Understanding the neural circuitry in Anorexia Nervosa
Neera Ghaziuddin
Affiliation
Department of Psychiatry, University of Michigan Medical School, Ann Arbor, MI
Corresponding Author
Shumaila Younas, M.D. Department of Psychiatry, University of Michigan Medical School, Ann Arbor, MI; E-mail: syounas@med.umich.edu
Citation
Shumaila Younas, M.D. Understanding the Neural Circuitry in Anorexia Nervosa. (2018) Clin Trials Pathol Case Stud 3(1): 1- 2.
Copy rights
© 2018 Shumaila Younas, M.D. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Abstract
I remember my frustration when I was consulted for a patient who was undergoing refeeding for a diagnosis of anorexia nervosa (AN) during an admission to a medical floor. Patient was a 17-year-old female who was an ambitious, straight A student with plans to become a business woman in the culinary industry. She was a pleasure to talk to until it was mealtime. I would look at her and wonder, “Why can’t she just eat?” Clearly, as I have had to learn, the problem or its fix is not simple!
Introduction
I remember my frustration when I was consulted for a patient who was undergoing re feeding for a diagnosis of anorexia nervosa (AN) during an admission to a medical floor. Patient was a 17-year-old female who was an ambitious, straight a student with plans to become a business woman in the culinary industry. She was a pleasure to talk to until it was mealtime. I would look at her and wonder, “Why can’t she just eat?” Clearly, as I have had to learn, the problem or its fix is not simple!
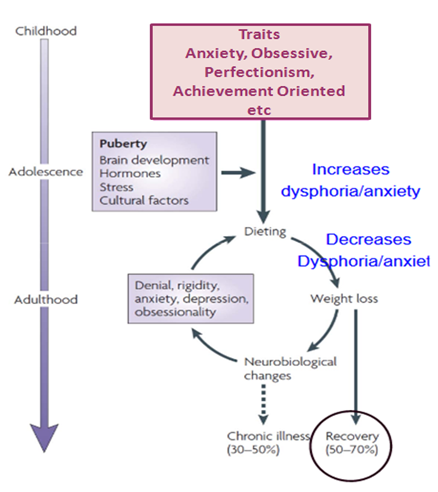
Similar to other psychiatric disorders, a bio psycho social understanding of an anorexic patient is essential to fully understand symptoms, which can appear irrational, even to a well-meaning psychiatrist. A broad underlying conceptualization of anorexia nervosa is that youth who are genetically predisposed, may develop symptoms when exposed to certain environmental and social factors[1]. In such instances, intra psychic conflicts, cognitive distortions and dissatisfaction with one’s body may result in a relentless, and often self-destructive, pursuit of thinness, thereby perpetuating a vicious cycle of weight loss, malnutrition and physiological changes[2].
Figure A: http://www.nature.com/nrn/journal/v10/n8/full/nrn2682.html
Twin studies by Bulik and colleagues[3] reported 50% heritability for anorexia nervosa. Certain gene mutations, such as those involving the serotonin system, result in serotonergic dysfunction. While mutations involving dopamine genes, implicated in there ward and affect pathways and in decision-making, result in decreased food intake in AN.(1 –b) Brain-derived neurotrophic factor (BDNF), which encodes for a neurotrophin, and has a main role in synaptic plasticity and neuronal growth and development, might influence eating behavior and weight regulation in anorexia nervosa[4]. This group of patients, who possess the genetic predisposition experience inactivation of the neural circuitry involved in the feeding process, when they are exposed to continuous social and environmental stressors[5]. Understanding this distorted neural circuitry involved in normal eating behavior also help treatment providers understand the pathophysiology of eating disorders and thus approach treatment with a greater understanding.
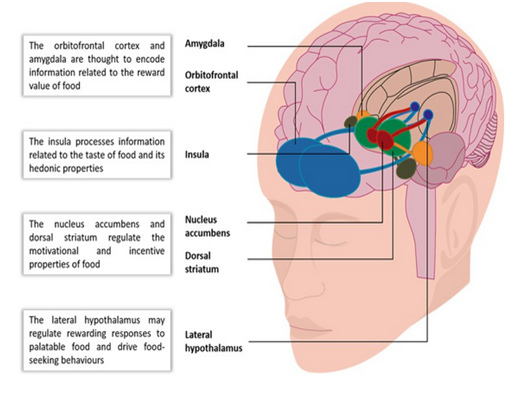
Another major advancement in this field has come from studies using Functional Magnetic Resonance Imaging (fMRI). fMRI studies have shown that in addition to the hypothalamus, which controls hunger and satiety, other higher centers such as the corticolimbic system are also involved[6]. The main neural pathways which have been implicated are, (1) network involving the insulin, anterior cingulate gyrus and the frontal operculum, which perceiveshunger, taste and food stimulus; (2) network involving the striatum, nucleus accumbens, putamen, caudate, orbito frontal cortex and the amygdale, which provides a dopamine surge in the reward system; (3) network involving the dorsal caudate, dorsal anterior cingulate gyrus, lateral prefrontal cortex and the parietal cortex which helps an individual regulate their food intake based on consideration for short and long term goals, such as energy needs and weight gain.
When a person, who does not have AN, eats food, sensations from her/his gut is transmitted to the thalamus and then to the insula, which perceive taste and experience hunger and satiety. The activation of the amygdale is contingent upon signals received from the insula. From the insula, signals are conveyed to activate nucleus accumbens resulting in a dopamine surge. Dopamine further activates dorsolateral prefrontal cortex (DLPFC), which is the main center for anticipation and provides an internal urge for the person to go for another mouthful of food. In the meantime, parietal cortex maintains balance between immediate and long-term effects of food on body and, therefore, food intake becomes a pleasurable experience. Simply stated, this delicate neural circuitry is essentially messed up in anorexia nervosa.
Figure B: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4530258/
These pathways work differently in patients with anorexia nervosa. For instance, in a patient with anorexia nervosa, due to inadequate serotonin registration at thalamus and insula, there is lack of perception of hunger for both homeostatic and hedonic need for food. This alter edinsular activity explains interoceptive dysfunction leading to impaired activation of striatum resulting in altered reward modulation. Food is interpreted as aversive and threatening, leading to activation of the amygdala. This increased firing from amygdala with resultant anxiety and back ground noise leads to impaired activation of the anticipation center i.e dorsolateral prefrontal cortex (DLPFC). Parietal cortex is also overactive making a patient with anorexia nervosa believe that any food consumption will result in gaining unrealistic amounts of weight. Due to food being interpreted as aversive and threatening- by not eating anorexics remove negative emotions associated with food.
Understanding anorexia nervosa from this perspective helps formulate treatment strategies. It likely helps clinicians understand the experiences of their patients who are in refeeding programs and are likely experiencing excessive anxiety when presented with food. During this weight restoration process, which is considered a corner stone in the effective treatment of AN, increased background noise and disturbed thought processes should be anticipated, therefore allowing clinicians to prepare their patients with challenges of refeeding. Some clinicians, using this understanding of complex circuits, have proposed using food as a medicine which should be pre-planned, pre-dosed and prescribed for this group of patients[7]. Theoretically, this approach may result in reduced intensity of distorted thoughts and anticipatory anxiety. It may also provide patients with an understanding that similar to a medication, food will come with some side effects such as disturbing thoughts but will likely improve over time.
The Maudsely model for treating anorexia nervosa is the most widely accepted treatment model[7]. A close examination of this model suggests that food is indeed being used as a “medicine” which is pre-calculated, controlled and planned by a parent and the clinician during refeeding. In addition to giving a clinician a better understanding of the neural pathophysiology, early identification of malfunctioning circuits has a future potential for early identification and early intervention.
References
1. Pinheiro, P.A., Root, T., Bulik, C.M. The Genetics of Anorexia Nervosa: Current Findings and Future Perspectives. Int J Child Adolesc health. (2009) Int J Child Adolesc health 2(2): 153–164.
2. Kaye, W.H., Fudge, J., Paulus, M. New Insights into Symptoms and Neurocircuit Function of Anorexia Nervosa. (2009) Nat Rev Neurosci 10(8): 573-584.
3. Mazzeo, S.E., Mitchell, K.S., Bulik, C.M., et al. Assessing the Heritability of Anorexia Nervosa Symptoms Using a Marginal Maximal Likelihood Approach. (2009) Psychol Med, 39(3): 463–473.
4. Kuipers, S.D., Bramham, C.R. Brain-derived Neurotrophic Factor Mechanisms and Function in Adult Synaptic plasticity: New Insights and Implications for Therapy. (2006) Current Opinion in Drug Discovery and Development, 9(5): 580-586.
5. Strober, M., Freeman, R., Lampert, C., et al. Males with Anorexia Nervosa: a controlled study of Eating Disorders in First- Degree Relatives. (2001) Int J Eat Disord 29(3): 263-269.
6. McAdams, C.J, Smith, W. Neural Correlates of Eating Disorders: Translational Potential. (2015) Neuroscience Neuroecon 4: 35-49.
7. Schmidt, U., Renwick, B., Lose, A., et al. The MOSAIC study - comparison of the Maudsley Model of Treatment for Adults with Anorexia Nervosa (MANTRA) with Specialist Supportive Clinical Management (SSCM) in outpatients with anorexia nervosa. (2013) Trials 14:160.