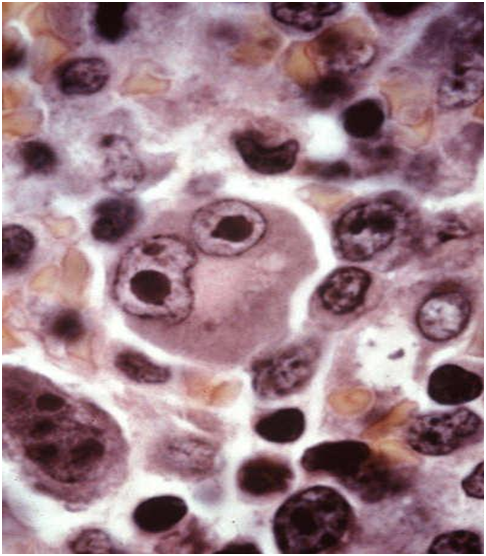
Figure 4: open chromatin, vesicular nuclei and basophilic cytoplasm [19].
Figure 5: pleomorphic immunoblast mimicking Reed Sternberg cell [20].
Figure 6: interfollicular egress of immunoblasts[21].
Figure 7: immunoblasts with conspicuous nucleoli and scanty cytoplasm[22].
Figure 8: sheets of pleomorphic immunoblasts with basophilic cytoplasm and vesicular nuclei[23].
Figure 9: lymphocytic aggregates admixed with pale –staining immunoblasts[23].
Figure 10: cytological elucidation of immunoblasts with basophilic rimming of cytoplasm[24].
Figure 11: atypical lymphocytes with admixed immunoblasts[24].
Figure 12: Reed Sternberg like cell with prominent nucleoli[25].
In situ Hybridization and Immune - Histochemistry: In situ hybridization for Epstein Barr virus encoded Ribo Nucleic Acid (EBER) may display a heterogeneous infiltrate of miniature to enlarged lymphoid cells with viral (EBV+) immune reactivity. The cellular egress may preponderantly appear within the centric inter-follicular zone and may majorly be composed of immunoblasts. Miniature lymphoid cells, immune reactive to the virus (EBV+) may infrequently emerge in the germinal centres[8,9]. Disseminated immune reactive lymphoid cells (EBV +) may exceptionally be situated within the mantle zones of the lymphoid follicles. However, immune reactive (EBV+) immunoblasts may be absent from the germinal centres. Majority of the immunoblasts may be B cells with immune reactivity to CD20+. Immunoblasts with immune reactivity to CD3+ may be elucidated in focal zones. An estimated half (> 50%) of the immunoblasts may depict an immune reactivity to MUM1 / IRF4. The particular immune reaction may be elucidated in all (100%) the instances of infectious mononucleosis. Immune markers for the germinal centre phenotype (CD10- and BCL6-) may be absent. The infected cells may display a post germinal centre phenotype (BCL6-, CD10-, MUM1/IRF4+)[3,5]. Immunoblasts may be mildly immune reactive to BCL2 in an estimated half (50%) of the instances. Majority of the immunoblasts may manifest reactivity to OCT2+, BOB1+ and CD30+ with a non reactive CD15-. Reed Sternberg like cells may depict an immune-phenotype resembling the immunoblasts in the instances (100%) of infectious mononucleosis. In situ hybridization employed for the detection of kappa and lambda light chains may exhibit innumerable plasma cells with a polyclonal manifestation of immunoglobulin. However, light chain enunciation within immunoblasts may be inadequately ascertained with a hybridization technique[9,10]. An immune histochemical assay for discovering kappa or lambda light chains within immunoblasts may display a polytypic manifestation in roughly 80% instances. Immunoblasts may be commingled with a polymorphous infiltrate preponderantly comprised of miniature CD3+ T lymphocytes along with CD20+ B lymphocytes and CD38+ plasma cells. The percentage of CD3+ T lymphocytes may be markedly overshadowed by the CD20+ B lymphocytes in a majority of instances[10,11]. Immune histochemistry for CD4+ and CD8+ markers may enunciate a markedly reduced or inverse CD4 / CD8 lymphocytic proportion in majority (85%) of instances. Plasma cells may exhibit a polyclonal immune response with in situ hybridization in infectious mononucleosis (100%). An immune histochemical assay for kappa and lambda immunoglobulin light chains may enunciate identical outcomes (100%). Plasma cells may manifest varied immunoglobulin molecules such as immunoglobulin G (Ig G), immunoglobulin M (Ig M) and heavy chains of immunoglobulin A (Ig A) with the exceptional appearance of immunoglobulin D (Ig D) secreting plasma cells. Polymerase Chain Reaction (PCR) may be reactive for the viral (EBV) infection[11,12].
Diagnostic Criterion: A primary phase of Epstein Barr viral infection may not be assessable purely on the basis clinical attributes. A laboratory confirmation with a heterophile antibody test may be necessitated. Paul Bunnell in 1932 described a heterophile antibody test which elucidated a competent antibody response towards specific and diverse antigens which may be unrelated to the particular antigens inciting an appropriate antibody response against infectious mononucleosis[13]. Haemolytic anaemia (detected with antibodies against mammalian erythrocytes) may ensue with the discernment of specific immunoglobulin M (Ig M) subclass of antibodies which may be induced with generalized immune enhancement secondary to the acute primary infection of infectious mononucleosis. An estimated half (40%) of the children below 4 years of age may be devoid of the heterophile antibodies following a primary viral (EBV) infection[14]. The antibodies generated may be non- specific and may arise on account of infections induced by secondary or alternative pathogens, malignancies and co-existent autoimmune disorders. The pertinent antibodies may persist for beyond a year, thus may not be confirmatory for acute Epstein Barr viral (EBV) infection. An appropriate testing modality may be the assay of viral capsid antigen immunoglobulin (VCA- Ig M, VCA – Ig G) and Epstein Barr nuclear antigen (EBNA -1 Ig G) immunoglobulin G. The parameters may be quantified with an enzyme immunoassay. The viral capsid antigen (VCA Ig M) immunoglobulin M may be discerned in approximately three fourths (75%) of the patients within the acute phase of disease (a false positive reaction may be generated with cytomegalovirus)[3,5]. Individuals with infectious mononucleosis may depict immunoglobulin G (Ig G) antibodies to Viral Capsid Antigen (VCA), an investigation considered to be superior for categorizing a preceding viral (EBV) infection. Antibodies against the nuclear capsid ( EBNA-1) antigen may be initiated gradually and may not be discernible for up to three months or beyond after onset of disease. Thus, antibodies to the nuclear antigen (EBNA-1) may exclude the presence of an acute illness or acute primary viral (EBV) infection. Majority of the viral (EBV) infections may be delineated with the aforementioned antibodies (VCA Ig M, VCA Ig G and EBNA-1). Early antigen (EA) antibodies of the category of immunoglobulin G( Ig G) may not be confirmatory of primary viral (EBV) infection as approximately 60% - 80% of the subjects may be reactive during the acute phase of infection and the specific antibodies may be detected in 20% of healthy individuals[14,15].
Additional Assays: Avidity assay of immunoglobulin G (Ig G) may be advantageous in instances of a debatable outcome of the aforementioned investigations. Immunoglobulin G (Ig G) antibodies when discovered during the acute phase of infection may not adhere to the antigenic targets as tightly as do the antibodies engendered in the convalescent phase. Enhanced avidity of the antibodies may signify a delayed stage of primary viral (EBV) infection[3,4].
Disease Complications: Acute phase of primary Epstein Barr viral infection may infrequently demonstrate severe complications. Airway obstruction on account of oro-pharayngeal inflammation, streptococcal pharyngitis, meningo encephalitis, haemolytic anaemia and thrombocytopenia may ensue in an estimated 1% individuals. A grave complication such as a splenic rupture or haemorrhage generally appears in below 1% instances. Public contact, athletics or sports may be permitted beyond three weeks in the absence of active, primary acute Epstein Barr viral infection[11,12]. The latent virus (EBV) may be reactivated and induce lymphoid malignancies, frequently in adults. Carcinoma concurrent with Epstein Barr virus (EBV)infection may be assessed by the proliferation of monoclonal lymphocytes infected by the virus (EBV) elucidating a restricted latent viral gene expression. Alteration in the mechanism of normal cellular expansion may induce an unchecked cellular division with the emergence of cancer. The virus (EBV) induced immunity may decline in order to impact the pathogenesis and evolution of virus (EBV) associated malignancies with secondary immune-deficiencies[14,15].
Asymptomatic and symptomatic primary viral (EBV) infection may be associated with several neoplasm and autoimmune diseases. A demonstrable history of pre-existing infectious mononucleosis may enhance the predilection of leukaemia, Hodgkin’s disease and multiple sclerosis. Lymphomas (Non Hodgkin’s and Hodgkin’s) may emerge from the affected lymphoid tissue. Viral (EBV) incrimination may depict an enhanced probability of lymphoma secondary to an immune compromised system, specific racial delineation and in elderly individuals[14,15].
Lymphomas may ensue on account of multiple interactions betwixt multitudinous genes and environmental triggers. Infection with the virus (EBV) may elevate the possible induction of specific malignancies such as the aggressive Burkitt’s lymphoma, nasopharyngeal carcinoma and variants of gastric carcinoma. The virus (EBV) may also incite a lymphoid malignancy in healthy, immune competent children[3,6].
Therapeutic Options: Applicable therapeutic modalities for treating infectious mononucleosis may be unsatisfactory. Various nucleoside analogues may prove too efficacious in treating the virus (EBV). The constitutional symptoms and disease severity may be markedly reduced with the administration of Valacyclovir, though the drug may demonstrate a few adverse reactions. Corticosteroids may be employed to treat the inflammatory complications such as airway obstruction or autoimmune reactions, anaemia and thrombocytopenia. However, an extensive use of corticosteroids may impede the viral clearance and induce a secondary malignancy. Appearance of a malignancy secondary to an overdose of corticosteroid may remain controversial[3,5].
Table 1: Immune Antibodies and Globulins discerned with Infectious Mononucleosis[5].
| Immune Antibody | Immunoglobulin Chains | In situ Hybridization |
|---|---|---|
| CD20 | Kappa | Kappa and Lambda chains |
| CD10 | Lambda | |
| BCL-2 | Mu | EBV-EBER |
| MUM1/IRF4 | Gamma | |
| BCL-6 | Alpha | |
| CD3 | Delta | |
| CD4 | ||
| CD8 | ||
| CD30 | ||
| CD15 | ||
| OCT-2 | ||
| BOB-1 |
EBV: Epstein Barr virus EBER: Epstein Barr virus encoded ribo nucleic acids (RNAs).
Table 2: Stages of EBV infection with enzymatic immunoassay[3].
| Stage of Infection | Onset of Infection | VCA Ig M | VCA Ig G | EBNA-1 Ig G |
|---|---|---|---|---|
| EBV naïve | ------ | Negative | Negative | Negative |
| Acute Primary Infection | 0 to 3 weeks | Positive | Negative or Positive | Negative |
| Sub-acute Infection | 4 to 12 weeks | Positive | Positive | Negative |
| Convalescence | 4 to 6 months | Negative or Positive | Positive | Negative or Positive |
| Previous Infection | Greater than 6 months | Negative | Positive | Positive |
EBV: Epstein Barr Virus, EBNA: EBV nuclear antigen, Ig M : Immunoglobulin M, VCA : Viral Capsid Antigen.
Table 3: Morphological Attributes of Infectious Mononucleosis[5].
| Histological Feature | Lymph Node | Waldeyer’s Ring |
|---|---|---|
| Paracortical Expansion | 100% | 100% |
| Architectural Distortion | 100% | 100% |
| Polymorphous Infitrate | 100% | 100% |
| Elevated Immunoblasts | 100% | 100% |
| Preserved Foci | 100% | 100% |
| Reed Sternberg like cells | 34% | 55% |
| Follicular Hyperplasia | 0% | 27% |
| Tissue abscess | 0% | 34% |
| Ulcerated Mucosa | N/A | 74% |
| Necrosis | 66% | 60% |
Table 4: Immune phenotypes of Immunoblasts[5].
| Immune Antibodies | Score 0+(absent) (%) | Score 1+(%) | Score 2+(%) | Score 3+(%) |
|---|---|---|---|---|
| CD20 | 0 | 0 | 39 | 61 |
| EBER | 0 | 0 | 83 | 17 |
| MUM1/IRF4 | 0 | 0 | 44 | 56 |
| OCT-2 | 0 | 0 | 56 | 44 |
| BOB-1 | 0 | 0 | 63 | 38 |
| CD30 | 0 | 8 | 77 | 15 |
| CD15 | 100 | 0 | 0 | 0 |
| BCL-2 | 47 | 18 | 35 | 0 |
| CD3 | 67 | 33 | 0 | 0 |
| BCL-6 | 88 | 12 | 0 | 0 |
| CD10 | 100 | 0 | 0 | 0 |
0+ : absent staining in immunoblasts , 1+:< 10% immune reactive immunoblasts, 2+: 10% - 80% immune reactive immunoblasts, 3+: >80% immune reactive immunoblasts. Kappa and Lambda immunoglobulin light chains maybe immune reactive with polyclonal antigens in 72% and devoid of immune staining in 19% instances. EBER with in situ hybridization may be reactive in 10% to 70% instances.( mean 35%, median 30%)
References
- 1. Sprunt, T.P., Evans, F.A. Mononuclear leucocytosis in reaction to acute infections. (1920) John Hopkins Hosp Bull 31: 410-417.
Pubmed|| Crossref|| Others
- 2. Epstein, M.A., Achong, B.G., Barr, Y.M. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. (1964) Lancet 1(7335): 702-703.
- 3. Balfour, H.H., Dunmire, S.K., Hogquist, K.A. Infectious mononucleosis. (2015) Clin Transl Immunology 4(2): 33.
Pubmed|| Crossref|| Others
- 4. Khodabandeh, M., Mohammadi, M., Borhani, K., et al. Lymphoma infectious mononucleosis by Epstein Barr virus: a case report. (2017) Caspian J Paediatr 3(2): 257-260.
Pubmed|| Crossref|| Others
- 5. Louissaint, A., Ferry, J.A., Soupir, C.P., et al. Infectious Mononucleosis mimicking lymphoma: distinguishing morphological and immunophenotypic features. (2012) Modern Pathology 25(8): 1149-1159.
- 6. Leem, S., Banihani, R., Wong, J., et al. Bilateral Hilar Lymphadenopathy with Infectious Mononucleosis. (2018) Int J Intern Emerg Med 1(1): 1004.
Pubmed|| Crossref|| Others
- 7. Jenson, H.B., Kliegman, R.M., Bonita, F., et al. Nelson‘s textbook of Paediatrics, 20th edition. (2016) Philadelphia Elsevier Health Sci 2: 1586-1590.
Pubmed|| Crossref|| Others
- 8. Gaddey, H.L., Riegel, A.M. Unexplained lymphadenopathy: evaluation and differential diagnosis. (2016) Am fam Physician 94(11): 896-903.
- 9. Zareifar, S., Kazemi, B., Arzanian, M.T., et al. Detection of Epstein Barr virus in a paediatric lymphoma: A single centre study. (2016) J Leuk 4(3): 213.
Pubmed|| Crossref|| Others
- 10. Bolis, V., Karadedos, C., Chiotis, I., et al. Atypical manifestations of Epstein Barr virus in children: a diagnostic challenge. (2016) J Paeditr 92(2): 113-121.
- 11. Epskamp, C., de Man, P., Libourel, E.J. Epstein Barr virus mimicking lymphoma: a case report. (2015) Neth J Med 73(9): 432-434.
- 12. Akkoc, G., Kadayifci, E.K., Karaaslan, A., et al. Epstein Barr virus encephalitis in an immunocompetent child: a case report and management of Epstein Barr virus encephalitis. (2016) Case Rep Infect: 7549252.
- 13. Paul, J.R., Bunnell, W.W. The presence of heterophile antibodies in infectious mononucleosis. (1932) Am J Med Sci 183: 90-104.
Pubmed|| Crossref|| Others
- 14. Son, S.M., Choi, M., Kim, W. S., et al. Case report: multifocal lymphadenopathy due to cytomegalovirus and Epstein Barr virus infection in lymphoma patients receiving chemotherapy: a report of two cases. (2016) Int J Clin Exp Pathol 9(8): 8745-8749.
Pubmed|| Crossref|| Others
- 15. Ansell, H.M. Hodgkin’s lymphoma: 2016 update on diagnosis, risk stratification and management. (2016) Am J Haematol 91(4): 434-442.
- 16. Image 1 Courtesy : Research gate
Pubmed|| Crossref|| Others
- 17. Image 2 Courtesy: Tops images.
Pubmed|| Crossref|| Others
- 18. Image 3 Courtesy: tpisupmc.
Pubmed|| Crossref|| Others
- 19. Image 4Courtesy: Lab roots.
Pubmed|| Crossref|| Others
- 20. Image 5 Courtesy: dnai.com
Pubmed|| Crossref|| Others
- 21. Image 6 Courtesy: Bedroom furniture.
Pubmed|| Crossref|| Others
- 22. Image 7 Courtesy: Time HD
Pubmed|| Crossref|| Others
- 23. Image 8 & 9 Courtesy: Springer link.com
Pubmed|| Crossref|| Others
- 24. Image 10 & 11 Courtesy: Eurocytology.
Pubmed|| Crossref|| Others
- 25. Image 12 Courtesy: Healthdoc.box
Pubmed|| Crossref|| Others