The role of lycopene as antioxidant and anti-inflammatory in protection of oxidative stress induced by metalaxyl
Marwa Fouad Hassan1*, Samy Ali Hussein2, Yakout El Senosi2, Mogda K Mansour1, Aziza Amin2
Affiliation
1Animal Health Research Institute (AHRI), Dokki, Egypt
2Faculty of Veterinary Medicine, Benha University, Egypt
Corresponding Author
Marwa, F.H, Animal Health Research Institute (AHRI), Dokki, Egypt, Tel: +2001006082481; E-mail: marwamora60@yahoo.com
Citation
Marwa, F.H., et al. The Role of Lycopene as Antioxidant and Anti-inflammatory in Protection of Oxidative Stress Induced by Metalaxyl. (2018) J Med Chem Toxicol 3(1): 26- 36.
Copy rights
© 2018 Marwa, F.H. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Metalaxyl; Lycopene; Oxidative stress; Pro-inflammatory cytokines; Apoptosis
Abstract
Pesticides play an important role in hepatotoxicity and liver damage. Lycopene is known to possess several beneficial properties including; antioxidant and anti-cancer effects. However, there is a lack of relevant information on its importance in chronic fungicide-induced hepatotoxicity. Therefore, our study was to assess the protective role of lycopene on metalaxyl-induced oxidative stress in liver tissue of male rats. The experiment was designed for 8 weeks and male albino rats were divided into 3 groups (n = 14). Group 1 served as normal control (with no treatment), group 2 metalaxyl exposed group; rats received metalaxyl at a dose level of 1/10 LD50 (130 mg/kg b.wt) orally three times per week, group 3 metalaxyl and lycopene;rats received metalaxyl (130 mg/Kg b.wt) orally three times per week and treated daily with lycopene (10 mg/kg b.wt/ orally). Our data showed that metalaxyl significantly increase serum Alanine AminoTransferase (ALT), Aspartate AminoTransferase (AST), Alkaline Phosphatase (ALP) activities, enhanced levels of L-MalonDiAldehyde (L-MDA), Nitric Oxide (NO), MyeloPerOxidase (MPO) and up-regulation of nuclear factor kappa B (NF-κB), tumor necrosis factor alpha (TNF-α), interlukin-6 (IL-6), caspase-8 gene expression, induced DNA damage. Meanwhile, down-regulation peroxisome proliferator activated receptor alpha (PPAR-α) and decreased the liver superoxide dismutase (SOD) and catalase (CAT) activities. Lycopene treatment was restored the hepatic antioxidant status which had indicated the significant protective effect against metalaxyl induced hepatotoxicity and finally confirmed by histopathologicaland immunohistochemical studies.
Introduction
Pesticides are used widely through the world for control agricultural pests and protect public health. Though these chemicals have many profitable purposes, they may also cause negative effects in both humans and animals. These chemicals are extensively used in industry, agriculture, home and gardens for several different purposes including the protection of seed grain during storage and germination[1]. Pesticidescontaminations of the environment have become one of the main problems in the region of Europe, Eastern Mediterranean and Africa, as well as worldwide importance. The presences of these toxic chemicals were recorded in water, air, house dust and in the tissues of non-occupationally exposed people, predominately in the adipose tissue, blood and urine[2]. Many pesticides extend their biological effects basically through electrophilic attack of cellular constituents with simultaneous production of Reactive Oxygen Species (ROS). ROS is a main cellular origin of oxidative stress[3,4].
Metalaxyl is a systemic benzenoid fungicide used in a mix of foliar spray for tropical and subtropical crops, such as a soil treatment for inhibit of soil-borne pathogens, and as a seed treatment to inhibit downy mildews, fungal diseases on cotton, fruits, peanuts, ornamentals and soybeans[1]. It is used in several states worldwide including USA, European nations, Australia and India[5]. The issues causing from metalaxyl originate from their high residual level in agriculture crops predominately cultivated vegetables under greenhouse situation and other components of the environment[6]. Metalaxyl exposure caused abnormal biochemical and hematologicalactivitiesinduce oxidative stress and an observable toxicityin liver[7,8].
Nitric oxide plays significant and diverse roles in the liver with possibilities for both liver cells protections from injury and aggravation of injury. The substantial factors in determining whether NO will be protecting or injurious are the location of NO generation and the amount of NO being produced[9]. It is an uncharged lipophilic molecule including a single unpaired electron, which leads it to be interactive with other molecules as glutathione, oxygen and superoxide radicals. Mean while NO is not a very reactive free radical it is fit to form other reactive intermediates, which have effect on entire organisms’ function and protein. These reactive intermediates may be trigger nitrative damage on biomolecules[10]. Excessive of NO in the mitochondria enhances the generation of ROS and Reactive Nitrogen Species (RNS) which can modify various processes activity such as oxidative stress, mitochondrial biogenesis and respiration[11].
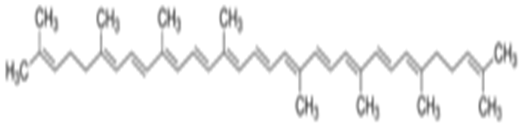
To control ROS leveland to protect cells under stress conditions, mammalian tissues contain many enzymatic and non-enzymatic antioxidants that scavenge ROS[12,13]. Due to persistent exposure of pesticides, these endogenous antioxidants level minimizes leading to cell death acceleration[12]. Natural and synthetic sources of antioxidants have proved to be highly effective to control the magnitude of free radicals’ production, to inhibit its unfavorable effects, as well as to prop the antioxidant of living organism and the mechanisms of detoxification[14]. Lycopene is one of the most potent antioxidants among the dietary carotenoids. It has an acyclic isomer of beta-carotene which gives tomatoes, pink guava, apricots, pink grapefruit, watermelon, rosehips and red oranges their red color. Humans and animals can’t synthesis lycopene and depend on dietary sources. It also has anti-inflammatory and anti-cancer effects[15,16]. Lycopene may have exerting protective influences to carcinogen-induced liver, lung and mammary tumors on experimental animals[17]. According to the structural formula of lycopene represented in Figure 1, various mechanisms involved in the health-beneficial effects of lycopene have been reported, due to a high number of conjugated dienes lycopene is particularly effective in singlet oxygen scavenging, and its ability is twice higher than that of β-carotene and ten times higher than that of α-tocopherol. Recently, it has been assumed that, lycopene directly modulates many redox-sensitive signaling pathways being responsible for cell regulatory function. As, antioxidant response element (ARE), ROS-producing enzymes, Mitogen-Activated Protein Kinases (MAPK), nuclear factor-κB (NF-κB) as well as redox-sensitive proteins participated in modulation of cell cycle and apoptosis (B-cell lymphoma-2 (Bcl-2) family proteins and Ku protein)[18]. A lot of studies about lycopene have been published, most of them focused on anti-apoptotic effect of lycopene in cancer cells, only few studiesindicatedthe possible beneficial effect of lycopene against deleterious effect of pesticide. Therefore, the present study was planned to investigate the protective role of lycopene in overcoming the oxidative damage induced in liver of male rats after exposure to metalaxyl fungicide.
Figure 1: Chemical structure of lycopene.
Materials and Methods
Chemicals
Metalaxyl: Metalaxyl[N-(2,6-Dimethylphenyl)-(methoxyacetyl) – DL-alanine methyl ester], 98% technical grade was obtained from Zhejiang Heben Pesticide and Chemicals Co., Ltd. China. Metalaxyl was dissolved in 430 μL of dimethyl sulfoxide (DMSO) and 5.6 mL of propylene glycol was added. Fresh metalaxyl preparation was administered orally three times per week at a dose of 130 mg/kg b.wt (1/10 of LD50)[19].
Lycopene: Lycopene(ψ,ψ-Carotene,2,6,10,14,19,23,27,31 – Octamethyl – dotriaconta-2,6,8,10,12,14,16,18,20,22,24,26,30-tridecaene), was obtained from Aktin Chemicals, Inc. company (Nature connecting health), Chengdu, China., and given orally at a dose level 10 mg/kg b.wt daily for 8 weeks[20].
Preparation of lycopene stock solution: 100 mg of lycopene was mixed with 2 ml of tween-80 at room temperature until a homogeneous paste was obtained. Then physiologic saline was added drop wise with continued vigorous stirring at room temperature, to reach a final concentration of 10 mg of lycopene/mL of suspension[21].
Animals and treatment: Forty-two male albino rats weighing between 150 to 200 g were purchased from laboratory Animals Research Center, Faculty of Veterinary Medicine, Benha University. Animals were housed in stainless steel cages and maintained on 12 hours, light/dark cycle, (23 ± 2°C) and 50 – 70% relative humidity. Water and food were provided ad libitum. Rats were adapted for 15 days before the start of experiment. All the animals received humans care according to the criteria outlined in the Guide for the care and used of laboratory animals prepared by the National Academy of Science and published by the National Institute of Health.
Male albino rats were divided into three groups each group containing fourteen rats. Total duration of experiment was 8 weeks. Group 1 served as normal control group (rats received no treatment), Group 2 metalaxyl treated group (rats received metalaxyl at a dose level of 1/10 LD50 (130 mg/kg b. wt) orally three times per week), Group 3 metalaxyl and lycopene group (rats received metalaxyl 130 mg/Kg b. wt orally three times per week and treated daily with lycopene 10 mg/kg b. wt/ orally).
Sampling: Blood samples and liver tissue specimens were collected from animals two times along the duration of experiment at 4 and 8 weeks from the onset of rats exposed to metalaxyl.
Blood samples preparation: The rats were anesthetized with intraperitoneal injection of sodium pentobarbital (35 mg/kg b. wt). Blood samples were collected by ocular vein puncture serum was separated by centrifugation at 3000 rpm for 15 minutes to estimate AST, ALT, ALP activities and NO concentration.
Tissue samples
Liver tissue homogenate for biochemical analysis: The rats were sacrificed and a portion of the liver tissues specimen was isolated. After isolation, liver tissues were weighed and minced into small homogenized pieces with a glass homogenizer with 9 volume of ice-cold of 0.05 mM potassium phosphate buffer (pH7.4) forgetting 10% homogenates. The liver tissue homogenates were centrifuged at 6000 r.p.m for 15 minutes at 4°C then the supernatant was used for the estimation of following parameters: levels of MPO, L-MDA, CAT and SOD.
Liver tissue preparation for molecular gene expression: Another portion of liver tissue were mincing into small pieces, they were placed in eppendorf tubes, snap-frozen in liquid nitrogen and storage at -80°C till RNA extraction for determination of: NF-κB, IL-6, TNF-α, PPAR-α, caspase-8 and DNA damage.
Histological analysis and immunohistochemical analysis: A portion of liver samples were fixed in 10% formalin immediately after harvesting for histopathological examination according to the technique described by Bancroft, and Stevens[22]. Immunohistochemical staining for the proapoptotic marker Bcl-2 antagonist-X (BAX) protein examination according to the technique described by Kiernan[23]; Sakr and Abdel-Samie[24].
The severity of the microscopical lesions fundamentally in the liver were classified to degree 1, slight changes including congestion of blood vessels, mild hepatocellular swelling due to hydropic degeneration; degree 2, moderate changes includes clear hepatocellular swelling in centrilobular and midzonal areas, in association with leukocytic cellular aggregation; degree 3, severe changes includes diffuse and severe hepatocellular swelling and necrotic areas[25].
Biochemical parameters
Biochemical of hepatic marker enzymes: Hepatic marker enzymes in serum namely, ALT and AST activities were estimated according to the kinetic method described by Schumann et al.[26] and serum ALP activity was determined using commercially available kits according to the enzymatic method described by EL-Aaser and EL-Merzabani[27]. AST, ALT and ALP activities are expressed as U/L.
Determination of NO level: The concentration of NO in serum was estimated according to the method described by Vodovotz[28]. Total nitrite was measured using cadmium-mediated reduction of NO3 to NO2then the Griess reagent producing a pink color and measured at 540 nm against reagent blank. NO is expressed as μmol/L.
Determination of L-MDA level: The estimation of L-MDA concentration (as marker of lipid peroxidation) in liver tissue was based on the method of Mesbah et al.[29]. L-MDA amount was calculated as nmol/g tissue.
Determination of MPO activity: Myeloperoxidase activity in liver tissue was estimated according to Bradley et al.[30]. More especially, assay mixture (3.0 ml) consisted of cell lysate (0.1 ml) prepared in 0.5% hexadecyltrimethylammonium bromide containing phosphate buffer and reaction buffer (50 mM phosphate (pH 6.0) buffer containing 0.167 mg/ml o-diaziridine hydrochloride and 0.0005% H2O2). After 1 min. the altered in absorbance was measured at 460 nm and the activity of enzyme is expressed as unit/milligram of tissue.
Free radical scavenging enzyme estimation: Catalase and superoxide dismutase activities in liver tissue were determined according to the methods suggested by Xu et al.[31] and Kakkar et al.[32] respectively.
Estimation of NF-κB, TNF-α, IL-6, PPAR-α and caspase-8 gene expression: Gene expression of NF-κB, TNF-α, IL-6, PPARα and caspase-8 levels were assessed in the liver using real-time quantitative polymerase chain reaction (real- time qPCR) analysis. Total RNA from liver was separated using the high pure RNA isolation kit (iNtRON Biotechnology, easy-RED Total RNA Extraction Kit) based on the manufacturer’s instructions. From each sample, cDNA was reversely transcribed using a Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, Fermentas, EP0451, and USA). Then, real-time quantitative PCR amplification carried out on Faststart Universal SYBR Green Master (Roche, GER), using specific primers gene (GSP) (Table 1), at 95 °C for 10 min followed by 40 cycles of 95 °C for 15 sec, 60°C/ 30 sec at the annealing temperature of GSP, and 30 sec at 72 °C. Target gene was normalizing with β –actin by used the 2-ΔΔCt method[33].
Table 1: Sequences of gene – specific primers.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| (/5 ------ /3) | (/5 ------ /3) | |
| NF-κB | CCTAGCTTTCTCTGAACTGCAAA | GGGTCAGAGGCCAATAGAGA |
| TNF-α | GCATGATCCGCGACGTGGAA | AGATCCATGCCGTTGGCCAG |
| IL-6 | TCCTACCCCAACTTCCAATGCTC | TTGGATGGTCTTGGTCCTTAGCC |
| PPAR-α | TCACACAATGCAATCCGTTT | GGCCTTGACCTTGTTCATGT |
| Caspase-8 | CTGGGAAGGATCGACGATTA | CATGTCCTGCATTTTGATGG |
| B-actin | CATGGATGACGATATCGCT | CATGAGGTAGTCTGTCAGGT |
Estimation of DNA damage by comet assay: DNA damage was estimated by alkaline single-cell gel electrophoresis (comet assay) based on the protocol recorded by Singh et al.[34]. Liver homogenates were scattered and immobilized in an agarose gel on microscope slides. The slides were placed in a lysis solution to lyse and disperse cell components; the DNA immobilized was leaved in the agarose. The DNA was denatured for a special period of time by immersing the slides in an alkaline solution. Strand breaks in the denatured cellular DNA resulted in super coil relaxation, the more breaks, the greater the degree of relaxation. Given a sufficient degree of relaxation the application of an electric field across the slides created a motive force by which the charged DNA may migrate through the surrounding agarose far from the immobilized main bulk of nuclear DNA. After electrophoresis, the slides were rinsed in neutral buffer and the gel and its contents were fixed using ethanol. The DNA in the fixed slides was stained with a fluorescent DNA-specific stain as SYBER green, ethidium bromide, propidium iodide, 4, 6 diamidino – 2-phenylindole hydrochloride (DAPI), Gel Red and benxoxazolium-4-quinolinum oxazole yellow homodimer (YOYO-1). Stained slides are checkup using a fluorescent microscope. The migration of DNA far from the nucleus, i.e. comet tail length, was measured by image analysis software which estimated different parameters of the comet, i.e. percentage of DNA in tail, tail length, tail moment = %DNA in tail X tail length[35].
Statistical analysis: The results were expressed as mean ± stander error using SPSS (13.0 software, 2009) program. The data were analyzed using one-way ANOVA to estimate the statistical significance of differences among groups[36]. Duncan’s test was used for making a numerous comparison among the groups for testing the inter-grouping homogeneity. Values were considered statistically significant when p < 0.05.
Results
ALT, AST and ALP activities: A significant increase in serum ALT, AST and ALP activities caused in metalaxyl exposed rats compared with normal group. Meanwhile, lycopene treatment to metalaxyl intoxicated male rats caused a significant decrease in elevated serum ALT, AST and ALP activities (Table 2).
Table 2: Effect of lycopene administration on serum ALT, AST and ALP activities in metalaxyl intoxicated male rats (U/L).
| Animal groups | ALT (U/L) | AST(U/L) | ALP(U/L) | |||
|---|---|---|---|---|---|---|
| 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | |
| Group Ι: | ||||||
| Normal control | 29.00 ± 1.53d | 27.67 ± 3.18d | 54.00 ± 0.58e | 57.00 ± 1.53d | 138.33 ± 3.84d | 178.67 ± 6.98d |
| Group II: | ||||||
| Metalaxyl group | 56.33 ± 4.09a | 59.33 ± 3.28a | 117.67 ± 1.86a | 121.00 ± 0.58a | 301.33 ± 8.69a | 317.67 ± 9.13a |
| Group III: | ||||||
| Metalaxyl+ Lycopene | 33.33 ± 1.86cd | 32.67 ± 1.33cd | 65.33 ± 1.20d | 74.67 ± 2.03c | 189.00 ± 5.51c | 210.67 ± 3.18c |
Data are presented as (Mean ± S.E). S.E = Standard error.
Mean values with different superscript letters in the same column are significantly different at (P ≤ 0.05).
NO, L-MDA, MPO, CAT, SOD contents: A significant increase in serum NO, liver L-MDA and MPO were observed in metalaxyl intoxicated rats after four and eight weeks. Meanwhile, a significant decrease in CAT activity was observed in metalaxyl intoxicated rats after four weeks followed by a non-significant decrease after eight weeks of the experiment associated with a significant decrease in SOD activity compared to normal rats. Conversely, lycopene treatment to metalaxyl intoxicated male rats showed a significant reduction of NO, L-MDA and MPO contents. On the other hand, there was a significant increase in liver CAT and SOD activities were observed compared to metalaxyl exposed group (Table 3).
Table 3: Effect of lycopene administration on serum NO level and liver tissue L-MDA, MPO, CAT and SOD activities in metalaxyl intoxicated male rats.
| Animal groups | NO (μmol/L) | L-MDA (nmol/ g tissue) | MPO (unit/milligram of tissue) | CAT (U/g.tissue) | SOD (u/g.tissue) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | |
| Group Ι: | ||||||||||
| Normal control | 19.36 ± 0.69c | 23.17 ± 1.28b | 4.08 ± 0.01d | 4.46 ± 0.05c | 0.05 ± 0.001d | 0.08 ± 0.002d | 1.14 ± 0.02a | 1.17 ± 0.03bc | 44.37 ± 2.19bc | 48.15 ± 3.02b |
| Group II: | ||||||||||
| Metalaxyl group | 28.88 ± 0.9a | 34.31 ± 1.42a | 7.64 ± 0.13a | 8.37 ± 0.07a | 0.54 ± 0.009a | 0.69 ± 0.010a | 1.03 ± 0.03b | 1.07 ± 0.01c | 31.14 ± 2.55d | 37.56 ± 2.03c |
| Group III: | ||||||||||
| Metalaxyl + Lycopene | 21.63 ± 1.0bc | 23.66 ± 0.88b | 4.87 ± 0.09c | 4.89 ± 0.23c | 0.15 ± 0.003c | 0.19 ± 0.004c | 1.25 ± 0.05a | 1.38 ± 0.05ab | 62.22 ± 1.21a | 69.09 ± 5.01a |
Data are presented as (Mean ± S.E). S.E = Standard error.
Mean values with different superscript letters in the same column are significantly different at (P ≤ 0.05).
Table 4: Effect of lycopene on the relative expression of NF-κB, TNF-α, IL-6, PPAR-α and caspase-8 gene in liver of metalaxyl-intoxicated rats.
| Animal groups | Fold change in NF-κB gene expression | Fold change in TNF-α gene expression | Fold change in IL-6 gene expression | Fold change in PPAR-α gene expression | Fold change in Caspase-8 gene expression | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | |
| Group Ι: | ||||||||||
| Normal control | 1.00 ± 0.09d | 1.00 ±0.08d | 1.00 ±0.08e | 1.00 ± 0.10e | 1.00 ± 0.07d | 1.00 ± 0.08d | 1.00 ± 0.07a | 1.00 ± 0.08a | 1.00 ± 0.08d | 1.00 ± 0.07d |
| Group II: | ||||||||||
| Metalaxyl group | 13.00 ± 0.34a | 17.75 ± 0.38a | 10.13 ± 0.37a | 12.82 ± 0.39a | 10.63 ± 0.25a | 15.45 ± 0.36a | 0.42 ± 0.04c | 0.32 ± 0.03c | 5.43 ± 0.17a | 9.32 ± 0.23a |
| Group III: | ||||||||||
| Metalaxyl + Lycopene | 3.86 ± 0.15c | 2.43 ± 0.13c | 3.61 ± 0.17d | 2.87 ± 0.16d | 3.01 ± 0.16c | 2.03 ± 0.14c | 0.66 ± 0.05b | 0.74 ± 0.06b | 2.45 ± 0.12c | 1.79 ± 0.11c |
Data are presented as (Mean ± S.E). S.E = Standard error.
Mean values with different superscript letters in the same column are significantly different at (P ≤ 0.05).
NF-κB, TNF-α, IL-6, PPAR-α and caspase-8 gene expression in liver: A significant up-regulation of NF-κB, TNF-α, IL-6 and caspase-8 genes expression level while a significant down-regulation of PPAR-α in livers of metalaxyl-intoxicated rats throughout of experiment compared to normal group. Conversely, lycopene treatment to metalaxyl intoxicated male rats showed a significant down-regulation of NF-κB, TNF-α, IL-6 and caspase-8. On the other hand, there was a significant up-regulation in liver PPAR-α level when compared with metalaxyl exposed group (Table 4).
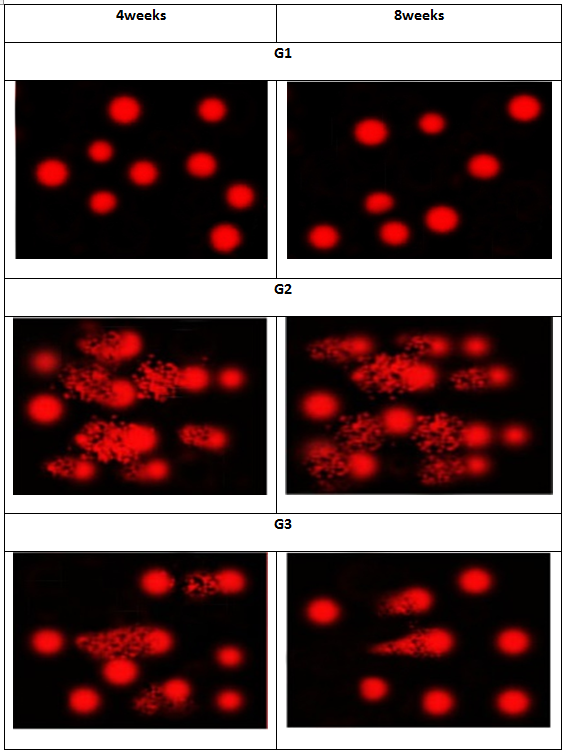
DNA damage: A significant increase in DNA damage that was indicated by an increase in tail length and tail DNA% in liver tissue was observed in metalaxyl-intoxicated rats compared to normal rats. Meanwhile, lycopene treatment significantly reduced DNA damage that was indicated by comet assay in metalaxyl intoxicated male rats (Figure 2).
Figure 2: Photomicrographs representation of liver DNA damage, using comet assay, in normal control (G1), and metalaxyl-intoxicated (G2) and lycopene (G3).
Histopathological examination: (Figure 3) showed various histopathological changes in the hepatic parenchyma of the examined rats. After 8 weeks livers of the rats treated with metalaxyl showed extensive dilatation and congestion of central veins and hepatic blood sinusoids. Additionally, mild peri-vascular leukocytic cellular aggregations with activation of Von Kuepfer’s cells were also observed. The portal areas showed marked congestion of portal vein with hyperplastic proliferation of the lining epithelium of the bile ducts in association with formation of newly formed bile ductules. Also, variable amounts of fibrous tissue proliferation in portal areas and around bile ductules were also detected. Additionally, extensive thickening of the hepatic capsule in association with sub-capsular hemorrhage was also demonstrated. Diffuse, marked hydropic degeneration of the hepatocytes characterized by swollen, pale, vacuolated cytoplasm with occasional pyknosis; rarely karyolysis or absence of nuclei of degenerated hepatocytes was noticed. In the centri-lobular zones of hepatic lobules, the hepatocytes showed degeneration characterized by enlargement of the cells by multiple variably sized discrete empty vacuoles that distend the cell cytoplasm and flattened, displaced nucleus to the periphery. Occasionally, coagulative necrosis of small groups of hepatocytes was characterized by retention of hepatic cell outline and shrunken hepatocytes with hyper eosinophilic cytoplasm and pyknotic nuclei in association with leukocytic cellular infiltrations mainly lymphocytes. Degree 3 of the severity microscopical lesions was observed in most treated animal’s liver. Accordingly, rats treated with metalaxyl had severe liver damage with a mean score of 3.
Figure 3: H&E stained sections of liver tissue taken from metalaxyl treated rat after 8-week (Group 2) showing (A) dilatation of central vein (cv) with peri-vascular leukocytic cellular aggregations (arrow). Notice also, activation of Von Kuepfer’s cells (zigzag arrow, x400), (B) mild peri-portal fibrosis with formation of newly formed bile ductules (arrow, x200), (C) extensive thickening of hepatic capsule (HC, arrow, x200), (D) marked diffuse hydropic degeneration of the hepatocytes with karyolysis or absence of nuclei (arrow, x400), (E) enlargement of hepatocytes by multiple variably sized discrete clear vacuoles that distend the cell cytoplasm with flattened, squeezed nucleus to the periphery (arrow, x200), (F) focal area of coagulative necrosis of hepatocytes (arrow, x400).
Meanwhile, the microscopical examination of liver obtained from rats treated with metalaxyl and lycopene for 8 weeks revealed marked improvement in the hepatocellular architecture with more regular and less altered hepatocytes when compared to metalaxyl treated rats (Figure 4). The hepatic tissue renovated its normal histological structure in comparison to the negative control group. Most of the hepatic displayed a certain degree of recovery besides the portal area appeared normal and contained normal bile duct with congested portal vein only. Congestion of the hepatic blood vessels and blood sinusoids was the most common pathological alterations detected in the rats of this group with activation of Von Kuepfer’s cells. Accidently, the hepatocytes in the periphero-lobular zones of hepatic lobules showed hydropic degeneration characterized by swollen pale vacuolated cytoplasm with pyknotic nuclei; while the hepatocytes in the centro-lobular zone of hepatic lobules showed normal histological appearance. Interestingly, the liver of rats obtained from this group appeared to be normal with a mean liver score 1, significantly better scores than animals had metalaxyl only congestion of the hepatic blood vessels with mild hydropic degeneration was observed in most rats.
Figure 4: H&E stained sections of liver tissue taken from metalaxyl and lycopene treated rat after 8 weeks (Group 3) showing normal hepatocytes showing (A) normal hepatocytes (x200), (B) enlargement and activation of Von Kupffer’s cells (arrow). Notice also, bi-nucleated hepatocytes (zigzag arrow, x400), (C) periphero-lobular mild hydropic degeneration of the hepatocytes (arrow, x200), (D)showing focal area of hydropic degeneration of the hepatocytes characterized by swollen, pale, vacuolated cytoplasm with pyknotic nuclei (arrow, x400).
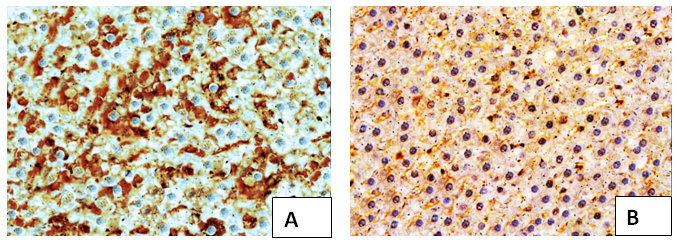
Immunohistochemistry: The microscopical evaluation of immunohistochemical stained hepatic sections for BAX revealed strong positive expression of BAX in most hepatocytes in metalaxyl induced rats. Meanwhile, in metalaxyl plus lycopene was detected weak positive immunoreaction for BAX in the cytoplasm of most hepatocytes (Figure 5).
Figure 5: Immunohistochemical stained liver sections of (A) metalaxyl treated after 8 weeks group revealed strong positive immunoreaction for BAX in most hepatocytes (x200), (B) metalaxyl and lycopene showing very weak positive immunoreaction for BAX in the cytoplasm of some hepatocytes. (x200).
Discussion
Pesticides compose a heterogeneous group of chemicals which are considered as one of the central factors involved in environmental contamination of today’s world. These chemicals designed for act as toxic to the pests are able to produce subversive influences on intoxicating non-target organisms, comprising animal and humans[37]. Metalaxyl is recorded to have dangerous effects on mammalian animals[38]. It induced apoptosis, biochemical, BAX expression and histological changes in the liver of albino mice[8,24].
The liver plays a major role in metabolism, and also has a number of functions in the body, involving plasma protein synthesis, glycogen storage and production of bile[39]. It is foreseeable not only to produce physiological functions but also to protect against the dangers of harmful chemicals and drugs[40]. Hepatotoxicity in most cases is because of free radical. Free radicals created by the metabolism of toxicant substance initiate the toxicity cascade[41]. In present study, elevation in hepatic ALT, AST and ALP indices could be a secondary event following metalaxyl-induced lipid peroxidation in liver tissue. The deleterious effects of metalaxyl may be resulting from its ROS generation that leads oxidative stress on different organs. In agreement with our results, several authors confirmed that oxidative stress, elevated lipid peroxidation, depletion of antioxidant defenses and increased pro-inflammatory mediators’ production are entangled in the pathogenesis of pesticide-induced hepatic damage[42,43]. Lipid peroxidation and ROS generation of cell membranes leads an increase in membrane permeability, loss of membrane fluidity and alters in membrane potential all of which lead to enzymes leakage from the liver cells[44].
Nitric oxide is extremely reactive signaling molecule and it is remarkable regulator for cellular functions. Nitrative stress also plays a main role in inflammation. NO modifies DNA directly and inhibits the DNA repair enzymes[45]. The increase in NO level may be due to the up-regulation of TNF-α and other cytokines[46]. TNF-α synergistically acts with other cytokines to lead up-regulation of inducible NO synthase (iNOS) and elevated NO production in bronchial epithelial cells[47].
Malondialdehyde, end point of lipid peroxidation process, is defined as an oxidative degradation of polyunsaturated lipids[48]. According to Calviello, et al.[49] fungicides-induced injury is closely associated with excess in lipid peroxidation and reduces in the antioxidant enzymes. Metalaxyl may be metabolized to a reactive metabolite which may start a chain reaction with regard to lipid peroxidation and other tissue damaging influences[50]. The decrease in SOD activity in metalaxyl treated rats, which might be because of an excessive formation of superoxide anions. These excrescent of superoxide anions might inhibit SOD and reduce its activity. In the absence of appropriate SOD activity, superoxide anions are not dismuted into H2O2, which is the substrate for CAT enzyme, leading to decreasing in CAT activity[51].
Myeloperoxidase activity elevated in metalaxyl exposed rats. This result was in agreement with Abolaji et al.[52], who suggested that fungicide carbendazim exposure resulted in significant increase in MPO activity in the liver, spleen and kidney when compared with normal rats. In present study, the increase of serum NO levels and liver MPO activity caused stimulation of pro-inflammatory cytokine expression. MPO activates neutrophils and enhances their recruitment leading to an increased pro-inflammatory immune response[53,54] suggests that, as with other pesticides, metalaxyl may mediate its effect through the NF-κB pathway in the chronic phase of inflammation.
Peroxisome proliferator activated receptor alpha in hepatocyte decreased in metalaxyl-exposed rats indicating that the fat burning machinery was compromised for causing hepatic steatosis and steatohepatitis. The decreased effectiveness of oxidation systems is because of genetic, toxic factors and metabolic disorders. In animal models, inefficient PPARα sensing enables the oxidation of the in fluxed fatty acids and causes severe hepatic steatosis development. Administration of PPARα agonists inhibits these processes and even reverses hepatic fibrosis in animal models[55]. Similarly, Al-Eryani[56] reported that, intraperitoneal injections of dichlorodiphenyltrichloro-ethane (DDT) pesticide caused down-regulation of hepatic mRNA levels of PPAR-α target gene.
DNA damage increase in this study may be because of the increase in NO, NF-κB, TNF-α levels and caspase-8 activity. Several reports have indicated that increase levels of NO induce apoptosis in various cell types. This influence seems to be mediated primarily through the effect of peroxynitrite on enhances mitochondrial permeability either directly, or by DNA damage with subsequent activation of the polyadenylate ribose synthase pathway[57-60]. This mitochondrial permeability transition results in liberate of cytochrome c from the mitochondria, which constitutes a signal for apoptosis[61]. Also, several cytokines may activate specific intracellular pathways, i.e., pro-apoptotic signals through caspase cascade[62,63]. Injured hepatocytes may liberate apoptotic bodies and activate Kupffer cells, and these activated cells mayenhance inflammatory and fibrogenic responses, leading to a vicious cycle of hepatic injury[64]. During inflammation, immune system cells caused an exacerbation of cellular respiration due to increased oxygen consumption causing an increase in generate and accumulation of ROS at damage site[65]. The production of ROS, inflammatory mediators, depletion of antioxidants and mitochondria damage is associated with morphological and functional alters that induce an acute inflammatory response leading to many clinical complications[63,64]. NF-κB binding sites have been identified in the promoter of TNF-α gene, which is commonly included in signal-induced programmed cell death. Many studies have clearly proved a pro-apoptotic role for NF - κB perhaps because it, along with activator protein 1, can induce Fas ligand (FasL) expression[66]. Ligation of FasL to Fas in the cell membrane triggers caspase-8 activation. Once activated, caspase-8 transduces a signal to effect or caspases, involving caspases 3, 6, and 7 and eventually causes hydrolysis of nuclear and cytosolic substrates[67]. Other studies reported that organochlorines may induce apoptosis of sertoli cells by a FasL-linked pathway including enhance of the FasL expression, nuclear translocation of NF-κB and caspase 8 and 3 activation[68,69].
The BAX protein is an important influence in cell apoptosis regulation. High Bax expression and formation of homo-or heterodimers with Bcl-2 may cause cell death[70]. The BAX protein undergoes changes in their structure after exposure to death signals and modifies the mitochondrial membrane structure leading the release of pro-apoptotic factors and cytochrome c[71]. In the present study, BAX appeared strong positive expression in most hepatocytes metalaxyl treated rats compared with normal rats. Apoptosis caused by metalaxyl has been suggested in hepatocytes cells of rats[72].
The present study, lycopene-inhibited hepatic oxidative stress caused by metalaxyl. This effect may be due to its stimulatory influence on the antioxidant parameters and its inhibitory effect on lipid peroxidation, which caused stabilization of hepatocyte membrane and enhancement of liver synthetic function[73]. Bose and Agrawal[74] reported that, lycopene has been shown to have the highest antioxidant activity among the carotenoids regarding cell protection against hydrogen peroxide radical components, singlet-oxygen and nitrogen dioxide. During singlet-oxygen quenching, energy is transferred from singlet-oxygen to the lycopene molecule, modifying it to the energy-rich triplet state. Trapping of other ROS, like OH-, NO2- or peroxynitrite, in the other hand, leads to oxidative breakdown of the lycopene molecule. Thus, lycopene may protect against oxidation of DNA, lipids and proteins[75,76].
In the present study, decreases L–MDA levelsin liver by lycopene may be originate from elevation of SOD and CAT activities and/or it act as a radical scavenger. Lycopene has function as an antioxidant by several mechanisms, and one of the best authenticated mechanisms is via strong singlet oxygen quencher[77]. Another mechanism for the antioxidant activity of lycopene is reaction with free radicals[78]. Likewise, lycopene may increase the antioxidant response element and thereby enhance the production of cellular enzymes as SOD and CAT that protect cells from ROS and other electrophilic molecules. For example, Ben-Dor et al.[79] suggested that, lycopene increases the Antioxidant Response Element (ARE) in human liver cancer (HepG2) and breast cancer (MCF-7) cell line through the nuclear factor erythroid 2-related factor 2 (Nrf2) nuclear transcription pathway. Nrf2 is a main transcription factor regulating the anti-oxidant genes such as SOD and CAT via binding to antioxidant response elements[80]. Additionally, lycopene reduces the oxidative stress and protects the kidney via decreasing MPO, L-MDA and nitrite levels[81]. Lycopene markedly decreased the increased MPO activity in liver indicating that suppression of neutrophil infiltration could be mechanism by which lycopene achieves its anti-inflammatory effect. The anti-inflammatory influence of lycopene as has been recorded in many other findings[82,83]. Possible mechanisms for its anti-inflammatory response may involve the inhibition of synthesis and liberate of pro-inflammatory cytokines and modulation of signal transduction pathways, involving that of the iNOS through its inhibitory effects on NF-κB[84].
Lycopene-mediated up-regulation PPAR-α and PPAR-γ associated genes in mesenteric adipose tissue may have enhanced mesenteric adipose tissue fatty acid utilization, and subsequently decreased lipid delivery from adipose tissue to the liver. Lycopene modulations in PPAR-associated signaling were observed in the adrenal glands and liver of rodents in preceding studies[85,86]. In present study, the anti-inflammatory and protective effect of lycopene may be via the activation of PPAR-α. Also, the decrease in ALT and AST after lycopene treatment may be because of PPAR-α activation. Kersten et al.[87] and Edgar et al.[88] reported that, PPAR-α governs metabolism of amino acids by inhibiting expression of genes included in transamination of AST and ALT in mice.
Our study showed that liver cell apoptosis, measured via caspase 8 and BAX protein expression levels, was inhibited by lycopene treatment implying an anti-apoptotic role for lycopene and correlating with protection from metalaxyl-induced liver damage. Similarly, He et al.[89] stated that, caspase 3, 8, 9 expression were markedly decreased following treatment with lycopene (10 mg/kg/day) for 4 weeks compared with myocardial infarction-induced mice group. Also, lycopene had preventive influence against oxidative damage to cell membranes and DNA strand, and that it significantly relieved histological alters caused by free radicals in the rat’s liver and in various cells[90,91]. Furthermore, Bayomy et al.[92] reported that, the Bax activity was significantly reduced in rats received gentamicin with lycopene (4 mg/kg/day for 12 days) when compared with gentamicin-induced renal oxidative stress group and result in apoptosis was inhibited.
Conclusion
The results of our study suggest that lycopene supplementation has hepato-protective effects in metalaxyl-induced liver damage. Lycopene can directly and rapidly scavenge free radicals and/or inhibit their formation, additionally; it can act by up-regulating endogenous antioxidant defenses. Lycopene protects the DNA of the cells from damage caused by free radicals. Results of the present study clearly indicate that feeding of antioxidant lycopene combats oxidative stress induced by metalaxyl in rats. Thus, lycopene may be effective in improving liver function, has a potential anti-inflammatory and antioxidant activities as well as diminishing liver injury complications.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The Corresponding author is grateful to Dr. Simon, Zhejiang Heben Pesticide and Chemicals Co., Ltd. company, Zhejiang, China, for providing us with Metalaxyl material and my husband Dr. Mostafa Mosalam Faculty of science, Cairo University for his continuous help and incorporeal support to me.
References
1. Ding, F., Li, X.N., Diao, J.X., et al. Chiral recognition of metalaxyl enantiomers by human serum albumin: evidence from molecular modeling and photophysical approach. (2012) Chirality 24(6): 471-480.
2. Gunnell, D., Eddleston, M., Phillips, M.R., et al. The global distribution of fatal pesticide self-poisoning: systematic review (2007) BMC Public Health 7-357.
3. Al-Attar, A. Effect of grapeseed oil on diazinon-induced physiological and histopathological alterations in rats (2015) Saudi J Biol Sci 22(3): 284-292.
4. Elzoghby, R.R., Hamuoda, A.F., Abdel, F., et al. Protective role of vitamin c and green tea extract on malathion-induced hepatotoxicity and nephrotoxicity in rats. (2014) Am J Pharmacol Toxicol 9: 177-188.
5. Clemens, M.G.The Liver: Biology and Pathobiology: 5th Edition. (2009)John Wiley & Sons.
6. Drew, B., Leeuwenburgh, C.Aging and the role of reactive nitrogen species. (2002) Ann N Y Acad Sci 959: 66-81.
Pubmed||Crossref||Others
7. Bolisetty, S., Jaimes, E.A. Mitochondria and reactive oxygen species: physiology and pathophysiology (2013) Int J Mol Sci 14(3): 6306-6344.
8. Sukul, P., Spiteller, M. Metalaxyl persistence, degradation, metabolism, and analytical methods. (2000) Rev Environ ContamToxicol 164: 1-26.
9. Pattanasupong, A., Nagase, H., Sugimoto, E., et al. Degradation of carbendazim and 2,4-dichlorophenoxyacetic acid by immobilized consortium on loofa sponge. (2004) J Biosci Bioeng 98(1): 28-33.
10. Al-Amoudi, W.M. Haematological and biochemical effects of metalaxyl fungicide on albino mice. (2012) Amer J Biochem 2(5): 62-66.
Pubmed||Crossref||Others
11. Hashem, H.E. Light and electron microscopic study of the possible protective effect of nigella sativa on metalaxyl induced hepatotoxicity in adult albino rats. (2012) J Cell Sc Ther 3:118.
12. Ojo, A.O., Oyinloye, B.E., Ajiboye,B., et al. Dichlorvos induced nephrotoxicity in rat kidney, protective effects of Alstoniaboonei stem bark extract. (2014) Indi J Pharmacol. 1(7): 429-437.
13. Newairy, A.A., Abdou, H.M. Effect of propolis consumption on hepatotoxicity and brain damage in male rats exposed to chlorpyrifos. (2013) Afr J Biotechnol 12(33): 5232-5243.
14. Martins, N., Barros, L., Ferreira, I.C.F.R., et al. In vivo antioxidant activity of phenolic compounds: facts and gaps (2016) Trends Food Sci Technol 48: 1-12.
15. Khan, N., Afaq, F. Mukhtar, H. Cancer chemoprevention through dietary antioxidants, progress and promise Antioxid (2008) Antioxid Redox Signal 10(3): 475-510.
16. Feng, D., Ling, W.H., Duan, R.D. Lycopene suppresses LPS-induced NO and IL-6 production by inhibiting the activation of ERK, p38MAPK, and NF-kappaB in macrophages. (2010) Inflamm Res 59(2):115-121.
17. Cohen, L.A. A Review of Animal Model Studies of Tomato Carotenoids, Lycopene, and Cancer Chemoprevention. (2002) Exp Bio Med 227(10): 864-868.
18. Palozza, P., Catalano, A., Simone, R., et al. Lycopene as a guardian of redox signaling. (2012) Acta Biochim Pol 59(1): 21-25.
19. Sakr, S.A., Lamfon, H.A.Effect of green tea on metalaxyl fungicide induced liver injury in albino mice. (2005) Oxford Res Forum J 2: 65-69.
Pubmed||Crossref||Others
20. Matos, R.H., Capelozzi, L.V., Gomes, F.O., et al. Lycopene inhibits DNA damage and liver necrosis in rats treated with ferric nitrilotriacetate. (2001) Arch Biochem Biophys 396(2): 171-177.
21. Matos, H.R., Di-Mascio, P., Medeiros, M.H. Protective effect of lycopene on lipid peroxidation and oxidative DNA damage in cell culture. (2000) Arch Biochem Biophys 383(1): 56-59.
22. Bancroft, J.D. Theory and Practice of Histological Techniques. (1996) 4th ed: 766.
Pubmed||Crossref||Others
23. Kiernan, J. Histological and histochemical methods: theory and practice, 3rd edition. (1999) Butterworth-Heinemann, Oxford.: 502.
Pubmed||Crossref||Others
24. Sakr, S., Abdel-Samie, H.A. Apoptosis related protein Bax in liver of metalaxyl fungicide-treated mice: The effect of antox. Ozean. (2008) J Appl Sci 1(1): 17-27.
Pubmed||Crossref||Others
25. Ortatatli, M., Oğuz, H. Ameliorative effects of dietary clinoptilolite on pathological changes in broiler chickens during aflatoxicosis. (2001) Res Vet Sci 71(1): 59-66.
26. Schumann, G., Bonora, R., Ceriotti, F., et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. (2002) Clin Chem Lab Med 40(7): 718-724.
27. EL-Aaser, A.A., EL-Merzabani, M.M. Simultaneous determination of 5’-nucleotidase and alkaline activities in serum. (1975) Z Klin Chem Klin Biochem1 3(10): 453-459.
28. Vodovotz, Y. Modified microassay for serum nitrite and nitrate. (1996) Bio Techniques 20(3): 390-394.
29. Mesbah, L., Soraya, B. , Narimane, S., et al. protective effect of flavonides against the toxicity of vinblastine cyclophosphamide and paracetamol by inhibition of lipid – peroxydation and increase of liver glutathione. (2004) Haema 7(1): 59-67.
Pubmed||Crossref||Others
30. Bradley, P.P., Priebat, D.A., Christensen, R.D., et al. Measurement of cutaneous inflammation, Estimation of neutrophil content with an enzyme marker. (1982) J Invest Dermatol 78(3): 206-209.
31. Xu, J.B., Yuan, X.F., Lang, P.Z. Determination of catalase activity and catalase inhibition by ultraviolet spectrophotometry. (1997) Chin Environ Chem 16: 73-76.
Pubmed||Crossref||Others
32. Kakkar, P., Das, B., Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. (1984) Indian J Biochem Biophys 21(2): 130-132.
33. Livak, K. J., Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. (2001) Methods 25(4): 402- 408.
34. Singh, N.P., McCoy, M.T., Tice, R.R., et al. A simple technique for the quantitation of low levels of DNA damage in individual cells. (1988) Exp Cell Res 175(1): 184-191.
35. Lee, R.F., Steinert, S. Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and fresh water) animals. (2003) Mutation Res 544(1): 43-64.
36. Steel, R., Torrie, J., Dickey, D. Principles and procedures of Statistics. (1997) A Biometrical Approach, 3rd ed.
Pubmed||Crossref||Others
37. Magnarelli, G., Fonovich, T. Protein phosphorylation pathways disruption by pesticides. (2013) Adv Biol Chem 3(5): 460- 474.
38. Walker, M.M., Keith, H.L. Environmental Protection Agency’s Pesticide Fact Sheet Database. (1992) Lewis Publishers.
Pubmed||Crossref||Others
39. Gartner, L.P., Hiatt, J.L. Color Atlas of Histology. (2000) Lippincott Williams & Wilkins. 3 rd edition.
Pubmed||Crossref||Others
40. Pang, S., Xin, X., Stpierre, M.V. Determinants of metabolic disposition. (1992) Annual Rev Pharmacology Toxicology32: 625-626.
Pubmed||Crossref||Others
41. Kumar, C.S., Balamurugan, B., Murugeswaran, S., et al. Hepatoprotective Activity of leaves and roots Extracts of loringaoleifera lam. (2010) Inter J Medicobiological Res 1: 90-93.
Pubmed||Crossref||Others
42. Nakatani, T., Tawaramato, M., Kennedy, D., et al. Apoptosis induced by chelation of interacellular zinc is associated with depletion of cellular reduced glutathione level in rat hepatocytes. (2000) Chem Biol Interact 125(3): 51-163.
43. Sakr, S.A. Ameliorative effect of ginger (Zingiberofficinale) on mancozeb fungicide induced liver injury in albino rats. (2007) Aust J Basic Appl Sci 1(4): 650-656.
Pubmed||Crossref||Others
44. Nehru, B., Anand, P. Oxidative damage following chronic aluminum exposure in adult and pup rat brains. (2005) J Trace Elem Med Biol 19(2-3): 203-208.
45. Salman, K.A., Ashraf, S. Reactive oxygen species: A link between chronic inflammation and cancer. (2013) Asia Pac J Mol Biol Biotech 21(2): 42-49.
Pubmed||Crossref||Others
46. Hassan, M., Hussein, S.A, El Senosi, Y., et al. Role of Ginger as Anti-inflammatory and Anti-apoptotic in Protection of Liver Damage Induced by Metalaxyl Fungicide in Male Albino Rats. (2018) 8(3):1-8.
47. Morris, S.M., Billiar, T.R. New insights into the regulation of inducible nitric oxide synthesis. (1994) Am J Physiol 266(6): 829-839.
48. Dar, M.A., Khan, A.M., Raina, R. et al. Effect of repeated oral administration of bifenthrin on lipid peroxidation and antioxidant parameters in wistar rats. (2013) Bull. Environ ContamToxicol 91(1):125-128.
49. Calviello, G., Piccioni, E., Boninsegan, A. et al. DNA damage and apoptosis induction by the pesticide mancozeb in rat cells involvement of oxidative mechanism. (2006) Carcinogenesis 28: 1202-1209.
Pubmed||Crossref||Others
50. Lamfon, H.A., Protective effect of ginger (Zingiberofficinale) against metalaxyl induced hepatotoxicity in albino mice (2011) J Am Sci 7: 1093-1100.
Pubmed||Crossref||Others
51. Panda, V., Ashar, H., Srinath, S. Anitioxidant and hepato-protective effect of Garcinia indica fruit rind in ethanolinduced hepatic damage in rodents. (2012) InterdiscipToxicol 5(4): 207-213.
52. Abolaji, A.O., Awogbindin, I.O., Adedara, I.A., et al. Insecticide chlorpyrifos and fungicide carbendazim, common food contaminants mixture, induce hepatic, renal, and splenic oxidative damage in female rats. (2017) Hum Exp Toxicol 36(5): 483-493.
53. Lau, D., Mollnau, H., Eiserich, J.P., et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. (2005) Proc Natl Acad Sci 102(2): 431- 436.
54. Klinke, A., Nussbaum, C., Kubala, L., et al. Myeloperoxidase attracts neutrophils by physical forces. (2011) Blood 117(4): 1350-1358.
55. Jp, E., Farrell, G.C., Robertson, G., et al. Central role of PPAR alpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. (2003) Hepatology 38(1): 123-132.
56. Al-Eryani, L. The role of pesticides in non-alcoholic fatty liver disease (NAFLD). (2014) Electronic Theses and Dissertations.
57. Balakirev, M.Y., Khramtsov, V.V., Zimmer, G. Modulation of the mitochondrial permeability transition by nitric oxide. (1997) Eur J Biochem 246(3): 710-718.
58. Hortelano, S., Dallaporta, B., Zamzami, N., et al. Nitric oxide induces apoptosis via triggering mitochondrial permeability transition. (1997) FEBS Lett 410(2-3): 373-377.
59. Szabo, C. DNA strand breakage and activation of poly-ADP ribosyltransferase: a cytotoxic pathway triggered by peroxynitrite. (1996) Free Radic Biol Med 21(6): 855-869.
60. Szabo, C., Ohshima, H. DNA damage induced by peroxynitrite, subsequent biological effects. (1997) Nitric Oxide 1(5): 373-385.
61. Costantini, P., Petronilli, V., Colonna, R., et al. On the effects of paraquat on isolated mitochondria. Evidence that paraquat causes opening of the cyclosporin A-sensitive permeability transition pore synergistically with nitric oxide. (1995) Toxicol 99(1-2): 77–88.
62. Center, S.A.Metabolic, antioxidant, nutraceutical, probiotic, and herbal therapies relating to the management of hepatobiliary disorders. (2004) Vet Clin Small Anim Pract 34(1): 67-172.
63. Compare, D., Coccoli, P., Rocco, A., et al. Gut-Liver axis: The impact of gut microbiota on non-alcoholic fatty liver disease. (2012) Nutr Metab Cardiovasc Dis 22(6): 471-476.
64. Czaja, A.J. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. (2014) World J Gastroenterol 20(10): 2515-2532.
65. Reuter, S., Gupta, S.C., Chaturvedi, M. M., et al. Oxidative stress, inflammation and cancer: How are they linked. (2010) Free Radic Biol Med 49(11): 1603-1616.
66. Wallach, D., Varfolomeev, E.E., Malinin, N.L., et al. Tumor necrosis factor receptor and Fas signaling mechanisms. (1999) Annu Rev Immunol 17: 331-367.
67. De-Maria, R., Lenti, L., Malisan, F., et al. Requirement for GD3 ganglioside in CD95- and ceramideinduced apoptosis. (1997) Science 277(5332): 1652-1655.
68. Song, Y., Liang, X., Hu, Y., et al. p, p’-DDE induces mitochondria-mediated apoptosis of cultured rat Sertoli cells. (2008) Toxicology 253(1-3): 53-61.
69. Shi, Y.Q., Wang, Y.P., Song, Y., et al. p, p’-DDE induces testicular apoptosis in prepubertal rats via the Fas/FasL pathway.(2010) Toxicol Lett 193(1): 79-85.
70. Liu, G., Wang, T., Song, J., et al. Effects of apoptosis-related proteins caspase 3, Bax and Bcl-2 on cerebral ischemia rats. (2013) Biome Rep1(6): 861-867.
71. Lalier, L., Cartron, P.F., Juin, P., et al. Bax activation and mitochondrial insertion during apoptosis. (2007) Apoptosis 12(5): 887-896.
72. EI-Ghonaimy, N.M. Role of ginger (zingiberofficinale) against metalaxyl induced hepatotoxicity in male albino rats: a histological and immune-histochemical study. (2015) J HistolHistopath 2: 1-10.
73. Sahin, K.,Orhan, C., Tuzcu, M. et al. Orally administered lycopene attenuates diethylnitrosamine-induced hepato-carcinogenesis in rats by modulating Nrf-2/HO-1 and Akt/m TOR pathways. (2014) Nutr Cancer 66(4): 590-598.
74. Bose, K.S., Agrawal, B.K. Effect of lycopene from cooked tomatoes on serum antioxi-dant enzymes, lipid peroxidation rate and lipid profile in coronary heart disease. (2007) Singapore Med J 48(5): 415-420.
75. Stahl, W., Sies, H. Uptake of lycopene and its geometrical isomers is greater from heatprocessed than from unprocessed tomato juice in humans. (1992) J Nutr 122(11): 2161-2166.
76. Wertz, K., Siler, U., Goralczyk, R, et al. Lycopene: modes of action to promote prostate health. (2004) Arch Biochem Biophys 430(1): 127-134.
77. Ojha, S., Goyal, S., Sharma, C., et al. Cardioprotective effect of lycopene against isoproterenol-induced myocardial infarction in rats. (2013) Hum Exp Toxicol 32(5): 492-503.
78. Krinsky, N.I. The antioxidant and biological properties of the carotenoids. (1998) Ann N Y Acad Sci 854: 443-447.
Pubmed||Crossref||Others
79. Ben-Dor, A., Steiner, M., Gheber, L., et al. Carotenoids activate the antioxidant response element transcription system. (2005) Mol Cancer Ther 4(1): 177-186.
80. Barcelos, R.P., Bresciani, G., Rodriguez-Miguelez, P., et al. Diclofenac pretreatment effects on the toll-like receptor 4/nuclear factor-kB-mediated inflammatory response to eccentric exercise in rat liver. (2016) Life Sci 148: 247-253.
Pubmed||Crossref||Others
81. Atilgan, H.I.,Aydin, A., Sadic, M., et al. The protective effect of lycopene on kidney against experimentally induced unilateral ureteral obstruction. (2016) Acta Medica Mediterranea 32(5): 1631-1636.
Pubmed||Crossref||Others
82. Jacob, K., Periago, M.J., Bohm, V., et al. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. (2008) Brit J Nutr 99(1): 137-146.
83. Hazewindus, M., Haenen, G., Weseler, A.R., et al. The anti-inflammatory effect of lycopene complements the antioxidant action of ascorbic acid and tocopherol. (2012) Food Chem 132(2): 954-958.
84. Palozza, P., Parrone, N., Catalano, A., et al. Tomato lycopene and inflammatory cascade: Basic interactions and clinical implications. (2010) Curr Med Chem 17(23): 2547-2563.
85. Ford, N.A., Elsen, A.C., Erdman, J.W. Genetic ablation of carotene oxygenases and consumption of lycopene or tomato powder diets modulate carotenoid and lipid metabolism in mice. (2013) Nutr Res 33(9): 733-742.
86. Tan, H.L., Moran, N.E., Cichon, M.J., et al. β-Carotene-9’, 10’-oxygenase status modulates the impact of dietary tomato and lycopene on hepatic nuclear receptor, stress, and metabolism related gene expression in mice. (2014) J Nutr 144(4): 431-439.
87. Kersten, S., Mandard, S., Escher, P., et al. The peroxisome proliferator-activated receptor alpha regulates amino acid metabolism. (2001) FASEB J 15(11): 1971-1978.
88. Edgar, A.D., Tomkiewicz, C., Costet, P., et al. Fenofibrate modifies transaminase gene expression via a peroxisome proliferator activated receptor α-dependent pathway. (1998) Toxicol Lett 98(1-2): 13-23.
89. He, Q., Zhou, W., Xiong, C., et al. Lycopene attenuates inflammation and apoptosis in post-myocardial infarction remodeling by inhibiting the nuclear factor-κB signaling pathway. (2015) Mol Med Rep 11(1): 374-378.
90. Sheik Abdulazeez, S., Thiruvengadam, D. Effect of lycopene on oxidative stress induced during d-galactosamine/ lipopoly saccharide-sensitized liver injury in rats. (2013) Pharm Biol 51(12): 1592-1599.
91. Collins, A.R., Olmedilla, B., Southon, S. et al. Serum carotenoids and oxidative DNA damage in human lymphocytes. (1998) Carcinogenesis 19(12): 2159-2162.
92. Bayomy, N.A., Elbakary, R.H., Ibrahim, M.A.A., et al. Effect of lycopene and rosmarinic acid on gentamicin induced renal cortical oxidative stress, apoptosis, and autophagy in adult male albino rat. (2017) Anat Rec 300(6): 1137-1149.