The Reliability of Blood Glucose Point-of-Care Testing (POCT) System in China Teaching Hospital
Feng-fei Li1#, Yan Cao2#, Bing-liLiu1#, Reng-na Yan1#, Hong-hongZhu1#, Wei Rong1, He-feng Diao1, Jie Lan1, Xue-mei Zheng3, Li Zhang4, Hai-yan Yang5, Bing Xia6, Hui Zhan7, Xiao-fei Su1, Jin-dan Wu1, Dan-Feng Zhang1, Shu-kui Wang8*#, Jian-hua Ma1*#
Affiliation
- 1Department of Endocrinology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- 2Department of quality management, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- 3Department of Cardiology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- 4Department of General Surgery, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- 5Emergency Intensive Care Unit, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- 6Department of Orthopedics, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- 7Department of Neuropathy, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- 8Central Laboratory, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
- #Authors Contributed equally to this article
Corresponding Author
Jian-hua Ma, M.D., PhD, Department of Endocrinology, Nanjing First Hospital, Nanjing Medical University, 32 Gongqingtuan Road, Nanjing, China, 210012, Tel: +862552887091, Fax: +862552887016; E-mail: majianhua196503@126.com
Shu-kui Wang, M.D, PhD, Central Laboratory, Nanjing First Hospital, Nanjing Medical University, 68 Changle Road, Nanjing 210006, Jiangsu, China, Tel: +862552271000, Fax: +862552271108; E-mail: shukwang@163.com
Citation
Jian-hua, M., et al. The Reliability of Blood Glucose Point-of-Care Testing (POCT) System in China Teaching Hospital. (2017) Int J Hematol Ther 3(2): 1- 4.
Copy rights
© 2017 Jian-hua, M. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Hematology
Abstract
Background: To observe the reliability of blood glucose Point-of-Care Testing (POCT) system in teaching hospital in China.
Methods: This was a single center, sectional study. Between Jun and Aug 2015, a total of 353 inpatients were recruited from Nanjing First Hospital, China. After 10-hr fasting, the finger point blood glucose concentrations were measured with POCT system. Venous blood specimens were collected by investigators within 5 min after the finger point blood glucose concentrations were measured. The venous blood samples were then divided into two parts, with one part for blood glucose concentration measurement in central laboratory, the other one part was for hematocrit measurement. The precision of POCT results was evaluated by the International Organization for Standardization (ISO) revised ISO15197:2013. The clinical accuracy of POCT results were assessed by Parkes Error Grid.
Results: A total of 372 blood glucose concentrations delivered from POCT were compared with venous serum blood glucose levels measured by central laboratory system. The precision of the POCT was 76.3 %, with the 81.8 % in patients with blood glucose concentrations > 5.55 mmol/L and 75.6 % in subjects with blood glucose concentrations ≤ 5.55 mmol/L. In addition, the precision of results of POCT in patients with lower (male 40 L/L and female 37 L/L) and normal (male 40 - 50 L/L and female 37-45 L/L) hematocrit concentrations were 73.4 % and 79.4 %, respectively. The Parkes Error Grid data showed that the results from POCT, being 89.58 % values in Zone A, 10.4 1% values in Zone B, and 100 % values in Zone A+B.
Conclusions: In this study, our data provided the real world of the reliability of results delivered from POCT was unfulfilled the standard of ISO15197:2013 of accuracy limits criteria in one hospital setting in China.
Introduction
Technical developments with the advantage of easy operation, the less volumes of blood specimen needed[1], the shorten turn-around time[2-5], and fulfill the accuracy limits criteria[6], make the utility of blood glucose Point-of-Care Testing (POCT) in hospital settings has the amazing increase in worldwide for years[7-9].
However, the POCT system running in hospital settings need more closer to adherence to a quality management framework. A survey uncovers that the staff directly associated to POCT with or without specific training and certification. In addition, the question of quality control and the reporting results of POCT were also raised even in Western Country[9].
POCT system in the hospital settings had numerous opportunities for errors, with the performance of POCT, the different kinds of blood-glucose meters, the patient, and environmental circumstances could be error sources of preanalytic, analytic and postanalytic[10,11]. However, studies highlight that the analytical phase of testing is the mainly error source POCT comparison with central laboratory blood-glucose testing[12,13].
Little information is available regarding the clinical accuracy and precision of results delivered from POCT in the real world in hospital settings. For patients with hypoglycemia or hyperglycemia tested by POCT system might mislead the decision-making and clinical outcome[14]. The aim of this study was to investigate the reliability of results delivered from POCT performed by nursing staff in hospital setting in China. To achieve this aim, a comparison of POCT results with central laboratory testing of blood glucose concentrations was conducted in one general hospital in China.
Materials and Methods
This was a pilot single center study. Between Jun and Aug 2015, a total of 353 inpatients were recruited from Emergency Intensive Care Unit (EICU), Department of Orthopedics, Department of General Surgery, Department of Neuropathy, Department of Cardiology, and Department of Endocrinology in Nanjing First Hospital, China. All study subjects provided written informed consent and the study was approved by the Ethics Committee of Nanjing First Hospital, Nanjing Medical University.
After 10-hr fasting, the finger point blood glucose concentrations were then measured by nursing staff with POCT (One Touch UltraVue, Johnson & Johnson Medical (China) Ltd)
system. Venous blood specimens were collected by investigators within 5 min after the finger point blood glucose concentrations were measured. The venous blood samples were then divided into two parts, one was centrifuged (4000 rpm, 15 min) at 4°C and stored at -20°C for 30 min to 1.5-hr for blood glucose concentration measurement in central laboratory of Nanjing First Hospital with a Hitachi 7600 - 120 analyzer (Hitachi Corp, Tokyo, Japan). The other one was stored at NaF anticoagulation tubes (3.1 mlDraw; Sarstedt AG & Co., N€ umbrecht, Germany), for which hematocrit values were analyzed. The precision of POCT results was evaluated by the International Organization for Standardization (ISO) revised ISO15197:2013, which requires the minimum error range of the results delivered from POCT within 15% at blood concentrations ≥ 5.55 mmol/L and less than 0.83 mmol/L at glucose concentrations ≤ 5.55 mmol/L. The clinical accuracy of POCT results were assessed by Parkes Error Grid, which was recommended by the International Organization for Standardization (ISO) 15197:2013.
Statistical Analysis
The blood concentrations were presented as mean. The relationship between the POCT system and Hitachi 7600 - 120 was analyzed by Parkers Error Grid.
Results
The precision of POCT
A total of 372 finger point blood glucose concentrations measured by POCT system and 372 venous blood glucose levels delivered from central laboratory were collected from 353 patients. The precision of POCT results was evaluated by the International Organization for Standardization (ISO) revised ISO15197:2013, which requires the minimum error range of the results delivered from POCT within 15% at blood concentrations ≥ 5.55 mmol/L and less than 0.83 mmol/L at glucose concentrations ≤ 5.55 mmol/L. Our data showed that only 76.3 % results of POCT were fulfilled the requirements. A stratified analysis comparing the results of POCT and Central Laboratory Testing (CLT) revealed that the precision of POCT in patients with blood glucose concentrations ≥ 5.55 mmol/L, ≤ 5.55 mmol/L were 81.8% and 75.6%, respectively (Table 1). In addition, the precision of results of POCT in patients with lower (male 40 L/L and female 37 L/L) and normal (male 40 - 50 L/L and female 37-45 L/L) hematocrit concentrations were 73.4% and 79.4%, respectively (Table 1).
Table 1: The accuracy of samples collected from patients with different blood glucose and hematocrit concentrations.
| Items | Total | Glucose levels (mmol/L) | Hematocrit concentrations (L/L) | ||
|---|---|---|---|---|---|
| > 5.55 | ≤ 5.55 | F < 37, M < 40 | F 37 - 45, M 40 - 50 | ||
| Total samples | 372 | 44 | 328 | 192 | 180 |
| Accuracy samples | 284 | 36 | 248 | 141 | 143 |
| Precision (%) | 76.3 | 81.8 | 75.6 | 73.4 | 79.4 |
F: Female, M: male
We conducted this study in six department departments in Nanjing First Hospital, China. The samples blood glucose concentrations delivered by POCT/CLT collected from EICU, Department of Orthopedics, Department of General Surgery, Department of Neuropathy, Department of Cardiology, and Department of Endocrinology were 45, 6, 51, 63, 51, and 156, respectively. The precision of POCT in Department of Orthopedics, Department of Neuropathy, Department of Cardiology, EICU, and department of endocrinology was 51.5%, 66.7%, 82.4%, 85.7%, 78.4%, 77.6%, respectively (Table 2).
Table 2: The accuracy of samples collected from different departments.
| Total | EICU | Orth | Surg | Neur | Card | Endo | |
|---|---|---|---|---|---|---|---|
| Total samples | 372 | 45 | 6 | 51 | 63 | 51 | 156 |
| Accuracy samples | 284 | 23 | 4 | 42 | 54 | 10 | 121 |
| Precision (%) | 76.3 | 51.5 | 66.7 | 82.4 | 85.7 | 78.4 | 77.6 |
EICU: Emergency Intensive Care Unit, Orth: Department of Orthopedics, Surg: Department of General Surgery, Neur: Department of Neuropathy, Card: Department of Cardiology, Endo: Department of Endocrinology.
The accuracy of POCT
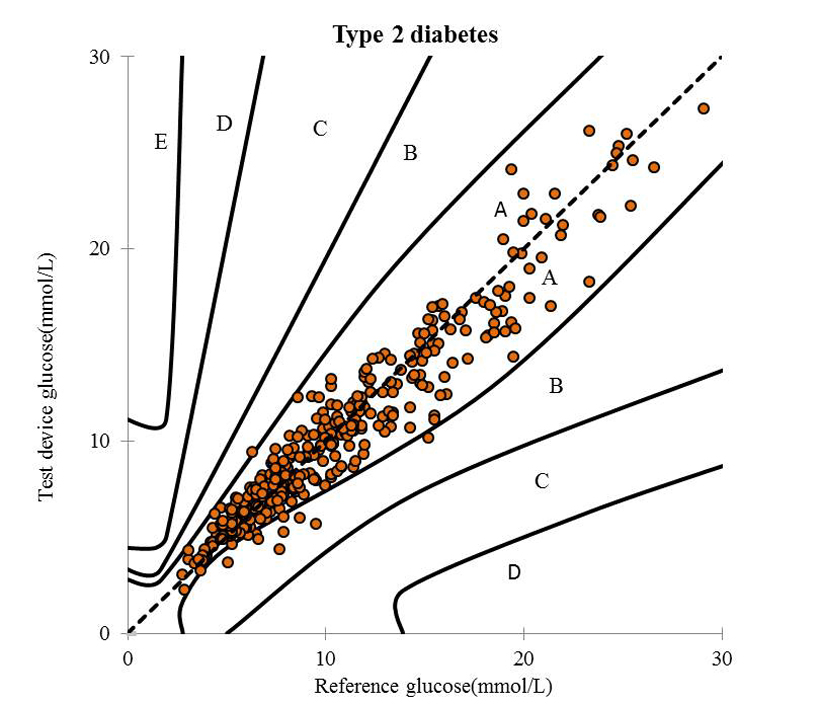
To investigate the accuracy of POCT system, we utilized the results of the venous blood specimen measured by central laboratory with a Hitachi 7600 - 120 analyzer. The Parkes Error Grid data showed that the results from POCT, being 89.58% values were within Zone A ("no effect on clinical action"), 10.41% values were within Zone B ("altered clinical action with little or no effect on clinical outcome"), and 100% values in Zone A+B (Figure 1). The ISO15197:2013 standard requirement of POCT results should be that more than 95% values were within Zone A. Our date indicated that the clinical accuracy of the results of POCT in this study did not meet the requirements of ISO 15197:2013 standard.
Figure 1: The Parkes Error Grid of POCT in this study.
Discussion
In this study, our data indicated that the real world of the reliability of results delivered from POCT was unfulfilled the standard of ISO15197:2013 of accuracy limits criteria in one hospital setting in China.
Results of POCT for blood glucose testing must fulfill ISO15197 released more stringent standards, which require 95% of the measurement results must be within 0.83 mmol/L or 15%. In this study, a total of 372 results of POCT collected from 353 subjects in 6 departments under routine clinical conditions directly compared with results delivered by CLT using venous samples in this study. A key observation was that only 79.3% of the POCT measurements in the clinical settings were matched the results of CLT. The accuracy of POCT was much lower than the ISO 15197:2013 standard requirement.
The much lower accuracy of results delivered by glucose POCT in this study raised several concerns under routine clinical conditions. Firstly, the accuracy might be the glucose POCT measurements may be affected by patient’s physiological parameters (hematocrit) and creatinine (Cr)[15-17]. Secondly, the environmental conditions, and hematocrit may affect the results[18-20]. Thirdly, the performance of POCT might be potential error sources in the hospital settings[10,11]. In this study, we collected specimens from six department, which including EICU. The data showed that the accuracy of POCT performed in patients without kidney dysfunction and abnormal hematocrit level was significantly higher than that of with kidney dysfunction and abnormal hematocrit levels (80.5%, 116 out of 144, and 30.8%, 4 out of 13, respectively, P = 0.01).
Our data also showed that the accuracy of POCT was different in the six departments. The POCT system lacks strict quality control and operator preformation system[21]. However, the more types of blood-glucose meters used in one hospital might offset to some degree the reliability of the results delivered by the POCT system. According to the stringent ISO standard of 15197:2013, two out of three POCT systems failed to meet the requirements in a China hospital performed very recently[22]. Furthermore, patients with hypoglycemia or hyperglycemia tested by POCT system might mislead the decision-making and clinical outcome[14]. The accuracy and precision of one blood-glucose testing meter is not necessarily equivalent to that of another glucose test, because the different methodology, environmental and operator effects[10,11]. In this study, the glucose testing meters used in the hospital were qualified to be used in China hospital and only one type of testing meters were used in the whole hospital. With the advantages of one type blood-glucose meter delivering the same blood glucose concentration unit, the convenience of operator training and the same quality control system, hospital might be benefited from selection of one type of blood-glucose meters.
Standard guidelines and the recommendations regarding the POCT system running are the importance elements in the total testing process[23]. Hospital staff directly performance of POCT think that the POCT system is an easy work, rather than more complex[9]. Moreover, there is ample opportunity for errors with POCT devices in hospital settings. Even under suitable situations, device can experience malfunction[24]. In addition, the liquid control, the validate and maintain the glucose meters good function to prevent errors from occurring[24]. Also, operators should pay attention to some extremely circumstances, such as extremely high and low temperatures, humidity and high altitude[25].
This study had some limitations. Firstly, this study was only conducted in a single center, which might not be the same of other hospitals. Secondly, the sample sizes were too small. Further prospective evaluation of these results is warranted.
In conclusion, our data provided the real world of the reliability of results delivered from POCT was unfulfilled the standard of ISO15197:2013 of accuracy limits criteria in one hospital setting in China.
Conflict of interest:
All authors declare that there is no conflict of interest.
References
- 1. Wisser, D., van Ackern, K., Knoll, E., et al. Blood loss from laboratory tests. (2003) Clin Chem 49(10): 1651-1655.
Pubmed || Crossref || Others - 2. Schimke, I. Quality and timeliness in medical laboratory testing. (2009) Anal Bioanal Chem 393(5): 1499-1504.
Pubmed || Crossref || Others - 3. Howanitz, P.J., Jones, B.A. Comparative analytical costs of central laboratory glucose and bedside glucose testing: a College of American Pathologists Q-Probes study. (2004) Arch Pathol Lab Med 128(7): 739-745.
Pubmed || Crossref || Others - 4. Hawkins, R.C. Laboratory turnaround time. (2007) Clin Biochem Rev 28(4): 179-194.
Pubmed || Crossref || Others - 5. Blick, K.E. The essential role of information management in point-of-care/critical care testing. (2001) Clinica Chimica Acta 307(1-2): 159-168.
Pubmed || Crossref || Others - 6. Ottiger, C., Gygli, N., Huber, A.R., et al. Performance of a Blood Glucose Monitoring System in a Point-of-Care Setting. (2016) J Diabetes Sci Technol 10(4): 939-946.
Pubmed || Crossref || Others - 7. Tonyushkina, K., Nichols, J.H. Glucose meters: a review of technical challenges to obtaining accurate results. (2009) J Diabetes Sci Technol 3(4): 971-980.
Pubmed || Crossref || Others - 8. Klonoff, D.C. Point-of-Care Blood Glucose Meter Accuracy in the Hospital Setting. (2014) Diabetes Spectr 27(3): 174-179.
Pubmed || Crossref || Others - 9. Sharp, L., Farrance, I., Greaves, R.F. The application of glucose point of care testing in three metropolitan hospitals. (2016) Pathology 48(1): 51-59.
Pubmed || Crossref || Others - 10. Klonoff, D.C., Perz, J.F. Assisted monitoring of blood glucose: special safety needs for a new paradigm in testing glucose. (2010) J Diabetes Sci Technol 4(5): 1027-1031.
Pubmed || Crossref || Others - 11. Ginsberg, B.H. We need tighter regulatory standards for blood glucose monitoring, but they should be for accuracy disclosure. (2010) J Diabetes Sci Technol 4(5): 1265-1268.
Pubmed || Crossref || Others - 12. O'Kane, M.J. The accuracy of point-of-care glucose measurement. (2012) Annals of clinical biochemistry 49(Pt 2): 108-109.
Pubmed || Crossref || Others - 13. Freckmann, G., Schmid, C., Baumstark, A., et al. System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197. (2012) J Diabetes Sci Technol 6(5): 1060-1075.
Pubmed || Crossref || Others - 14. Voulgari, C., Tentolouris, N. Accuracy and precision of glucose monitoring are relevant to treatment decision-making and clinical outcome in hospitalized patients with diabetes. (2011) Diabetes Technol Ther 13(7): 723-730.
Pubmed || Crossref || Others - 15. Kanji, S., Buffie, J., Hutton, B., et al. Reliability of point-of-care testing for glucose measurement in critically ill adults. (2005) Critic Care Med 33(12): 2778-2785.
Pubmed || Crossref || Others - 16. Shearer, A., Boehmer, M., Closs, M., et al. Comparison of glucose point-of-care values with laboratory values in critically ill patients. (2009) Am J Crit Care 18(3): 224-230.
Pubmed || Crossref || Others - 17. Cook, A., Laughlin, D., Moore, M., et al. Differences in glucose values obtained from point-of-care glucose meters and laboratory analysis in critically ill patients. (2009) Am J Crit Care 18(1): 65-71.
Pubmed || Crossref || Others - 18. Heinemann, L. Quality of glucose measurement with blood glucose meters at the point-of-care: relevance of interfering factors. (2010) Diabetes Technol Ther 12(11): 847-857.
Pubmed || Crossref || Others - 19. Nerhus, K., Rustad, P., Sandberg, S. Effect of ambient temperature on analytical performance of self-monitoring blood glucose systems. (2011) Diabetes Technol Ther 13(9): 883-892.
Pubmed || Crossref || Others - 20. Oberg, D., Ostenson, C.G. Performance of glucose dehydrogenase-and glucose oxidase-based blood glucose meters at high altitude and low temperature. (2005) Diabetes care 28(5): 1261.
Pubmed || Crossref || Others - 21. Dyhdalo, K.S., Howanitz, P.J., Wilkinson, D.S., et al. Documentation of quality control and operator training at point-of-care testing: a College of American Pathologists Q-Probes study of 106 institutions. (2014) Arch Pathol Lab Med 138(11): 1444-1448.
Pubmed || Crossref || Others - 22. Wei, H., Lan, F., He, Q., et al. A Comparison Study between Point-of-Care Testing Systems and Central Laboratory for Determining Blood Glucose in Venous Blood. (2016) J Clin Lab Anal 31(3).
Pubmed || Crossref || Others - 23. Tirimacco, R., Koumantakis, G., Erasmus, R., et al. Glucose meters - fit for clinical purpose. (2013) Clin Chem Lab Med 51(5): 943-952.
Pubmed || Crossref || Others - 24. Nichols, J.H. Blood glucose testing in the hospital: error sources and risk management. (2011) J Diabetes Sci Technol 5(1): 173-177.
Pubmed || Crossref || Others - 25. Clarke, S.F., Foster, J.R. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. (2012) Br J Biomed Sci 69(2): 83-93.
Pubmed || Crossref || Others