The challenge of cancer induced neuropathic pain
Affiliation
Palliative Medicine, Specialist in neuropathic pain relief, St Raphael’s Hospice, 801, London Road, North Cheam, Surrey, SM3 9DX, UK
Corresponding Author
Marie Joseph, Medical Director and Lead Consultant in Palliative Medicine, Specialist in neuropathic pain relief, St Raphael’s Hospice, 801, London Road, North Cheam, Surrey, SM3 9DX, UK Tel: +44 (0)208 099 7777, Fax: +44 (0)208 099 1724; E-mail: mariejoseph@straphaels.org.uk
Citation
Joseph, M. The Challenge of Cancer Induced Neuropathic Pain. (2016) J Palliat Care Pediatr 1(1): 5- 8.
Copy rights
© 2016 Joseph, M. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Neuropathic pain; Complex pathophysiology; Poly-pharmacological therapeutic approach; Maximise efficacy; Minimise medication induced toxicity
Abstract
Neuropathic pain is often the most distressing type of pain that can be suffered by any human being. In the clinical context of cancer, both detection and appropriate management lead not only to improved quality of life but also enables the patient to receive appropriate oncological management.
The clinician needs to be aware of the complex pathophysiology of neuropathic pain which necessitates a rational, multi-modal, poly-pharmacological therapeutic approach in order to maximize efficacy and minimize medication induced toxicity.
Introduction
Neuropathic pain is defined as pain caused by any disease or lesion affecting the central or the peripheral nervous system[1]. It is by far the worst possible pain experience that can be suffered by any human being. In severe instances it can result in devaluation of self, despondency, depression and consequent isolation of the suffering patient. The clinician needs to be vigilant in recognising neuropathic pain in a complex, severe pain situation in a cancer patient, in order to tailor appropriate therapeutic management.
Cancer induced neuropathic pain arises from tumour infiltration of the peri-neural sheath[2,3]. As the tumour progresses, the pain often escalates[3]. The patient often becomes fearful of the prognostic implications of the worsening pain which adds to the suffering. Empathy by the caring clinician is crucial in this situation.
Neuropathic pain occurs in a significant percentage of patients with cancer[3]. It is more common in certain cancers than in others. Head & neck cancer, apical lung cancer, Malignant Mesothelioma of the pleura, cancer of the Pancreas, rectal cancer and spinal metastases are common examples. In these clinical situations, the clinician needs to exercise vigilance in order to diagnose neuropathic pain and then formulate an appropriate analgesic plan.
The characteristic clinical description of intermittent, intense ‘crisis pain episodes’ should alert the clinician that the pain is primarily neuropathic in nature[3]. In addition, the patient often describes a constant, background pain and nocturnal pain which wake an already disturbed sleep.
Box 1
- Neuropathic Pain Characteristics
| * Constant, background pain * ‘Crisis pain episodes’ often providing clue to diagnosis * Nocturnal pain wakes a disturbed sleep |
Recognition of neuropathic pain leads to trust between the patient and the clinician. It will also reassure the patient and family.
Neuropathic pain is caused by a complex, specialised, multi-mechanistic path pathophysiology[2,4]. Hence, unsurprisingly a poly pharmacological therapeutic approach is required for its successful management. Opioid analgesics have useful yet a limited role in severe neuropathic pain management.
The primary biochemical trigger for pain following any nerve injury is excess intra-cellular calcium ion influx. This leads to release of the main excitatory neuro-transmitter, Glutamate which acts on NMDA (N- Methyl, D- Aspartate) receptor. This receptor activation leads to escalation and self perpetuation of the hyper excitation process within the central nervous system resulting in neuropathic pain.
In the above state, there exists a relative opioid resistance to analgesia, which is an important therapeutic fact. Indeed, it is known that very high doses of opioids, particularly opioids that act purely on mu opioid receptors (such as Morphine, Fentanyl) can accelerate this process of central hyper excitation and thus lead to more pain. This phenomenon is termed opioid induced hyper-algesia[5].
It is understandable that, drugs which reduce the initial intra cellular calcium ion influx by acting on the voltage gated calcium channels will be beneficial in neuropathic pain management. Gabapentin and Lyrica® (Pregabalin) are in this category of analgesics.
In addition to the excitatory process, there exists in the brain stem, a descending pain inhibitory process which is served by the inhibitory neuro transmitters, Norepinephrine (NorAdrenaline) and Serotonin (5 HydroxyTryptamine).
Hence, drugs that augment this pain inhibitory process will also contribute to analgesia. Tricyclic anti- depressants (example Amitriptyline), selective Norepinephrinergic and Serotonergic re-uptake inhibitors (example Duloxetine), Norepinephrinergic and specific Serotonergic anti- depressants (example Mirtazapine) fall into this category of analgesics.
If neuropathic pain remains severe, despite treatment with above mentioned analgesics, the use of NMDA antagonist, for example Ketamine is likely to be beneficial[6]. Ketamine is known to improve opioid analgesia in neuropathic pain.
If despite the use of opioid and adjuvant analgesics, the patient experiences opioid toxicity and pain escalation, it is worth cautiously considering ‘combined opioid therapy’[7,8]. This treatment strategy is based on the therapeutic principle of ‘Fractional Receptor Occupancy’(FRO)[8]. FRO = Ro/Rt, Ro implying receptors occupied, and Rt implying total number of receptors. The smaller the value of FRO, the greater is the efficacy of the opioid concerned. Hence combining two opioids with opportune FRO, analgesic efficacy can be enhanced and adverse effects minimised.
The clinician can thus safely tailor an appropriate analgesic strategy, without undue reliance on high doses of a single opioid analgesic. In fact in severe neuropathic pain situations, it is best to prevent escalation of opioid doses as aforementioned, since there is clinical evidence of the phenomenon of ‘Opioid Induced Hyper-Algesia’ (OIH)[5]. OIH leads to a vicious circle of increasing neuropathic pain with escalating opioid doses which can result in inadvertent and serious opioid induced toxicity.
Instead in these clinical situations, the clinician can commence co-analgesic adjuvants, which use the therapeutic principle of SYNERGY[8] thus relatively opioid sparing the patient and thereby maximising efficacy and minimising medication induced toxicity.
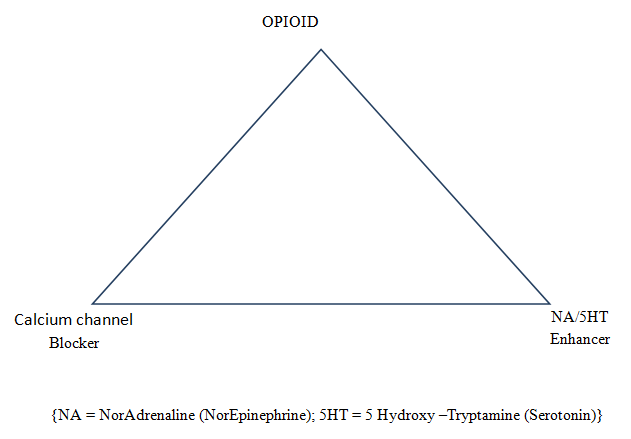
Extensive clinical experience in neuropathic pain management suggests Opioid, voltage gated calcium channel blocker (by reducing intra-cellular calcium ion influx) and drug enhancing the pain inhibitory process (by enhancing Noradrenalin and Serotonin {5 Hydroxytryptamine}) form a successful analgesic triad in the management of severe neuropathic pain.
Figure 1: Neuropathic analgesic triad
Mirtazapine which augments the pain inhibitory process, despite causing initial sedation, improves appetite, mood and pain and is virtually devoid of muscarinic antagonistic effects unlike Tricyclic antidepressants[9].
If the analgesic triad does not adequately improve pain, then consideration should be given to the use of Ketamine preferably with Clonazepam. The Clonazepam, being a Benzodiazepine is likely to provide analgesic as well as anxiolytic effects and reduce the psycho-mimetic effects of Ketamine[10].
It is thus evident that appropriate analgesic management of severe neuropathic pain requires a rational, multi-modal, poly pharmacological therapeutic approach aimed at maximising analgesic efficacy while minimising drug induced toxicity. Therapeutic vigilance is always recommended to combine efficacy with safety.
The detection of neuropathic pain in a cancer setting does have further advantages in addition to carefully selected therapeutic management described above. Particularly in the context of Head & Neck tumours, the development of neuropathic pain can
In the clinical context of cancer induced radicular neuropathic pain from spinal metastases, the clinician is likely to detect impending spinal cord compression or cauda-equina compression well before neurological complications occur[12]. This early detection has huge clinical and qualitative advantages to the patient.
The following clinical case scenarios highlight the above mentioned principles in clinical practice.
Clinical case scenario 1
A 55 year old gentleman underwent right nephrectomy for a renal cell carcinoma. Unfortunately he also developed a uro-thelial malignancy and a prostate malignancy. He then developed thoracic spinal metastases which caused spinal cord compression at the spinal level of T8. He therefore underwent spinal decompression surgery followed by single fraction radiotherapy to this site.
Despite palliative radiotherapy to the spinal metastatic site, he experienced excruciating thoracic spinal pain with ‘girdle distribution pain’ under his rib cage implying radicular neuropathic pain. He was transferred to a hospice palliative care setting from an acute hospital for pain management. He was paraplegic and bed bound.
On admission to the hospice, it was noted that he was receiving high doses of opioids in the form of OxyContin® (Oxycodone modified release) 800 mg 12 hourly. He was receiving high doses of steroid, namely Dexamethasone. He was not receiving any co-analgesic adjuvants for analgesic relief. His pain was intense and he showed features of hand tremors implying opioid induced neurotoxicity.
His pain management was altered in the hospice, by the palliative care clinician with a special interest in cancer induced neuropathic pain.
The high dose of OxyContin® was changed to regular four hourly, immediate release preparation of Oxycodone, initially 200 mg. Co-analgesic adjuvants were commenced, namely Lyrica® (Pregabalin) and Mirtazapine. The Lyrica® was up-titrated gradually to an eventual dose of 75 mg in the morning and 150 mg in the evening. Mirtazapine was gradually up-titrated to an eventual dose of 30 mg at night.
High dose Dexamethasone 8mg twice daily was used for a week with a proton pump inhibitor, namely Lansoprazole to provide gastro-duodenal protection from the steroid. A Steroid reduction plan was in place.
The dose of Oxycodone immediate release was gradually and safely reduced to 50 mg four hourly (without any escalation of pain). Combined opioid therapy was initiated with twice weekly Transtec® (Buprenorphine) TD patch, initially 35 microgram/ hour and increased to an eventual dose of 52.5 microgram/hour. The immediate release preparation of Oxycodone was converted to OxyContin® 160 mg 12 hourly (a reduction from the original dose of 800 mg 12 hourly). This combined opioid therapy utilised the principle of ‘Fractional Receptor Occupancy’ (FRO)[8] which was described earlier.
The patient achieved a good clinical outcome. The opioid toxicity resolved. Although the patient remained paraplegic, his neuropathic pain greatly improved and he was discharged home.
Clinical case scenario 2
A 65 year old gentleman had an anterior resection for a rectal malignant tumour. The surgery was followed by palliative radiotherapy and chemotherapy. Despite this management, his tumour progressed and he developed extensive metastases involving his right pelvic bone eroding the periosteum. This resulted in severe bony and neuropathic pain in his right pelvis which was compromising his mobility.
He had several weeks of analgesic stability at home while receiving OxyContin® (Oxycodone modified release) 160 mg twelve hourly together with adjuvant analgesics namely, Lyrica® (Pregabalin) 75 mg mane and 150 mg evening as well as Zispin SolTab® (Mirtazapine) 45 mg at night, Ketamine oral solution 50 mg TDS and Clonazepam 1mg at night. He was receiving oral steroid, namely Dexamethasone 4 mg mane (with gastric protection with a proton pump inhibitor) with the intention of reducing peri-tumoural oedema in order to further reduce pain. He had also received three doses of a Bisphosphonate, namely Aredia® (Pamidronate disodium), at four weekly intervals in an attempt to reduce the bone pain in his right pelvis.
Since his neuropathic pain escalated and he was showing features suggestive of borderline opioid toxicity, he was admitted to the hospice where his opioid regime was altered.
Combined opioid therapy was commenced with Transtec® (Buprenorphine) TD patch 35 microgram/hour twice weekly and a reduced dose of OxyContin® 120 mg twelve hourly. The co-analgesic adjuvants were continued. Despite this regime his pain could not be controlled. It was feared that due to inadvertent sedation that occurred, he was likely reaching the terminal stage of his illness.
Hence the combined opioid therapy was altered. Opioid switch was undertaken with regard to both opioids in the regime. Diamorphine 100 mg over twenty four hours via a continuous sub cutaneous infusion (CSCI) was combined with oral Methadone 10 mg BD. His existing co-analgesic adjuvants were unaltered. With this analgesic regime, the patient achieved sufficient analgesia without medication induced toxicity. He was able to slowly mobilise to the wash room with the aid of a walking frame. He enjoyed quality time with his grand children much to the relief of his family.
Discussion
These two clinical scenarios exemplify the importance of recognition of neuropathic pain which resulted in appropriate pharmacological management.
In the first scenario, the patient was clearly experiencing severe, radicular neuropathic pain associated with mid thoracic spinal cord compression caused by spinal metastases. Despite radiotherapy, the pain increased even after few weeks when oedema due to the radiotherapy should have resolved. It was indeed clear in this scenario that the patient was experiencing Opioid Induced Hyper-Algesia (OIH) with very high doses of the opioid, namely OxyContin® alone without any adjuvant analgesics. The immediate introduction of adjuvant analgesics, namely Lyrica® and Mirtazapine together with a cautious reduction of the opioid dose in a combined opioid therapy resulted in good analgesia without opioid withdrawal or medication induced adverse effects. Despite the paraplegia, the patient obtained his wish to return home.
In the second scenario the recognition of neuropathic pain arising from tumour infiltration of the heavily innervated periosteum aided the eventual successful pain management. In this scenario, opioid induced toxicity resulted in pain escalation and further toxicity in a vicious circular manner (OIH). The recognition of this toxic situation resulted in the utilisation of the therapeutic principle of ‘fractional receptor occupancy’[8]. Hence two opioids were combined, namely Diamorphine via a continuous sub-cutaneous infusion (CSCI) and oral Methadone. This principle utilises the combined efficacy of the two opioids using smaller doses of each, which will inevitably reduce medication induced toxicity. Thus pain control improved and opioid induced hyper-algesia resolved. Analgesic adjuvants were already successfully in place, namely, Lyrica®, Mirtazapine, Clonazepam and Ketamine which also helped to keep the opioid doses to a minimum (opioid sparing effect).
Thus in both the situations, recognition of neuropathic pain resulted in formulating a rational, poly pharmacological pain management. This careful clinical management improved therapeutic efficacy and reduced medication induced toxicity. The entire process improved the patients’ quality of life in the palliative care setting.
Conclusion
Neuropathic pain is experienced by a significant number of patients with cancer. Its recognition is crucial since it leads to appropriate and carefully tailored pharmacological management. Over reliance on opioids alone is dangerous as it can result in opioid toxicity and vicious escalation of the pain (OIH). Adjuvant analgesics are crucial in this clinical setting. In addition, in extremely painful situations where opioid toxicity is experienced despite adequate analgesic adjuvants, the therapeutic principle of ‘fractional receptor occupancy’ is recommended. Accordingly, two opioids can be combined at smaller individual doses in order to enhance efficacy and minimise toxicity.
The ultimate aim of the clinician in these palliative care situations is to maximally improve the quality of life, comfort and dignity of these patients with cancer whose overall prognosis is unfortunately considered to be a few months at most. The clinician needs to always combine clinical excellence with empathy in caring and to recognise it as a privilege to walk in the trajectory of these patients.
Conflict of Interest:
None
References
- 1. Wall, P., Melzack, R. Text book of pain. (1994) Churchill Livingstone Pub.
- 2. McCance, K., Huether, S. Understanding Pathophysiology; Elsevier Mosby Pub., 2012
- 3. Hoskin, P., Makin, W. Oncology for Palliative Medicine. (1998) Oxford University Press
- 4. Melzack, R., Wall, P. The challenge of pain Penguin Science (2008).
- 5. Bannister, K., Dickenson, A. Opioid Hyperalgesia. (2010) Current Opinion Supportive Palliative Care 4(1): 1-5.
- 6. Prommer, E.E. Ketamine for pain: an update of uses in palliative care. (2012) J Pallat Med 15(4): 474-483.
- 7. Reddy, A., Yennurajalingam, S., Bruera, E. Dual opioid therapy using Methadone as a coanalgesic. (2015) Expert Opin Drug Saf 14(1): 181-182.
- 8. Bantel, C. Combination of opioids: the evidence The 7th Annual Royal Marsden Pain and Opioid Conference, London, 2014
- 9. Montgomery, S.A. Safety of Mirtazapine: a review. (1995) Internat Clin Psychopharm 10(4): 37-45.
- 10. Smith, H. Drugs for pain. (2003) Hanley & Belfus.
- 11. Greer, S., Joseph, M. Palliative Care: A Holistic Discipline. (2015) Integrative Cancer Therapies Sagepub.
- 12. Joseph, M., Tayar, R. Spinal cord compression requires early detection. (2005) Eur J Palliat Care 12: 141-143.